Abstract
Exposure to carbon monoxide (CO) during general anesthesia can result from volatile anesthetic degradation by carbon dioxide absorbents as well as re-breathing of endogenously produced CO. Although adherence to the Anesthesia Patient Safety Foundation guidelines reduces the risk of CO poisoning, patients may still experience a sub-toxic CO exposure during low-flow anesthesia. The consequences of such exposures are relatively unknown. In contrast to the widely recognized toxicity of high CO concentrations, the biological activity of low concentration CO has recently been shown be cytoprotective. As such, low dose CO is being explored as a novel treatment for a variety of different diseases. Here we review the concept of anesthesia-related CO exposure, identify the sources of production, detail the mechanisms of overt CO toxicity, highlight the cellular effects of low dose CO, and discuss the potential therapeutic role for CO as a part of routine anesthetic management.
Keywords: carbon monoxide, exposure, low-flow anesthesia, general anesthesia, toxicity, cytoprotection, therapy, exogenous, endogenous
Introduction
Carbon monoxide (CO) is a colorless, odorless, and tasteless gas that exerts a number of biologic effects in a variety of different tissues and organs.1,2 Historically, CO was regarded as a poison due to the well-characterized tissue hypoxia and overt toxicity that result following exposure to high concentrations.2 Classification of CO as a life-threatening toxin is certainly warranted given that it continues to be the major cause of poison-related death in the United States.1,2 However, CO also has biological activity at sub-toxic levels, affecting several cellular pathways in a more complex manner. Such CO-mediated effects can be either cytoprotective or pathologic, depending on the context, duration, and concentration of exposure. Anesthesiologists should be aware of CO and its effects given that CO exposure can occur during general endotracheal anesthesia.3–6 Although the consequences of such anesthesia-related exposures are relatively unknown and investigative efforts to understand them are just beginning, low dose CO is being developed more generally to treat a variety of conditions. Here we review the concept of CO exposure in the setting of an anesthetic and identify the sources of production, detail the mechanisms of overt CO toxicity, highlight the cellular effects of low dose CO, and discuss the potential therapeutic role for CO as a part of routine anesthetic management.
CO Exposure During General Endotracheal Anesthesia
The first report of CO detection within a closed circuit anesthesia system was published in 1965.7 In this study, the authors documented levels of CO as high as 810 parts per million (ppm) within the breathing circuit and concluded that “complete closure of the anesthetic system [allowed] build up” of CO.7 Although it is over 50 years old, this work insightfully identified the patient's own alveolar gas as a potential source of CO.7
In the early to mid-1990s, generation of CO within the anesthesia breathing circuit and patient exposure became more widely recognized.8 Several studies and anecdotal case reports during that period described high CO concentrations within the anesthesia circuit along with clinical signs of CO toxicity and elevated carboxyhemoglobin (COHb) levels.9–11 The earliest case report of such toxicity described a rise in COHb in a 76 year old, non-smoking woman undergoing general endotracheal anesthesia for thyroid resection.10 An arterial blood gas measured 25 minutes following anesthesia induction and 1 hour later demonstrated COHb levels of 9.1% and 28%, respectively.10 These findings were somewhat serendipitous given that co-oximetry measurement was performed on all blood gas samples as a standard and the anesthesiology care providers did not intend to assess such values as part of routine clinical care.10 The patient experienced a headache postoperatively which resolved with hyperbaric oxygen therapy.10 Fresh gas contamination was suspected, but was ruled out by the absence of CO in the oxygen and nitrous oxide supply lines.10
Several weeks later, a second case of intraoperative CO exposure was identified at the same institution.10 In this case, a COHb level of 24.7% was measured in a patient undergoing total hip replacement under general anesthesia.10 Although initial investigation failed to locate an exogenous source of CO, thorough assessment of the anesthesia circuit identified CO concentrations in excess of 500 ppm in the gas exiting the carbon dioxide absorbent canister.10 Eight cases of anesthetic-related CO exposure were subsequently reported in 1990 and, in all, a total of 31 cases were reported.3,12 In some instances, CO concentrations within the breathing circuit exceeded 1000 ppm and patient COHb levels reached 30% or greater.3
At the annual meeting of the American Society of Anesthesiologists in 1990, a review of these cases revealed one shared characteristic: most occurred on a Monday morning as the first anesthetic of the day or with an anesthesia machine that had been idle for at least two days previously.10 This finding raised the possibility of a chemical reaction between the conventional carbon dioxide absorbent and volatile anesthetic agents.10 Subsequent investigation demonstrated definitively that CO is generated when volatile anesthetics are degraded by conventional carbon dioxide absorbents.8,11,13–15 Importantly, the magnitude of CO production inversely correlated with the water content of the absorbent.8
In 2003, Abbott Laboratories, in collaboration with the Food and Drug Administration, released a “Dear Health Care Professional” letter to notify practitioners of the risk of adverse events when sevoflurane interacts with desiccated absorbent.16,17 This letter focused mostly on the risk of fire due to the exothermic reaction, but the reader was also alerted to the risk of CO production. In 2005, the Anesthesia Patient Safety Foundation (APSF) convened a meeting of medical experts and industry representatives to address the risk of fire within the anesthesia breathing circuit and the potential for CO exposure as a result of the chemical reaction.18 This safety conference focused mostly on formation of toxic products via the chemical breakdown of volatile anesthetics.18 As a result of the meeting, the APSF made two recommendations: first, they suggested the use of absorbents that lack strong alkali hydroxides and do not degrade inhalation anesthetics.18,19 Second, they provided several recommendations aimed at preventing conventional absorbent desiccation.18 Although adherence to these guidelines reduces the risk of overt CO poisoning, limiting volatile agent degradation by using hydrated absorbent does not necessarily prevent sub-toxic anesthesia-related CO exposure.
Exogenous Sources
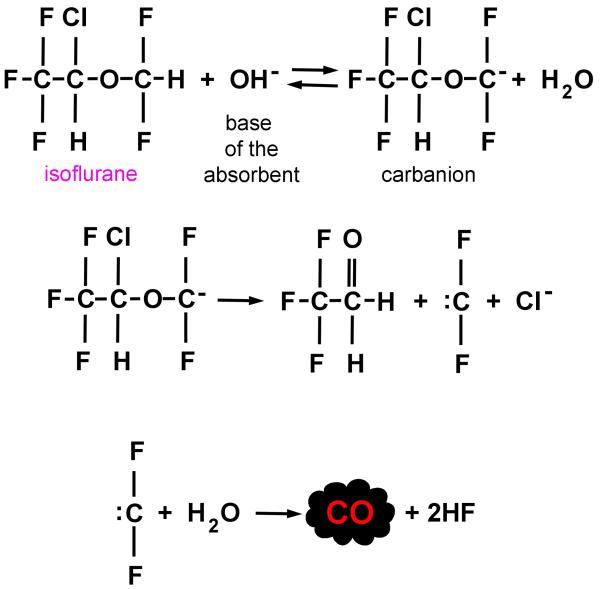
Exogenous CO is generated within the anesthesia breathing circuit when volatile anesthetic agents are degraded by conventional carbon dioxide absorbents.3,20 One mechanism believed to contribute to CO production is proton abstraction of the difluoromethyl ether moiety of halogenated agents (such as desflurane and isoflurane), catalyzed by the base of the absorbent to yield a carbanion intermediate (figure 1).20 In the absence of adequate water, the carbanion decomposes and subsequently interacts with hydroxide or residual water, generating CO.20 Sevoflurane and halothane can also generate CO, yet lack a difluoromethyl ether group, suggesting that other, less well defined mechanisms may be involved.21 Importantly, CO production via degradation by conventional absorbents has been demonstrated with all of the volatile anesthetic agents currently in use today.13,21,22
Figure 1. A proposed mechanism of carbon monoxide (CO) generation from isoflurane.
Proton (H+) abstraction is catalyzed by the base (OH−) of the carbon dioxide absorbent. In the absence of adequate water, the carbanion decomposes and subsequently interacts with hydroxide or residual water (H2O) within the absorbent, generating CO. Adapted from reference 20.
As mentioned earlier, water content is a key factor in determining the magnitude of anesthetic degradation and exogenous CO production within the anesthesia breathing circuit.8,13 Specifically, the amount of CO generated is inversely proportional to the amount of water in the absorbent.13 Completely dried absorbent produces the greatest amount of CO compared to partially hydrated absorbent.8,13 Reducing the water content in barium hydroxide lime from 13% (fully hydrated) to 8–10% by drying overnight for up to 14 hours with a fresh gas flow of 10 liters per minute, for example, leads to only minimal amounts of CO generation.23 On the other hand, reducing the water content of the absorbent to 5% or less by drying for 24 hours or more results in the production of significant concentrations of CO.23 This property likely explains the severe CO exposures seen on Monday mornings following use of anesthesia machines that were idle over the weekend with fresh gas flowing.23
Desiccated Baralyme® (Chemetron Medical Division, Allied Healthcare Products, St. Louis, MO), now obsolete and no longer commercially available, was shown to generate up to 20,000 ppm CO during a desflurane anesthetic while Baralyme® containing 1.6% or 3.2% water produced lower concentrations of CO (peak concentrations of 15,000 ppm or less) (table).13 With soda lime, fully desiccated absorbent generates approximately 2500 ppm CO from desflurane and ~500 ppm from isoflurane while partially hydrated absorbent produces only ~60 ppm CO and ~100 ppm with each agent, respectively.13 Rehydrating desiccated barium hydroxide lime prevented CO formation from desflurane which suggested that fully hydrated conventional absorbents would be completely safe.8,13 However, in vitro experimentation demonstrated that fully hydrated soda lime could, indeed, degrade desflurane during a 2-hour exposure and generates up to 23 ppm CO.24 Although the magnitude of CO produced from hydrated absorbent is markedly less than that generated by desiccated absorbent, CO formation still occurs and fresh gas flow (FGF) inversely correlates with CO concentration.24 A potential explanation is that fresh gas dilutes the CO that is formed during anesthetic degradation and lower FGFs may limit this dilutional effect within the breathing circuit, resulting in higher concentrations.24
Table.
Carbon dioxide absorbent composition and peak carbon monoxide generation.
| Chemical composition (%) | Peak CO (ppm) when fully desiccated | |||||||
|---|---|---|---|---|---|---|---|---|
| Absorbent | Ca(OH)2 | KOH | NaOH | LiOH | Ba(OH)2 | H2O | Refs | |
| Baralyme® | 74 | 4.6 | 0 | 11 | 14 | ~20,000 | 13,20 | |
| Classic Soda lime | 2.6 | 1.3 | 15 | ~2,500 | 13,20 | |||
| Medisorb® | 81 | 0.003 | 1.0 | 18 | ~13,000 | 31 | ||
| Spherasorb® | 84.5 | 0.003 | 1.5 | 14 | ~9,000 | 31 | ||
| LoFloSorb® | 84 | 16 | 525 | 31 | ||||
| Superia® | 79.5 | 17.5 | ~30 | 31 | ||||
| Amsorb® | 83.2 | 14.4 | 0 | 31 | ||||
| Lithium Hydroxide | 99 | 1 | 0 | 31 | ||||
Ba(OH)2, barium hydroxide; Ca(OH)2, calcium hydroxide; CO, carbon monoxide; H20, water; KOH, potassium hydroxide; LiOH, lithium hydroxide; NaOH, sodium hydroxide; ppm, parts per million; refs, references. Peak CO generation determined with various volatile anesthetics as detailed in indicated reference(s). Amsorb® is a product of Armstrong Ltd., Coleraine, Northern Ireland; Baralyme® was a product of Chemetron Medical Division, Allied Healthcare Products, St. Louis, MO; Medisorb® is a product of Datex Ohmeda, Helsinki, Finland; Spherasorb® and LoFloSorb® are products of Intersurgical, Wokingham, Berkshire, United Kingdom; Superia® is a product of Datex Ohmeda, Hoevelaken, The Netherlands.
In addition to the water content within the absorbent, other important variables that affect the degree of CO production include type of volatile agent used, anesthetic concentration, absorbent temperature, patient carbon dioxide production, and the chemical composition of the specific carbon dioxide absorbent in use.13,24 The amount of CO generated at equipotent anesthetic concentrations for the different volatile agents is generally described as: desflurane ≥ enflurane > isoflurane ≥ halothane = sevoflurane, with higher volatile agent concentrations producing higher peak concentrations of CO for each anesthetic.3,13,20,24 However, this relationship is not absolute given that the CO generating capacity of each agent depends on a number of variables and specific conditions. For example CO production from sevoflurane was shown to be exponential, reaching levels greater than 11,000 ppm when the temperature of desiccated Baralyme® rose to 80 °C.25 Such levels of CO exceed those produced by equipotent enflurane and isoflurane at temperatures of 45 °C with the same absorbent.13 Thus, comparisons made between anesthetic agents should be interpreted cautiously with regard to CO generation.
With regard to temperature, degradation of anesthetics and CO production are accelerated by heat.13 This attribute is particularly important because volatile agent degradation is an exothermic process.25,26 Thus, heat resulting from anesthetic breakdown raises the temperature of the absorbent, leading to further anesthetic degradation and greater CO formation.13,25,26 This effect is magnified when desiccated or partially dried absorbent is used.13 For example, sevoflurane undergoes 13% degradation over 1 hour at 40 °C while 56% is degraded over the same period of time when the temperature of the absorbent is increased to 60 °C.27
Carbon dioxide absorption is also exothermic and can reduce the water content in absorbent within the upstream canister over time.28 The combined effects of heating and drying the absorbent may indirectly increase the amount of CO produced and may explain why patients who produce more carbon dioxide may encounter higher concentrations of CO during an anesthetic.24,28 Although absorbent temperature is an important variable, the lack of a rise in soda lime temperature does not predict the amount of CO that is formed.21
Chemical composition of the carbon dioxide absorbent is another critical factor that influences volatile anesthetic breakdown and CO formation. Specifically, strong alkali hydroxides, such as potassium hydroxide and sodium hydroxide, are key components within conventional carbon dioxide absorbents responsible for anesthetic degradation.29 Since base-catalyzed proton abstraction occurs to a greater degree with potassium versus sodium hydroxide, dried barium hydroxide lime will produce more CO than desiccated soda lime.3,20 This is because Baralyme® contained 4.6% potassium hydroxide while conventional soda lime is composed of 2.6% potassium hydroxide and ~1.5% sodium hydroxide (table).20 Of note, Baralyme® was voluntarily removed from the United States market in 2004 by the manufacturer due to “concerns regarding [it's] use…in conjunction with…newer inhalation anesthetics when Baralyme® [was]…allowed, contrary to recommended practice, to become desiccated”.30
More modern carbon dioxide absorbents lack potassium and sodium hydroxide (table).31 These newer absorbents are composed of calcium or lithium hydroxide and generate approximately one tenth as much CO as conventional absorbents.3 However, CO production may still occur and clinical reports suggest significant CO generation with some of the calcium containing absorbents when they become desiccated.31 On the other hand, lithium hydroxide based absorbents appear to lack CO-generating capability, even when completely desiccated.31
Endogenous Sources
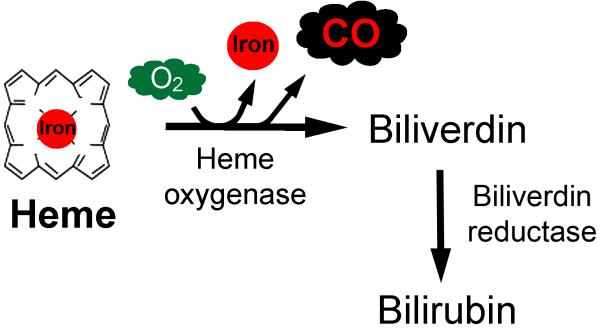
In addition to exogenous formation within the anesthesia breathing circuit, CO can also arise from endogenous patient sources.32 CO is produced naturally within the liver, spleen, and kidney and in tissues within the central nervous system and reticuloendothelial system during heme catabolism (figure 2).33 Following formation, CO diffuses into the circulation, binds to hemoglobin to form COHb, and is ultimately excreted by the lungs via exhalation.33.
Figure 2. Heme catabolism pathway.
In the presence of oxygen (O2), heme oxygenase catalyzes the breakdown of heme, generating equimolar amounts of free iron, biliverdin, and carbon monoxide (CO).
Low-flow anesthesia (LFA) is a commonly used, cost-saving paradigm to permit re-breathing and conservation of volatile anesthetics.34 Because exhaled CO is not scavenged or removed from the breathing circuit, patients may re-breathe exhaled CO during LFA.9 Children inspire up to 20 ppm CO with a concomitant rise in COHb during general endotracheal anesthesia when FGF is set below the rate of minute ventilation.5,6 CO re-breathing has also been demonstrated in adults during LFA and concentrations within the circuit may reach levels as high as 145 ppm CO in smokers.4 CO exposure inversely correlates with FGF rates such that higher CO levels result from lower FGFs.4,5 Importantly, little or no CO is re-breathed by children when FGFs exceed patient minute ventilation rates and CO has been predicted to decrease within the circuit by an average of 5.9 ppm for each 1 liter per minute increase in FGF in adults.4,5
Endogenously produced CO is thought to yield about 0.5% COHb and levels of up to 3% COHb in non-smoking city dwellers are considered normal.35 Increased endogenous CO production can occur in several clinical scenarios, leading to elevated COHb levels. This is seen with high heme turnover diseases such as autoimmune-mediated hemolysis, sickle cell anemia, and conditions that upregulate heme oxygenase activity and with heme breakdown states such as trauma, sepsis, and shock.36–39 Furthermore, environmental CO exposure, as occurs with smoke inhalation and with exposure to air pollution or tobacco smoke (including recreational water pipe smoking), also increases circulating COHb.40 In addition, patient COHb levels rise following transfusion with homologous packed red blood cells that have an elevated COHb content.41 The increase in COHb in these disease processes and following such exogenous exposures leads to greater CO concentrations in exhaled breath. As a result, these patients may experience an even more profound CO exposure when re-breathing is permitted during LFA.
Biologic effects of CO
Policy
In 1971, the United States Environmental Protection Agency established the National Ambient Air Quality Standard for CO which set the limit for a time-weighted average exposure at 9 ppm for 8 hours and 35 ppm for 1 hour in outdoor environments.42 This limit was based on research that demonstrated a correlation between an acute CO exposure and a rise in COHb with a concomitant reduction in time to the onset of angina in adults with coronary artery disease during exercise.43–50 There are currently no established limits for anesthesia-related CO exposure, in part because of a paucity of research assessing the risk of such exposures. However, one prior investigation demonstrated that preoperative exposure to tobacco smoke was a cardiac risk factor in patients undergoing general endotracheal anesthesia.51 In this work, acute smoking prior to surgery resulted in a mean exhaled CO concentration of 52.4 ppm (versus 9.4 ppm CO in non-smokers) and these concentrations along with rate pressure product were significant predictors of intraoperative ST segment depression when simultaneously considered.51 CO exposure during a general endotracheal anesthetic, thus, has the potential to interrupt myocardial tissue oxygenation.51 Such findings raise the question of whether safety limits for anesthesia-related CO exposure should be investigated and considered.
With regard to indoor ambient levels of CO, no legal regulations currently exist. However, several US agencies have put forward guidelines regarding the upper limits of exposure. For example, the Occupational Safety and Health Administration (OSHA) suggests limiting CO exposure to 50 ppm for 8 hours, The National Institute for Occupational Safety and Health (NIOSH) recommends a time-weighted limit of 35 ppm over 8 hours with an upper ceiling of 200 ppm, and The American Conference of Governmental Industrial Hygienists (ACGIH) recommends limiting CO exposure to 25 ppm for 8 hours.42
It should be noted that CO concentrations produced by completely desiccated and partially dehydrated conventional soda lime may exceed these recommended concentration limits.13,31 However, with strict adherence to the APSF guidelines, the risk of anesthesia-related CO poisoning can be reduced. With regard to LFA, the amount of CO inspired (from either exogenous or endogenous sources) can also meet or exceed these time-weighted limits depending on the concentration of CO in the circuit, the duration of the anesthetic, and the length of exposure.4–6,24 Unfortunately, anesthesia-related CO exposure has been poorly studied, so the consequences of such exposures remain relatively unknown.
Overt toxicity
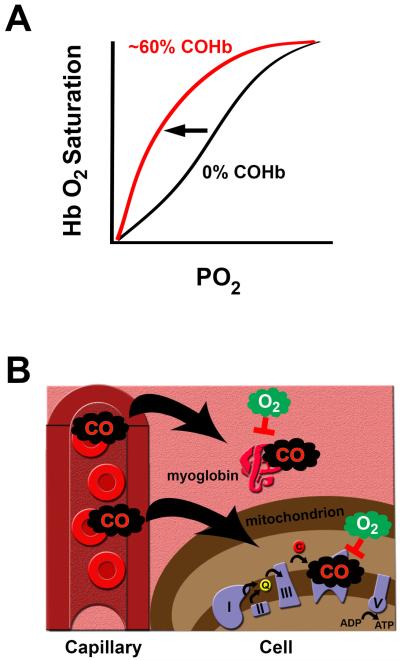
Following inspiration, CO diffuses across the alveolar capillary membrane and rapidly binds to hemoglobin to form COHb.52 The affinity of hemoglobin for CO is about 240 times greater than that for oxygen, and formation of COHb can interfere with the ability of oxygen to bind to and dissociate from hemoglobin.53,54 High levels of COHb shift the oxygen-hemoglobin dissociation curve to the left and impair tissue oxygen delivery (figure 3).54 Furthermore, CO can bind to hemoproteins such as myoglobin within the cytosol and cytochrome oxidase within the electron transport chain of mitochondria and high concentrations interrupt aerobic cellular energy production (figure 3).55,56 Thus, overt CO toxicity manifests as a result of tissue and cellular hypoxia and causes clinically detectable signs and symptoms when COHb is greater than 10-20%.2 In addition to tissue hypoxia, a number of other mechanisms of overt CO toxicity have recently been elucidated. These include CO-induced oxidative stress, peroxynitrite formation, inflammation, apoptosis, and immune-mediated injury.57 Thus, clinically relevant CO toxicity results from both tissue hypoxia and direct cellular effects.57
Figure 3. Overt carbon monoxide (CO) toxicity.
A. CO shifts the oxygen (O2)-hemoglobin (Hb) dissociation curve to the left (as indicated by the arrow) when critical levels of carboxyhemoglobin (COHb) are formed. Representative curves for 60% COHb (red curve) and 0% COHb (black curve) are depicted. PO2 is partial pressure of oxygen. The consequence of a left-shifted curve is impaired tissue delivery of oxygen. B. Following exogenous exposure, CO diffuses from the blood into tissues and cells and can bind to myoglobin within the cytosol and cytochrome oxidase (complex IV of the electron transport chain) within mitochondria. High concentrations of CO impair O2 availability and inhibit aerobic cellular energy (adenosine triphosphate [ATP]) production.
Organs with the greatest aerobic activity, such as the brain and the heart, are most vulnerable to overt CO toxicity.2 Cardiovascular effects of acute CO poisoning include hypotension due to vasodilation, arrhythmias, ischemia, infarction, and cardiac arrest while neurologic manifestations include headache, dizziness, impaired judgment, confusion, altered mental status, seizures, syncope, stroke, and coma.2,58 Such physiologic derangements occur regardless of the source of CO and can be seen in patients following toxic exposure to indoor or outdoor pollution as well as in burn-injured patients following smoke inhalation.2 Symptoms of CO toxicity in children may include nausea, vomiting, and lethargy.59,60 Interestingly, many of these symptoms mimic the adverse effects of a variety of anesthetic agents as well as clinical signs seen during routine emergence from general anesthesia. Thus, without monitoring inspired CO concentrations, determining CO content in exhaled breath, or measuring COHb levels, toxic anesthesia-related CO exposure can easily be overlooked. It should be noted that anesthesia-related CO exposure due to desiccated carbon dioxide absorbent reported in the 1990s often resulted in COHb levels that exceeded the 10-20% threshold and was associated with clinical signs of overt CO toxicity.8–10,12 In at least one of these cases, hyperbaric oxygen therapy led to resolution of the patient's symptoms.10
The magnitude of COHb formed and the manifestation of toxic effects depend on the concentration of CO that is inhaled and the duration of exposure.58,61 This time-weighted relationship is critical in determining the degree of toxicity following exposure to CO.58 Environmental exposure to less than 120 ppm CO for up to 4 hours, for example, is usually asymptomatic, does not result in tissue hypoxia, and is not life threatening.58,62,63 Lack of clinically detectable signs and symptoms defines such an exposure as sub-clinical and sub-toxic.58 On the other hand, exposure to an environment containing concentrations of CO that exceed 200 ppm readily elicits symptoms of overt toxicity and inspiring greater than 800 ppm CO leads to COHb levels that exceed 60% and can rapidly result in death.58
With regard to anesthesia-related CO exposure, the magnitude of COHb formation has previously been determined using a validated mathematical model that accounts for clinically relevant conditions during a general anesthetic with isoflurane or desflurane in the context of desiccated barium hydroxide lime.22 Calculated COHb levels were found to be higher in patients who were smaller, had anemia, and were breathing lower inspired oxygen concentrations.22 For example, with 7.5% desflurane and partially desiccated barium hydroxide lime, COHb levels exceeded 60% within 20 minutes of exposure in a 25 kg patient, but only reached 30% in a 100 kg patient during the same time period.22
Because anesthesia-related CO exposure was not commonly recognized in the era of routine Baralyme® use and prior to the institution of guidelines aimed at preventing desiccation of conventional soda lime, the role of such toxic CO exposures in causing perioperative morbidity and mortality is, historically, unknown. Importantly, removal of Baralyme® from the market and strict adherence to the APSF guidelines greatly reduces the risk of anesthesia-related overt CO toxicity. However, institutions continue to use conventional carbon dioxide absorbents and complete and partial desiccation can still occur. Therefore, exposure to high concentrations of CO during an anesthetic remains plausible and a potential risk. Nevertheless, the current rate of toxic anesthesia-related CO exposure is unknown, partly because monitoring for CO exposure is not a standard of care during anesthetic management.
As mentioned earlier, because the signs and symptoms of a toxic CO exposure can mimic those seen following a routine general anesthetic, CO poisoning can easily go undetected. From a safety standpoint, it seems logical that CO measurement technology should be incorporated into the anesthesia breathing system to monitor for and detect CO levels within the circuit. Devices that use an electrochemical oxidation principle to quantify CO concentrations currently exist and have been validated with the use of volatile anesthetic agents.64 Such monitoring is, thus, feasible. Furthermore, technology that measures COHb levels via non-invasive pulse co-oximetry has also been developed.65 Although COHb levels determined with this device compare favorably to those obtained via standard co-oximetry with acceptable limits of bias and precision, some studies suggest that the non-invasive technology is less accurate and limited to states of normoxia.65–70
Although evaluating COHb (invasively or non-invasively) may not be as useful in providing timely information regarding instantaneous exposure, monitoring gaseous CO levels within the circuit would certainly protect patients from a known anesthesia-related toxin by providing real-time data to the anesthesiologist (for example, elevated inspired CO levels could indicate use of desiccated soda lime and anesthetic degradation, prompting a change in absorbent). The primary goal of monitoring for CO within the anesthesia breathing circuit would be to prevent a toxic exposure.
With regard to sub-toxic CO exposures, inspiring low concentrations of CO commonly occurs during low-flow endotracheal anesthesia due to exogenous CO generation, re-breathing of endogenously produced exhaled CO, or both. The impact of such an exposure during an anesthetic is not known and has been poorly studied. Unlike toxic CO concentrations, however, low dose CO acts as a signaling molecule and can exert a range of diverse and complex cytoprotective effects.71 As such, low dose CO is currently being investigated and developed as a novel therapy for use in a variety of disease processes. Because patients often inspire low concentrations of CO during LFA, the beneficial effects of CO may be clinically relevant in the perioperative setting and could carry therapeutic potential. However, future study is necessary to determine if CO represents a common anesthesia-related pollutant with deleterious effects or if sub-toxic exposure during an anesthetic influences and improves patient outcomes.
A suggestion of a biologic effect of low-flow anesthesia
Despite the lack of rigorous scientific investigation of the effect of low dose CO exposure during LFA, several publications suggest a potential clinical impact of LFA, itself. For example, when compared with a high-flow approach, LFA with desflurane preserved pulmonary function and mucociliary clearance in adults undergoing tympanomastoidectomy.72 Such potential effects of LFA on respiratory function are consistent with known protective effects of low dose CO in the context of lung inflammation and oxidative injury.73 In another observational study evaluating the effect of fresh gas flow rates, LFA with sevoflurane was associated with lower plasma viscosity and prolonged activated partial thromboplastin times compared with high-flow anesthesia.74 In other work, LFA with desflurane in adults undergoing thyroidectomy was associated with a relative preservation of and significant increase in plasma nitric oxide (NO) levels 24 hours after surgery compared with a high-flow anesthesia approach.75 Although CO can cause a rapid release of NO from the hemoproteins that bind it, the relative increase in circulating NO seen postoperatively with LFA could be consistent with CO-mediated activation of nitric oxide synthase (NOS).76–81
Effects of low concentration CO
Although CO was once believed to be an insignificant toxic byproduct of endogenous heme catabolism, it is now evident that nanomolar concentrations can exert important biological activity in a variety of cells and tissues.82 CO was first identified as a signaling molecule following discovery of its ability to weakly stimulate soluble guanylate cyclase (sGC) to produce cyclic guanosine 3′,5′-monophosphate (cGMP).83–85 CO binds directly to the heme iron of the enzyme to stimulate cGMP generation in a manner similar to the well-known sGC agonist, NO.86 However, the process of CO binding is more simplistic than that of NO and CO-mediated activation of sGC is less potent than NO-mediated activation by approximately 100 fold.86,87 In addition, indirect and dynamic interactions between the two molecules exist. For example, in the presence of low concentrations of NO, CO modestly activates sGC, but with high concentrations of NO, CO acts a partial antagonist.87 Thus, although there are similarities between CO and NO, the biologic effects of each are complex and may differ when there is interaction between the two gaseous mediators.87
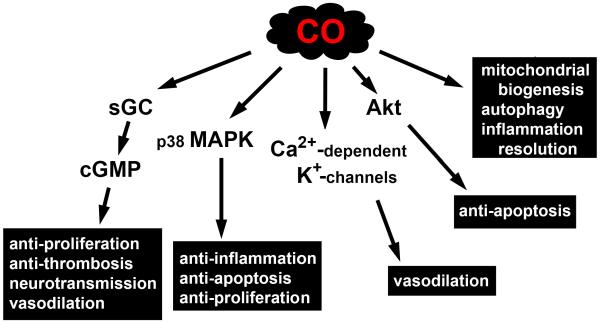
Since its identification as a gasotransmitter, CO has also been shown to modulate a number of p38 mitogen-activated protein kinase (MAPK)-related signaling pathways via both cGMP-dependent and independent processes, directly activate calcium-dependent potassium channels, induce protein kinase B (Akt) phosphorylation via the phosphatidylinositol 3-kinase/Akt pathway, and regulate the activity of a number of hemoproteins by binding to the iron center within their heme prosthetic groups.71, 88 Examples of CO-mediated cGMP-dependent signaling include inhibition of smooth muscle cell proliferation, inhibition of platelet aggregation, neurotransmission, and vasodilation, while CO-mediated cGMP-independent effects include anti-inflammation, anti-apoptosis, and anti-proliferation (figure 4).89–91 In addition, low dose CO promotes host and cell survival by stimulating mitochondrial biogenesis, inducing autophagy via mitochondrial reactive oxygen species generation, and accelerating the resolution of inflammation via a proresolving mechanism (figure 4).92–94
Figure 4. Biological activity of low concentration carbon monoxide (CO).
Various cellular pathways known to be affected by low dose CO are depicted. CO-mediated signaling includes stimulation of soluble guanylate cyclase (sGC) to produce cyclic guanosine 3′,5′-monophosphate (cGMP), modulation of p38 mitogen-activated protein kinase (MAPK), activation of calcium (Ca2+)-dependent potassium (K+) channels, induction of protein kinase B (Akt) phosphorylation via the phosphatidylinositol 3-kinase/Akt pathway, stimulation of mitochondrial biogenesis, induction of autophagy, and acceleration of inflammation resolution.
Potential therapeutic role of CO
Interpretation of studies evaluating the potential therapeutic effect of CO should be carried out cautiously given the importance of oxygen tension and oxyhemoglobin saturation in determining the tissue delivery of CO.95,96 Drawing definitive conclusions about the effects of specific CO concentrations becomes difficult without explicit measurement of such variables. Furthermore, the lack of hemoglobin and its impact on CO binding and diffusion with in vitro experimentation must be taken into account when interpreting the effect of CO exposure of cells and tissue in culture. As such, in vitro exposure to specific concentrations of CO may not exactly reflect similar time-weighted exposures in vivo. In addition, many of the experiments studying low dose exposure used CO concentrations in excess of those inspired during routine LFA. Although technically sub-toxic, such CO exposures may not be directly translatable to the setting of an anesthetic. In lieu of these limitations, preclinical investigation using various animal models of injury and disease suggests that inhaled exogenous CO confers cytoprotection in many different tissues and organ systems.89,97,98 The clinical scenarios that have been experimentally modeled represent human disease states that are commonly encountered in the perioperative setting. Thus, the therapeutic potential of CO may prove to be clinically relevant to anesthesiologists in the near future.
In a rat model of hyperoxia-induced lung injury, for example, inhaled CO (250 ppm) attenuated neutrophil accumulation and apoptosis in the lungs following exposure and significantly improved survival.97 In a rodent model of ventilator-induced lung injury, various concentrations of exogenous CO (up to 250 ppm) reduced tumor necrosis factor-α (TNF- α) and total cell count in bronchoalveolar lavage fluid while increasing levels of the anti-inflammatory cytokine, interleukin (IL)-10.99 The mechanisms of CO-mediated protection from ventilator-induced lung injury appear to involve p38 MAPK signaling, differential regulation of anti- and pro-inflammatory transcription factors such as the proliferator-activated receptor (PPAR)-gamma and early growth response (Egr)-1, and increased caveolin-1 expression.99–101
In mechanically ventilated baboons with pneumococcal pneumonia, inhaled CO (100–300 ppm) given along with antibiotics accelerated the resolution of acute lung injury.102 In a mouse model of aspiration, inhalation of 500 ppm CO for 6 hours significantly reduced the number of neutrophils in the lavage fluid and decreased the degree of lung injury following intratracheal injection of an acidic solution.103 Furthermore, in an aeroallergen model of asthma, inhaled CO (250 ppm) markedly reduced the amount of eosinophils and IL-5 in the bronchoalveolar lavage fluid and reduced airway reactivity via a cGMP-dependent mechanism.104,105
In addition, inhaled low dose CO protects the lungs following ischemia and reperfusion by attenuating the inflammatory response and by preventing apoptosis.106–111 These pro-survival effects have been demonstrated in clinically relevant animal models of lung transplantation, cardiopulmonary bypass (CPB), and secondary lung injury due to remote ischemia/reperfusion.108,110,112,113 The mechanisms of such cytoprotection involve the p38 MAPK pathway, the phosphatidylinositol 3-kinase/Akt pathway, and Egr-1 expression.107,109–111 The CO-mediated effects could potentially benefit mechanically ventilated patients routinely cared for by anesthesiologists in the operating room environment and in the intensive care unit (ICU).
In humans, the anti-inflammatory effects of inhaled CO have been preliminarily tested in patients with chronic obstructive pulmonary disease.114 In a recent pilot study, for instance, inspiring 100–125 ppm CO for 2 hours a day for 4 consecutive days resulted in a trend toward reduced eosinophils within the sputum and improved airway responsiveness to methacholine.114 In other work, a Phase I trial is currently assessing the safety of inhaled CO (100–200 ppm) in intubated ICU patients with sepsis-induced Acute Respiratory Distress Syndrome.115
The cardiovascular system is another successful therapeutic target of low dose CO. In a rat model of left anterior descending coronary artery occlusion, for example, pre-occlusion exposure to CO reduced the infarct area size, suppressed macrophage and monocyte migration into the area at risk, and decreased the expression of TNF- α.116 CO-mediated protection was related to cGMP and endothelial NOS and activation of the p38 MAPK and Akt pathways within myocardium.116 In a CPB model in pigs that included 2 hours of cardiac arrest, exposure to 250 ppm CO for 2 hours prior to application of the aortic cross clamp and administration of CO-saturated cardioplegia solution resulted in enhanced myocardial bioenergetics, less interstitial edema and cardiomyocyte apoptosis, and fewer number of electrical cardioversions required post reperfusion.117 Thus, exogenous CO may prevent injury to the heart following ischemia and reperfusion.
As with the lungs, low dose CO also protects the heart in the setting of organ transplantation. In a model of mouse-to-rat cardiac transplantation, exposure of the donor and recipient to 250–400 ppm CO prior to and following transplant, respectively, suppressed rejection and promoted long-term graft survival via inhibition of platelet aggregation, thrombosis, myocardial infarction, and cardiomyocyte apoptosis.118 In related work, exposing rat donors to 400 ppm CO prior to transplantation and placing the explanted heart in storage solution containing 1000 ppm CO protected the graft from ischemia/reperfusion injury via an anti-apoptotic mechanism.119 Furthermore, exposing rat heterotopic heart transplant recipients to 20 ppm CO for up to 100 days post-transplant, markedly prolonged allograft survival.120 In this model, exogenous CO reduced the amount of vascular inflammation, fibrosis, and cellular infiltration in the donated heart, and inhibited the expression of pro-inflammatory cytokines and mediators.120 Thus, CO may be developed in the near future as a novel therapeutic adjunct to standard myocardial protective strategies in the setting of cardiac surgery and cardiac transplantation.
Pulmonary arterial hypertension (PAH), a pathologic state that manifests from a wide range of etiologies and disease processes, carries significant perioperative risk.121 In a rat model of PAH, chronic exposure to 50 ppm CO attenuated hypoxia-induced PAH via activation of calcium-activated potassium channels within pulmonary artery smooth muscle cells.122 In other work, exposure to 250 ppm CO for 1 hour per day reversed PAH and right ventricle hypertrophy and restored pulmonary vascular architecture to near normal in a variety of rat PAH models.123 CO-mediated reversal of PAH in these studies involved endothelial NOS and was associated with an increase in smooth muscle apoptosis and a decrease in cellular proliferation.123
With regard to clinical studies, two ongoing trials are currently evaluating CO as a potential therapy for PAH.124,125 The first, a Phase I/Phase II trial in adults with severe PAH, is evaluating the safety and efficacy of inspiring 150 ppm CO for 3 hours at variable frequencies over a 16 week study period.124 The second study is a Phase I trial in neonates with PAH evaluating for adverse events associated with inspiring exogenous CO.125 Depending upon trial outcomes, it is possible that inhaled CO could be developed as a therapeutic intervention for patients with PAH.
The brain is another potential target of low concentration CO. Exposure to 250 ppm CO, for example, preconditioned mouse neurons cultured in vitro and provided protection from experimentally-induced cell death via an anti-apoptotic manner.126 The pro-survival mechanism in this work involved activation of GC and NOS and was dependent on CO-mediated generation of reactive oxygen species.126 Preconditioning with low dose CO in vivo also protects neuronal tissue in a variety of animal models. For instance, in a piglet model of cardiopulmonary bypass, exposure to 250 ppm CO for 3 hours, 1 day prior to surgery, completely prevented cell death in the neocortex and hippocampus following deep hypothermic circulatory arrest.127 In other work, preconditioning with 250 ppm CO prevented hypoxia- and ischemia-induced apoptosis in the hippocampus of newborn mice.128 Post-conditioning with CO also provides some degree of neuroprotection in models of permanent brain ischemia. For example, exposing mice to 250 ppm CO for 18 hours following complete occlusion of the middle cerebral artery reduced infarct size by about 30%.129 Thus, low concentration CO has potential as a future therapy to protect the brain from injury.
In contrast, however, low concentration CO can pathologically disrupt proliferation, differentiation, myelination, and natural apoptosis in the brain and neuronal tissue during development.130–134 Low dose CO exposure in the postnatal period, for example, permanently impaired the developing rodent auditory system and CO-mediated inhibition of natural programmed cell death in the newborn mouse brain resulted in excess number of neurons and long-lasting cognitive and behavioral defects.134–137 These disruptive effects of CO exposure are likely due to the unique sensitivity and vulnerability of the developing brain to a variety of biologically active agents that can interrupt the critical processes involved in neurodevelopment.138,139
Juxtaposed with the deleterious effect on natural cell death, however, is the ability of low dose CO to protect the developing brain against certain neurotoxic pro-apoptotic stimuli.140,141 For example, in a mouse model of anesthesia-induced neurodegeneration, postnatal exposure to 100 ppm CO limited and prevented isoflurane-induced neurotoxicity via an anti-apoptotic mechanism and modulated lipid peroxidation within forebrain mitochondria.140,141 Thus, the pro-survival effects of low concentration CO may have differential effects in the developing brain depending on the context of exposure. As such, further work will be necessary to determine the neurodevelopmental consequences of CO exposure during states of health and disease before low concentration CO can be adopted as a therapeutic agent for infants and children.
Conclusions
Because patients can be exposed to CO during the administration of a general endotracheal anesthetic, anesthesiologist should appreciate the history of anesthesia-related CO exposure, understand the conditions for exposure, and be aware of the sources of CO within the anesthesia breathing system. Although the risk of anesthesia-related overt CO toxicity can be reduced by strict adherence to APSF guidelines, generation of high concentrations of CO within the circuit can still occur with the use of certain carbon dioxide absorbents when they become desiccated. Thus, anesthesia care providers should know the type of carbon dioxide absorbent they are using, understand their institutional and departmental practice with regard to changing the absorbent, and maintain vigilance to ensure that policies are followed to prevent conventional absorbent desiccation. Furthermore, CO monitoring and detection technology within the anesthesia breathing circuit should be considered as a standard safety measure to prevent toxic exposures.
Anesthesiologists should also understand that the practice of low-flow general endotracheal anesthesia may result in low concentration CO exposure. Although the effects of such sub-toxic exposures are not well understood, animal studies indicate that inspiring low dose CO might be beneficial from a cytoprotective standpoint, but might also have pathologic consequences depending on the context of exposure. Because of the lack of clinical information and formal study regarding such anesthesia-related CO exposures in patients, further investigation is necessary and anesthesiologists should not change their practice. Instead, they should be aware of ongoing clinical trials and await the results of studies aimed at determining the safety and therapeutic efficacy of low concentration CO. Furthermore, anesthesiologists should recognize that the phenomenon of anesthesia-related CO exposure provides us with the opportunity to be at the forefront of translational investigation evaluating the effect of such exposures in our patient populations and unique clinical settings.
The preclinical evidence demonstrating the potential pro-survival benefits of low dose CO is certainly intriguing. However, because sub-toxic CO exposure has the potential for both beneficial and deleterious consequences, further understanding of the biologic effects is necessary. In order for CO to be developed as a novel perioperative therapeutic agent, ideal patient populations and clinical contexts will need to be identified and the benefits of exposure will need to outweigh the risks. If successful, CO could be seamlessly incorporated into to the armamentarium of gaseous agents that we routinely administer and titrate to effect each day. Intended CO exposure, whether achieved by a LFA technique or by exogenous CO administration, could be guided by real-time monitoring within the anesthesia breathing circuit to permit exact dosing and precise time-weighted exposures. With future investigation and better understanding, we may find that perioperative CO exposure has the potential to impact our patients by improving their recovery from various disease processes, preventing perioperative-related injury, and enhancing outcome and survival.
Acknowledgments
Supported by NIH/NIGMS R01GM103842-01, NIEHS Center for Environmental Health in Northern Manhattan
Footnotes
Conflicts of interest: None
References
- 1.Iqbal S, Clower JH, Hernandez SA, Damon SA, Yip FY. A review of disaster-related carbon monoxide poisoning: surveillance, epidemiology, and opportunities for prevention. Am J Public Health. 2012;102:1957–63. doi: 10.2105/AJPH.2012.300674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kao LW, Nañagas KA. Carbon monoxide poisoning. Med Clin N Am. 2005;89:1161–1194. doi: 10.1016/j.mcna.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Coppens MJ, Versichelen LF, Rolly G, Mortier EP, Struys MM. The mechanisms of carbon monoxide production by inhalational agents. Anaesthesia. 2006;61:462–8. doi: 10.1111/j.1365-2044.2006.04536.x. [DOI] [PubMed] [Google Scholar]
- 4.Tang CS, Fan SZ, Chan CC. Smoking status and body size increase carbon monoxide concentrations in the breathing circuit during low-flow anaesthesia. Anesth Analg. 2001;92:542–7. doi: 10.1097/00000539-200102000-00048. [DOI] [PubMed] [Google Scholar]
- 5.Levy RJ, Nasr VG, Rivera O, Roberts R, Slack M, Kanter JP, Ratnayaka K, Kaplan RF, McGowan FX. Detection of carbon monoxide during routine anesthetics in infants and children. Anesth Analg. 2010;110:747–53. doi: 10.1213/ANE.0b013e3181cc4b9f. [DOI] [PubMed] [Google Scholar]
- 6.Nasr VG, Emmanuel J, Deutsch N, Slack M, Kanter J, Ratnayaka K, Levy R. Carbon monoxide re-breathing during low-flow anaesthesia in infants and children. Br J Anaesth. 2010;105:836–41. doi: 10.1093/bja/aeq271. [DOI] [PubMed] [Google Scholar]
- 7.Middleton V, Van Poznak A, Artusio JF, Jr, Smith SM. Carbon monoxide accumulation in closed circle anesthesia systems. Anesthesiology. 1965;26:715–9. doi: 10.1097/00000542-196511000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Baxter PJ, Kharasch ED. Rehydration of desiccated Baralyme prevents carbon monoxide formation from desflurane in an anesthesia machine. Anesthesiology. 1997;86:1061–5. doi: 10.1097/00000542-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Lentz RE. Carbon monoxide poisoning during anesthesia poses puzzles. J Clin Monit. 1995;11:66–7. doi: 10.1007/BF01627423. [DOI] [PubMed] [Google Scholar]
- 10.Moon RE. Cause of CO poisoning, relation to halogenated agents still not clear. J Clin Monit. 1995;11:67–71. doi: 10.1007/BF01627425. [DOI] [PubMed] [Google Scholar]
- 11.Woehlck HJ, Dunning M, 3rd, Nithipatikom K, Kulier AH, Henry DW. Mass spectrometry provides warning of carbon monoxide exposure via trifluoromethane. Anesthesiology. 1996;84:1489–93. doi: 10.1097/00000542-199606000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Moon RE, Ingram C, Brunner EA, Meyer AF. Spontaneous generation of carbon monoxide within anesthetic circuits. Anesthesiology. 1991;75(suppl 3A):A873. [Google Scholar]
- 13.Fang ZX, Eger EI, 2nd, Laster MJ, Chortkoff BS, Kandel L, Ionescu P. Carbon monoxide production from degradation of desflurane, enflurane, isoflurane, halothane, and sevoflurane by soda lime and Baralyme. Anesth Analg. 1995;80:1187–93. doi: 10.1097/00000539-199506000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Woehlck HJ, Dunning M, 3rd, Gandhi S, Chang D, Milosavljevic D. Indirect detection of intraoperative carbon monoxide exposure by mass spectrometry during isoflurane anesthesia. Anesthesiology. 1995;83:213–7. doi: 10.1097/00000542-199507000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Frink EJ, Jr, Nogami WM, Morgan SE, Salmon RC. High carboxyhemoglobin concentrations occur in swine during desflurane anesthesia in the presence of partially dried carbon dioxide absorbents. Anesthesiology. 1997;87:308–16. doi: 10.1097/00000542-199708000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Laster M, Roth P, Eger EI., 2nd Fires from the interaction of anesthetics with desiccated absorbent. Anesth Analg. 2004;99:769–74. doi: 10.1213/01.ANE.0000136553.69002.C9. [DOI] [PubMed] [Google Scholar]
- 17. [Accessed November 16, 2015]; http://www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/%20SafetyAlertsforHumanMedicalProducts/UCM169499.pdf.
- 18. [Accessed November 16, 2015];Carbon Dioxide Absorbent Desiccation Safety Conference Convened by APSF. http://www.apsf.org/newsletters/pdf/summer2005.pdf.
- 19.Murray JM, Renfrew CW, Bedi A, McCrystal CB, Jones DS, Fee JP. Amsorb: a new carbon dioxide absorbent for use in anesthetic breathing systems. Anesthesiology. 1999;91:1342–8. doi: 10.1097/00000542-199911000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Baxter PJ, Garton K, Kharasch ED. Mechanistic aspects of carbon monoxide formation from volatile anesthetics. Anesthesiology. 1998;89:929–41. doi: 10.1097/00000542-199810000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Keijzer C, Perez RS, De Lange JJ. Carbon monoxide production from five volatile anesthetics in dry sodalime in a patient model: halothane and sevoflurane do produce carbon monoxide; temperature is a poorpredictor of carbon monoxide production. BMC Anesthesiol. 2005;5:6. doi: 10.1186/1471-2253-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woehlck HJ, Mei D, Dunning MB, 3rd, Ruiz F. Mathematical modeling of carbon monoxide exposures from anesthetic breakdown: effect of subject size, hematocrit, fraction of inspired oxygen, and quantity of carbon monoxide. Anesthesiology. 2001;94:457–60. doi: 10.1097/00000542-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Woehlck HJ, Dunning M, 3rd, Raza T, Ruiz F, Bolla B, Zink W. Physical factors affecting the production of carbon monoxide from anesthetic breakdown. Anesthesiology. 2001;94:453–6. doi: 10.1097/00000542-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Fan SZ, Lin YW, Chang WS, Tang CS. An evaluation of the contributions by fresh gas flow rate, carbon dioxide concentration and desflurane partial pressure to carbon monoxide concentration during low fresh gas flows to a circle anaesthetic breathing system. Eur J Anaesthesiol. 2008;25:620–6. doi: 10.1017/S0265021508003918. [DOI] [PubMed] [Google Scholar]
- 25.Holak EJ, Mei DA, Dunning MB, 3rd, Gundamraj R, Noseir R, Zhang L, Woehlck HJ. Carbon monoxide production from sevoflurane breakdown: modeling of exposures under clinical conditions. Anesth Analg. 2003;96:757–64. doi: 10.1213/01.ANE.0000049584.64886.39. [DOI] [PubMed] [Google Scholar]
- 26.Dunning MB, 3rd, Bretscher LE, Arain SR, Symkowski Y, Woehlck HJ. Sevoflurane breakdown produces flammable concentrations of hydrogen. Anesthesiology. 2007;106:144–8. doi: 10.1097/00000542-200701000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Strum DP, Johnson BH, Eger EI., II Stability of sevoflurane in soda lime. Anesthesiology. 1987;67:779–81. doi: 10.1097/00000542-198711000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Strum DP, Eger EI., II The degradation, absorption and solubility of volatile anaesthetics in soda lime depend on water content. Anesth Analg. 1994;78:340–348. doi: 10.1213/00000539-199402000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Neumann MA, Laster MJ, Weiskopf RB, Gong DH, Dudziak R, Förster H, Eger EI., 2nd The elimination of sodium and potassium hydroxides from desiccated soda lime diminishes degradation of desflurane to carbon monoxide and sevoflurane to compound A but does not compromise carbon dioxide absorption. Anesthesia and Analgesia. 1999;89:768–73. doi: 10.1097/00000539-199909000-00046. [DOI] [PubMed] [Google Scholar]
- 30. [Accessed March 3, 2015]; http://www.sec.gov/Archives/edgar/data/874710/000095013704007365/c87912e8vk.txt.
- 31.Keijzer C, Perez RS, de Lange JJ. Carbon monoxide production from desflurane and six types of carbon dioxide absorbents in a patient model. Acta Anaesthesiol Scand. 2005;49:815–8. doi: 10.1111/j.1399-6576.2005.00690.x. [DOI] [PubMed] [Google Scholar]
- 32.Woehlck HJ. Carbon monoxide rebreathing during low flow anesthesia. Anesth Analg. 2001;93:516–7. doi: 10.1097/00000539-200108000-00058. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi M, Takahashi T, Morimatsu H, Fujii H, Taga N, Mizobuchi S, Matsumi M, Katayama H, Yokoyama M, Taniguchi M, Morita K. Increased carbon monoxide concentration in exhaled air after surgery and anaesthesia. Anesth Analg. 2004;99:444–8. doi: 10.1213/01.ANE.0000123821.51802.F3. [DOI] [PubMed] [Google Scholar]
- 34.Baum JA, Aitkenhead AR. Low-flow anaesthesia. Anaesthesia. 1995;50(Suppl):37–44. doi: 10.1111/j.1365-2044.1995.tb06189.x. [DOI] [PubMed] [Google Scholar]
- 35.Marshall MD, Kales SN, Christiani DC, Goldman RH. Are reference intervals for carboxyhemoglobin appropriate? A survey of Boston area laboratories. Clin Chem. 1995;41:1434–8. [PubMed] [Google Scholar]
- 36.Sylvester KP, Patey RA, Rafferty GF, Rees D, Thein SL, Greenough A. Exhaled carbon monoxide levels in children with sickle cell disease. Eur J Pediatr. 2005;164:162–5. doi: 10.1007/s00431-004-1605-8. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan M, Vreman HJ, Hammerman C, Leiter C, Rudensky B, MacDonald MG, Stevenson DK. Combination of ABO blood group incompatibility and glucose-6-phosphate dehydrogenase deficiency: effect on hemolysis and neonatal hyperbilirubinemia. Acta Paediatr. 1998;87:455–7. doi: 10.1080/08035259850157093. [DOI] [PubMed] [Google Scholar]
- 38.Wohlfeil ER, Woehlck HJ, Gottschall JL, Poole W. Increased carboxyhemoglobin from hemolysis mistaken as intraoperative desflurane breakdown. Anesth Analg. 2001;92:1609–10. doi: 10.1097/00000539-200106000-00051. [DOI] [PubMed] [Google Scholar]
- 39.Moncure M, Brathwaite CE, Samaha E, Marburger R, Ross SE. Carboxyhemoglobin elevation in trauma victims. J Trauma. 1999;46:424–7. doi: 10.1097/00005373-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Levy RJ. Carbon monoxide pollution and neurodevelopment: A public health concern. Neurotoxicol Teratol. 2015;49:31–40. doi: 10.1016/j.ntt.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehlers M, Labaze G, Hanakova M, McCloskey D, Wilner G. Alarming levels of carboxyhemoglobin in banked blood. J Cardiothorac Vasc Anesth. 2009;23:336–8. doi: 10.1053/j.jvca.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 42.US Environmental Protection Agency [Accessed November 16, 2015];Carbon monoxide. 2015 [Online]. Available at: http://www.epa.gov/airquality/carbonmonoxide/
- 43.Adams KF, Koch G, Chatterjee B, Goldstein GM, O'Neil JJ, Bromberg PA, Sheps DS, McAllister S, Price CJ, Bissette J. Acute elevation of blood carboxyhemoglobin to 6% impairs exercise performance and aggravates symptoms in patients with ischemic heart disease. J. Am. Coll. Cardiol. 1988;12:900–909. doi: 10.1016/0735-1097(88)90452-4. [DOI] [PubMed] [Google Scholar]
- 44.Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J. Short-term effects of carbon monoxide exposure on the exercise performance of subjects with coronary artery disease. NEJM. 1989;321:1426–1432. doi: 10.1056/NEJM198911233212102. [DOI] [PubMed] [Google Scholar]
- 45.Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J. Effects of carbon monoxide on myocardial ischemia. Environ. Health Perspect. 1991;91:89–132. doi: 10.1289/ehp.919189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson EW, Andelman RJ, Strauch JM, Fortuin NJ, Knelson JH. Effect of low-level carbon monoxide exposure on onset and duration of angina pectoris. A study in ten patients with ischemic heart disease. Ann Intern Med. 1973;79:46–50. doi: 10.7326/0003-4819-79-1-46. [DOI] [PubMed] [Google Scholar]
- 47.Aronow WS, Isbell MW. Carbon monoxide effect on exercise-induced angina pectoris. Ann Intern Med. 1973;79:392–5. doi: 10.7326/0003-4819-79-3-392. [DOI] [PubMed] [Google Scholar]
- 48.Kleinman MT, Davidson DM, Vandagriff RB, Caiozzo VJ, Whittenberger JL. Effects of short-term exposure to carbon monoxide in subjects with coronary artery disease. Arch. Environ. Health. 1989;44:361–369. doi: 10.1080/00039896.1989.9935908. [DOI] [PubMed] [Google Scholar]
- 49.Kleinman MT, Leaf DA, Kelly E, Caiozzo V, Osann K, O'Niell T. Urban angina in the mountains: effects of carbon monoxide and mild hypoxemia on subjects with chronic stable angina. Arch. Environ. Health. 1998;53:388–397. doi: 10.1080/00039899809605726. [DOI] [PubMed] [Google Scholar]
- 50.Sheps DS, Adams KF, Jr., Bromberg PA, Goldstein GM, O'Neil JJ, Horstman D, Koch G. Lack of effect of low levels of carboxyhemoglobin on cardiovascular function in patients with ischemic heart disease. Arch. Environ. Health. 1987;42:108–116. doi: 10.1080/00039896.1987.9935805. [DOI] [PubMed] [Google Scholar]
- 51.Woehlck HJ, Connolly LA, Cinquegrani MP, Dunning MB, 3rd, Hoffmann RG. Acute smoking increases ST depression in humans during general anesthesia. Anesth Analg. 1999;89:856–60. doi: 10.1097/00000539-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Smithline HA, Ward KR, Chiulli DA, Blake HC, Rivers EP. Whole body oxygen consumption and critical oxygen delivery in response to prolonged and severe carbon monoxide poisoning. Resuscitation. 2003;56:97–104. doi: 10.1016/s0300-9572(02)00272-1. [DOI] [PubMed] [Google Scholar]
- 53.Hauck H, Neuberger M. Carbon monoxide uptake and the resulting carboxyhemoglobin in man. Eur J Appl Physiol Occup Physiol. 1984;53:186–90. doi: 10.1007/BF00422585. [DOI] [PubMed] [Google Scholar]
- 54.Gorman D, Drewry A, Huang YL, Sames C. The clinical toxicology of carbon monoxide. Toxicology. 2003;187:25–38. doi: 10.1016/s0300-483x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 55.Brown SD, Piantadosi CA. In vivo binding of carbon monoxide to cytochrome c oxidase in rat brain. J.Appl.Physiol. 1990;68:604–610. doi: 10.1152/jappl.1990.68.2.604. [DOI] [PubMed] [Google Scholar]
- 56.Iheagwara KN, Thom SR, Deutschman CS, Levy RJ. Myocardial cytochrome oxidase activity is decreased following carbon monoxide exposure. Biochim Biophys Acta. 2007;1772:1112–6. doi: 10.1016/j.bbadis.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hampson NB, Piantadosi CA, Thom SR, Weaver LK. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am J Respir Crit Care Med. 2012;186:1095–101. doi: 10.1164/rccm.201207-1284CI. [DOI] [PubMed] [Google Scholar]
- 58.Winter PM, Miller JN. Carbon monoxide poisoning. JAMA. 1976;236:1502. [PubMed] [Google Scholar]
- 59.Baker MD, Henretig FM, Ludwig S. Carboxyhemoglobin levels in children with nonspecific flu-like symptoms. J Pediatr. 1988;113:501–4. doi: 10.1016/s0022-3476(88)80638-3. [DOI] [PubMed] [Google Scholar]
- 60.Foster M, Goodwin SR, Williams C, Loeffler J. Recurrent acute life-threatening events and lactic acidosis caused by chronic carbon monoxide poisoning in an infant. Pediatrics. 1999;104:e34. doi: 10.1542/peds.104.3.e34. [DOI] [PubMed] [Google Scholar]
- 61.Raub J. [Accessed November 16, 2015];Carbon Monoxide (2nd Edition) Environmental Health Criteria 213. World Health Organization. 1999 [Online] Available at: http://apps.who.int/iris/bitstream/10665/42180/1/WHO_EHC_213.pdf.
- 62.Raub JA, Benignus VA. Carbon monoxide and the nervous system. Neurosci Biobehav Rev. 2002;26:925–40. doi: 10.1016/s0149-7634(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 63.Tomaszewski C. In: Carbon monoxide, Goldfrank's toxicologic emergencies. 7th edition Goldfrank LR, Flomenbaum NE, Lewin NA, et al., editors. McGraw-Hill; New York: 2002. pp. 1478–97. [Google Scholar]
- 64.Bermudez JA. Investigation of electrochemical carbon monoxide sensor monitoring of anesthetic gas mixtures. Anesthesiology. 2003;99:1233–5. doi: 10.1097/00000542-200311000-00037. [DOI] [PubMed] [Google Scholar]
- 65.Barker SJ, Curry J, Redford D, Morgan S. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry: a human volunteer study. Anesthesiology. 2006;105:892–7. doi: 10.1097/00000542-200611000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Zaouter C, Zavorsky GS. The measurement of carboxyhemoglobin and methemoglobin using a non-invasive pulse CO-oximeter. Respiratory physiology & neurobiology. 2012;182:88–92. doi: 10.1016/j.resp.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 67.Piatkowski A, Ulrich D, Grieb G, Pallua N. A new tool for the early diagnosis of carbon monoxide intoxication. Inhal Toxicol. 2009;21:1144–7. doi: 10.3109/08958370902839754. [DOI] [PubMed] [Google Scholar]
- 68.Roth D, Herkner H, Schreiber W, Hubmann N, Gamper G, Laggner AN, Havel C. Accuracy of noninvasive multiwave pulse oximetry compared with carboxyhemoglobin from blood gas analysis in unselected emergency department patients. Ann Emerg Med. 2011;58:74–9. doi: 10.1016/j.annemergmed.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 69.Touger M, Birnbaum A, Wang J, Chou K, Pearson D, Bijur P. Performance of the RAD-57 pulse CO-oximeter compared with standard laboratory carboxyhemoglobin measurement. Ann Emerg Med. 2010;56:382–8. doi: 10.1016/j.annemergmed.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 70.Feiner JR, Rollins MD, Sall JW, Eilers H, Au P, Bickler PE. Accuracy of carboxyhemoglobin detection by pulse CO-oximetry during hypoxemia. Anesth Analg. 2013;117:847–858. doi: 10.1213/ANE.0b013e31828610a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauer I, Pannen BH. Bench-to-bedside review: Carbon monoxide--from mitochondrial poisoning to therapeutic use. Crit Care. 2009;13:220. doi: 10.1186/cc7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bilgi M, Goksu S, Mizrak A, Cevik C, Gul R, Koruk S, Sahin L. Comparison of the effects of low-flow and high-flow inhalational anaesthesia with nitrous oxide and desflurane on mucociliary activity and pulmonary function tests. Eur J Anaesthesiol. 2011;28:279–83. doi: 10.1097/EJA.0b013e3283414cb7. [DOI] [PubMed] [Google Scholar]
- 73.Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid Redox Signal. 2007;9:2157–73. doi: 10.1089/ars.2007.1811. [DOI] [PubMed] [Google Scholar]
- 74.Binici O, Kati I, Goktas U, Soyaral L, Aytekin OC. Comparing effects of low and high-flow anesthesia on hemorheology and coagulation factors. Pak J Med Sci. 2015;31:683–7. doi: 10.12669/pjms.313.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalaycı D, Dikmen B, Kaçmaz M, Taşpınar V, Ornek D, Turan O. Plasma levels of interleukin-10 and nitric oxide in response to two different desflurane anesthesia flow rates. Braz J Anesthesiol. 2014;64:292–8. doi: 10.1016/j.bjane.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Thom SR, Bhopale VM, Fisher D, Zhang J, Gimotty P. Delayed neuropathology after carbon monoxide poisoning is immune-mediated. Proc Natl Acad Sci U S A. 2004;101:13660–5. doi: 10.1073/pnas.0405642101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thom SR, Fisher D, Zhang J, Bhopale VM, Cameron B, Buerk DG. Neuronal nitric oxide synthase and N-methyl-D-aspartate neurons in experimental carbon monoxide poisoning. Toxicol Appl Pharmacol. 2004;194:280–95. doi: 10.1016/j.taap.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 78.Thom SR, Ohnishi ST, Ischiropoulos H. Nitric oxide released by platelets inhibits neutrophil B2 integrin function following acute carbon monoxide poisoning. Toxicol Appl Pharmacol. 1994;128:105–10. doi: 10.1006/taap.1994.1186. [DOI] [PubMed] [Google Scholar]
- 79.Thom SR, Xu YA, Ischiropoulos H. Vascular endothelial cells generate peroxynitrite in response to carbon monoxide exposure. Chem Res Toxicol. 1997;10:1023–31. doi: 10.1021/tx970041h. [DOI] [PubMed] [Google Scholar]
- 80.Thom SR, Fisher D, Xu YA, Garner S, Ischiropoulos H. Role of nitric oxide-derived oxidants in vascular injury from carbon monoxide in the rat. Am J Physiol. 1999;276:H984–92. doi: 10.1152/ajpheart.1999.276.3.H984. [DOI] [PubMed] [Google Scholar]
- 81.Truss NJ, Warner TD. Gasotransmitters and platelets. Pharmacol Ther. 2011;132:196–203. doi: 10.1016/j.pharmthera.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Kapetanaki SM, Silkstone G, Husu I, Liebl U, Wilson MT, Vos MH. Interaction of carbon monoxide with the apoptosis-inducing cytochrome c-cardiolipin complex. Biochemistry. 2009;48:1613–9. doi: 10.1021/bi801817v. [DOI] [PubMed] [Google Scholar]
- 83.Ignarro LJ, Degnan JN, Baricos WH, Kadowitz PJ, Wolin MS. Activation of purified guanylate cyclase by nitric oxide requires heme. Comparison of heme-deficient, heme-reconstituted and heme-containing forms of soluble enzyme from bovine lung. Biochim. Biophys. Acta. 1982;718:49–59. doi: 10.1016/0304-4165(82)90008-3. [DOI] [PubMed] [Google Scholar]
- 84.Furchgott RF, Jothianandan D. Endothelium-dependent and –independent vasodilation involving cyclic GMP: Relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 85.Morita T, Mitsialis SA, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J. Biol. Chem. 1997;272:32804–9. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 86.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 87.Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M. Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal. 2010;13:157–92. doi: 10.1089/ars.2009.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–49. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 89.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 90.Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, Choi AM. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38 beta MAPK and heat shock factor-1. J Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 91.Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, Ryter SW, Choi AM. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci USA. 2005;102:11319–11324. doi: 10.1073/pnas.0501345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rhodes MA, Carraway MS, Piantadosi CA, Reynolds CM, Cherry AD, Wester TE, Natoli MJ, Massey EW, Moon RE, Suliman HB. Carbon monoxide, skeletal muscle oxidative stress, and mitochondrial biogenesis in humans. Am J Physiol Heart Circ Physiol. 2009;297:H392–9. doi: 10.1152/ajpheart.00164.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, Kim YS. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am J Respir Cell Mol Biol. 2011;45:867–73. doi: 10.1165/rcmb.2010-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AM, Serhan CN. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol. 2013;190:6378–88. doi: 10.4049/jimmunol.1202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bissonnette JM, Wickham WK. Placental diffusing capacity for carbon monoxide in unanesthetized guinea pigs. Respir Physiol. 1977;31:161–8. doi: 10.1016/0034-5687(77)90099-8. [DOI] [PubMed] [Google Scholar]
- 96.Jain KK. Carbon Monoxide and Other Tissue Poisons. In: Jain KK, editor. Textbook of Hyperbaric Medicine. Hogrefe Publishing; Cambridge, MA: 2009. pp. 111–133. [Google Scholar]
- 97.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol. 1999;276:L688–94. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 98.Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, Bach FH. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–77. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 99.Dolinay T, Szilasi M, Liu M, Choi AM. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;170:613–620. doi: 10.1164/rccm.200401-023OC. [DOI] [PubMed] [Google Scholar]
- 100.Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, Kaynar AM, Schmidt R, Ryter SW, Choi AM. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med. 2008;177:1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoetzel A, Schmidt R, Vallbracht S, Goebel U, Dolinay T, Kim HP, Ifedigbo E, Ryter SW, Choi AM. Carbon monoxide prevents ventilator-induced lung injury via caveolin-1. Crit Care Med. 2009;37:1708–15. doi: 10.1097/CCM.0b013e31819efa31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fredenburgh LE, Kraft BD, Hess DR, Harris RS, Wolf MA, Suliman HB, Roggli VL, Davies JD, Winkler T, Stenzler A, Baron RM, Thompson BT, Choi AM, Welty-Wolf KE, Piantadosi CA. Effects of inhaled CO administration on acute lung injury in baboons with pneumococcalpneumonia. Am J Physiol Lung Cell Mol Physiol. 2015;309:L834–46. doi: 10.1152/ajplung.00240.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nemzek JA, Fry C, Abatan O. Low-dose carbon monoxide treatment attenuates early pulmonary neutrophil recruitment after acid aspiration. Am J Physiol Lung Cell Mol Physiol. 2008;294:L644–L653. doi: 10.1152/ajplung.00324.2007. [DOI] [PubMed] [Google Scholar]
- 104.Chapman JT, Otterbein LE, Elias JA, Choi AM. Carbon monoxide attenuates aeroallergen-induced inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2001;281:L209–L216. doi: 10.1152/ajplung.2001.281.1.L209. [DOI] [PubMed] [Google Scholar]
- 105.Ameredes BT, Otterbein LE, Kohut LK, Gligonic AL, Calhoun WJ, Choi AM. Low-dose carbon monoxide reduces airway hyperresponsiveness in mice. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1270–L1276. doi: 10.1152/ajplung.00145.2003. [DOI] [PubMed] [Google Scholar]
- 106.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 108.Kohmoto J, Nakao A, Stolz DB, Kaizu T, Tsung A, Ikeda A, Shimizu H, Takahashi T, Tomiyama K, Sugimoto R, Choi AM, Billiar TR, Murase N, McCurry KR. Carbon monoxide protects rat lung transplants from ischemia-reperfusion injury via a mechanism involving p38 MAPK pathway. Am J Transplant. 2007;7:2279–2290. doi: 10.1111/j.1600-6143.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- 109.Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, Minamoto K, Yoshikawa Y, Harada H, Pinsky DJ. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proc Natl Acad Sci USA. 2006;103:5191–5196. doi: 10.1073/pnas.0600241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, Alber SM, Watkins SC, Zuckerbraun BS, Otterbein LE, Ning W, Oury TD, Lee PJ, McCurry KR, Choi AM. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol. 2003;163:231–242. doi: 10.1016/S0002-9440(10)63646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, Lee PJ. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem. 2003;278:22061–22070. doi: 10.1074/jbc.M301858200. [DOI] [PubMed] [Google Scholar]
- 112.Boutros C, Zegdi R, Lila N, Cambillau M, Fornes P, Carpentier A, Fabini JN. Carbon monoxide can prevent acute lung injury observed after ischemia reperfusion of the lower extremities. J Surg Res. 2007;143:437–442. doi: 10.1016/j.jss.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 113.Goebel U, Siepe M, Mecklenburg A, Stein P, Roesslein M, Schwer CI, Schmidt R, Doenst T, Geiger KK, Pahl HL, Schlensak C, Loop T. Carbon monoxide inhalation reduces pulmonary inflammatory response during cardiopulmonary bypass in pigs. Anesthesiology. 2008;108::1025–1036. doi: 10.1097/ALN.0b013e3181733115. [DOI] [PubMed] [Google Scholar]
- 114.Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J. 2007;30:1131–1137. doi: 10.1183/09031936.00163206. [DOI] [PubMed] [Google Scholar]
- 115. [Accessed November 16, 2015]; https://clinicaltrials.gov/ct2/show/NCT02425579?term=carbon+monoxide&rank=7.
- 116.Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, Yoshida K, Nagai R. Carbon monoxide protects against cardiac ischemia-reperfusion injury in vivo via MAPK and Akt-eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848–1853. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- 117.Lavitrano M, Smolenski RT, Musumeci A, Maccherini M, Slominska E, Di Florio E, Bracco A, Mancini A, Stassi G, Patti M, Giovannoni R, Froio A, Simeone F, Forni M, Bacci ML, D'Alise G, Cozzi E, Otterbein LE, Yacoub MH, Bach FH, Calise F. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004;18:1093–1095. doi: 10.1096/fj.03-0996fje. [DOI] [PubMed] [Google Scholar]
- 118.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 119.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graça-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 120.Nakao A, Toyokawa H, Abe M, Kiyomoto T, Nakahira K, Choi AM, Nalesnik MA, Thomson AW, Murase N. Heart allograft protection with low-dose carbon monoxide inhalation: effects on inflammatory mediators and alloreactive T-cell responses. Transplantation. 2006;81:220–230. doi: 10.1097/01.tp.0000188637.80695.7f. [DOI] [PubMed] [Google Scholar]
- 121.McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65:1976–97. doi: 10.1016/j.jacc.2015.03.540. [DOI] [PubMed] [Google Scholar]
- 122.Dubuis E, Potier M, Wang R, Vandier C. Continuous inhalation of carbon monoxide attenuates hypoxic pulmonary hypertension development presumably through activation of BKCa channels. Cardiovasc Res. 2005;65:751–761. doi: 10.1016/j.cardiores.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 123.Zuckerbraun BS, Chin BY, Wegiel B, Billiar TR, Czsimadia E, Rao J, Shimoda L, Ifedigbo E, Kanno S, Otterbein LE. Carbon monoxide reverses established pulmonary hypertension. J Exp Med. 2006;203:2109–2119. doi: 10.1084/jem.20052267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. [Accessed November 17, 2015]; https://clinicaltrials.gov/ct2/show/NCT01523548?term=carbon+monoxide&rank=4.
- 125. [Accessed November 17, 2015]; https://clinicaltrials.gov/ct2/show/NCT01818843?term=carbon+monoxide&rank=10.
- 126.Vieira HL, Queiroga CS, Alves PM. Pre-conditioning induced by carbon monoxide provides neuronal protection against apoptosis. J Neurochem. 2008;107:375–84. doi: 10.1111/j.1471-4159.2008.05610.x. [DOI] [PubMed] [Google Scholar]
- 127.Mahan VL, Zurakowski D, Otterbein LE, Pigula FA. Inhaled carbon monoxide provides cerebral cytoprotection in pigs. PLoS One. 2012;7:e41982. doi: 10.1371/journal.pone.0041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Queiroga CS, Tomasi S, Widerøe M, Alves PM, Vercelli A, Vieira HL. Preconditioning triggered by carbon monoxide (CO) provides neuronal protection following perinatal hypoxi-aischemia. PLoS One. 2012;7:e42632. doi: 10.1371/journal.pone.0042632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang B, Cao W, Biswal S, Doré S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–10. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Benagiano V, Lorusso L, Coluccia A, Tarullo A, Flace P, Girolamo F, Bosco L, Cagiano R, Ambrosi G. Glutamic acid decarboxylase and GABA immunoreactivities in the cerebellar cortex of adult rat after prenatal exposure to a low concentration of carbon monoxide. Neuroscience. 2005;135:897–905. doi: 10.1016/j.neuroscience.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 131.Carratù MR, Cagiano R, Desantis S, Labate M, Tattoli M, Trabace L, Cuomo V. Prenatal exposure to low levels of carbon monoxide alters sciatic nerve myelination in rat offspring. Life Sci. 2000;67:1759–72. doi: 10.1016/s0024-3205(00)00761-x. [DOI] [PubMed] [Google Scholar]
- 132.Fechter LD. Neurotoxicity of prenatal carbon monoxide exposure. Res Rep Health Eff Inst. 1987;12:3–22. [PubMed] [Google Scholar]
- 133.Fechter LD, Karpa MD, Proctor B, Lee AG, Storm JE. Disruption of neostriatal development in rats following perinatal exposure to mild, but chronic carbon monoxide. Neurotoxicol Teratol. 1987;9:277–81. doi: 10.1016/0892-0362(87)90013-4. [DOI] [PubMed] [Google Scholar]
- 134.Cheng Y, Thomas A, Mardini F, Bianchi SL, Tang JX, Peng J, Wei H, Eckenhoff MF, Eckenhoff RG, Levy RJ. Neurodevelopmental Consequences of Sub-clinical Carbon Monoxide Exposure in Newborn Mice. PLoS One. 2012;7:e32029. doi: 10.1371/journal.pone.0032029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lopez I, Acuna D, Webber DS, Korsak RA, Edmond J. Mild carbon monoxide exposure diminishes selectively the integrity of the cochlea of the developing rat. J Neurosci Res. 2003;74:666–75. doi: 10.1002/jnr.10813. [DOI] [PubMed] [Google Scholar]
- 136.Stockard-Sullivan JE, Korsak RA, Webber DS, Edmond J. Mild carbon monoxide exposure and auditory function in the developing rat. J Neurosci Res. 2003;74:644–54. doi: 10.1002/jnr.10808. [DOI] [PubMed] [Google Scholar]
- 137.Webber DS, Korsak RA, Sininger LK, Sampogna SL, Edmond J. Mild carbon monoxide exposure impairs the developing auditory system of the rat. J Neurosci Res. 2003;74:655–65. doi: 10.1002/jnr.10809. [DOI] [PubMed] [Google Scholar]
- 138.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 139.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng Y, Levy RJ. Subclinical carbon monoxide limits apoptosis in the developing brain after isoflurane exposure. Anesth Analg. 2014;118:1284–92. doi: 10.1213/ANE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng Y, Mitchell-Flack MJ, Wang A, Levy RJ. Carbon monoxide modulates cytochrome oxidase activity and oxidative stress in the developing murine brain during isoflurane exposure. Free Radic Biol Med. 2015;86:191–9. doi: 10.1016/j.freeradbiomed.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]