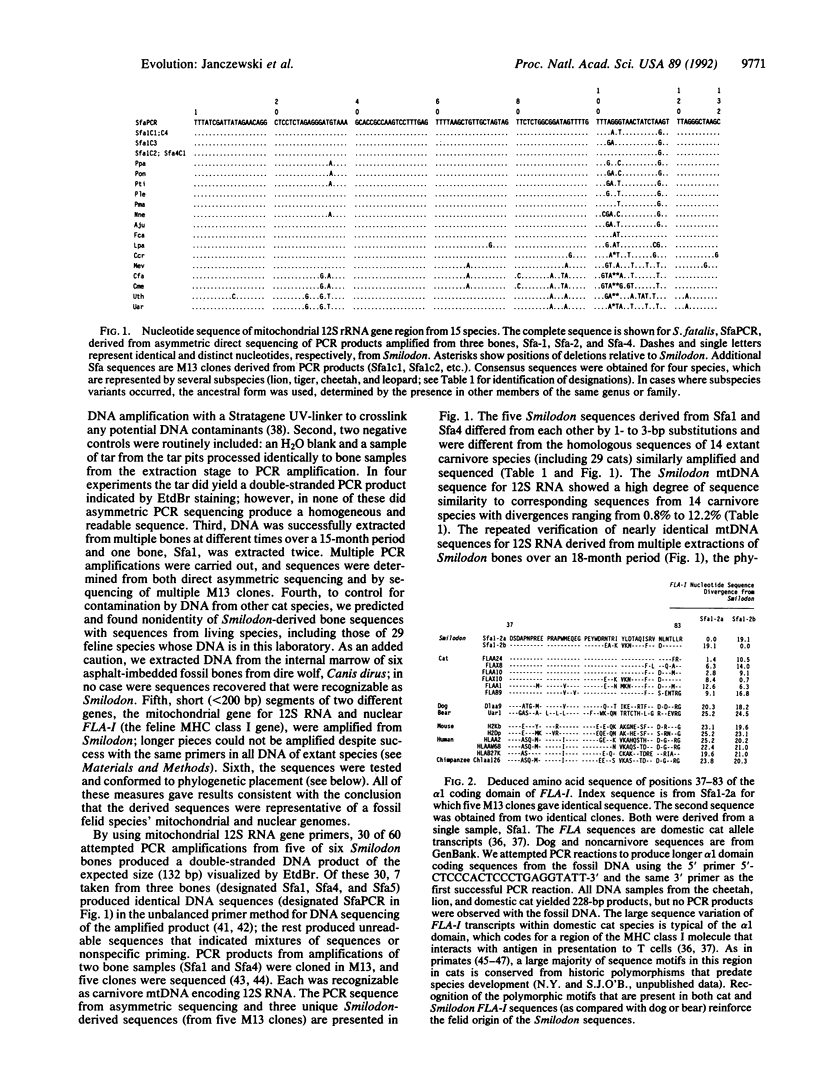
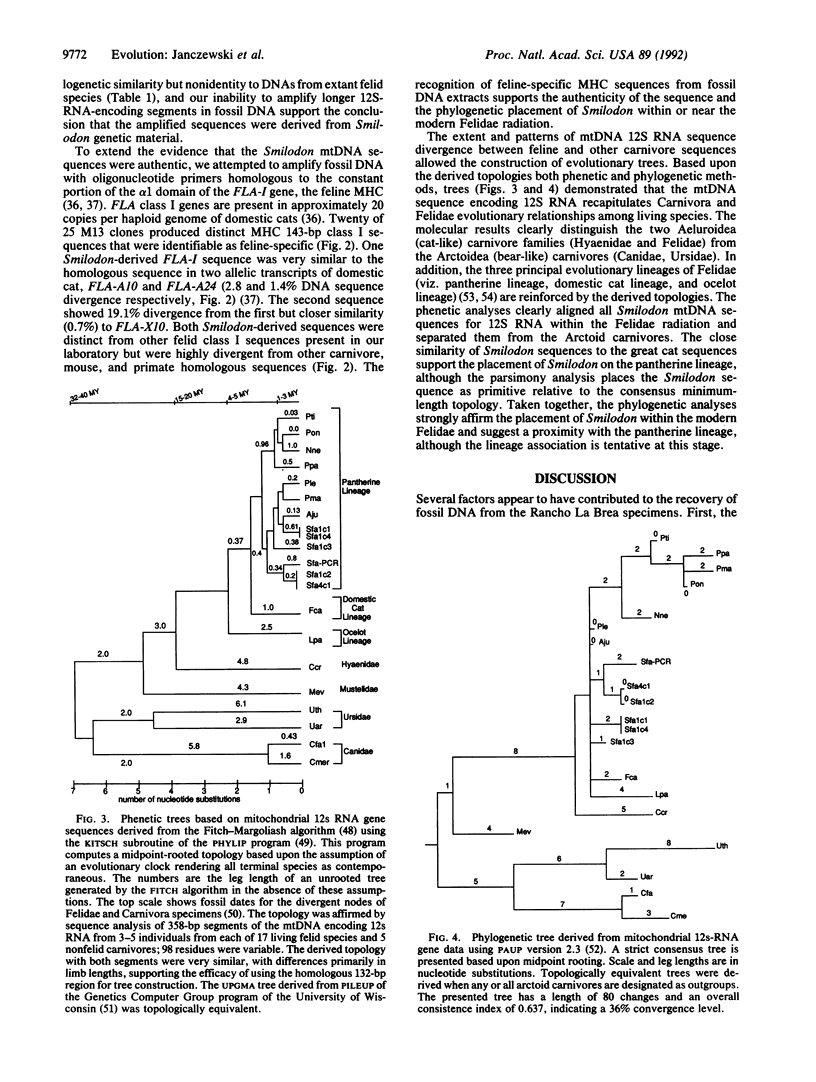
Abstract
A method for the successful extraction of sequestered cellular DNA from 14,000-year-old fossil bones was developed and applied to asphalt-preserved specimens of the extinct saber-toothed cat, Smilodon fatalis. Two distinct gene segments, the mitochondrial gene for 12S rRNA and nuclear FLA-I (the feline class I major histocompatibility complex gene), from three different individual fossil specimens were cloned and sequenced after PCR amplification. Comparison of fossil-derived DNA sequences to homologous regions in 15 living carnivorous species, including 9 species of Felidae and 6 nonfelids, affirmed the phylogenetic placement of Smilodon within the modern radiation of Felidae distinct from the Miocene paleofelid (Nimravidae) saber-toothed "cat" species. These results raise the prospect of obtaining genetically informative DNA from preserved bones of extinct fossil species, particularly among the 2 million specimens excavated from the asphaltic sediments at Rancho La Brea in metropolitan Los Angeles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Doran G. H., Dickel D. N., Ballinger W. E., Jr, Agee O. F., Laipis P. J., Hauswirth W. W. Anatomical, cellular and molecular analysis of 8,000-yr-old human brain tissue from the Windover archaeological site. 1986 Oct 30-Nov 5Nature. 323(6091):803–806. doi: 10.1038/323803a0. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Hoener P. A., Collins F. S. Direct sequencing of enzymatically amplified human genomic DNA. Proc Natl Acad Sci U S A. 1988 Jan;85(2):544–548. doi: 10.1073/pnas.85.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich H. A., Gelfand D., Sninsky J. J. Recent advances in the polymerase chain reaction. Science. 1991 Jun 21;252(5013):1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Gilbert D. A., Packer C., Pusey A. E., Stephens J. C., O'Brien S. J. Analytical DNA fingerprinting in lions: parentage, genetic diversity, and kinship. J Hered. 1991 Sep-Oct;82(5):378–386. doi: 10.1093/oxfordjournals.jhered.a111107. [DOI] [PubMed] [Google Scholar]
- Golenberg E. M., Giannasi D. E., Clegg M. T., Smiley C. J., Durbin M., Henderson D., Zurawski G. Chloroplast DNA sequence from a miocene Magnolia species. Nature. 1990 Apr 12;344(6267):656–658. doi: 10.1038/344656a0. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagelberg E., Sykes B., Hedges R. Ancient bone DNA amplified. Nature. 1989 Nov 30;342(6249):485–485. doi: 10.1038/342485a0. [DOI] [PubMed] [Google Scholar]
- Higuchi R. G., Wrischnik L. A., Oakes E., George M., Tong B., Wilson A. C. Mitochondrial DNA of the extinct quagga: relatedness and extent of postmortem change. J Mol Evol. 1987;25(4):283–287. doi: 10.1007/BF02603111. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Bowman B., Freiberger M., Ryder O. A., Wilson A. C. DNA sequences from the quagga, an extinct member of the horse family. Nature. 1984 Nov 15;312(5991):282–284. doi: 10.1038/312282a0. [DOI] [PubMed] [Google Scholar]
- Klein J., Gutknecht J., Fischer N. The major histocompatibility complex and human evolution. Trends Genet. 1990 Jan;6(1):7–11. doi: 10.1016/0168-9525(90)90042-5. [DOI] [PubMed] [Google Scholar]
- Kocher T. D., Thomas W. K., Meyer A., Edwards S. V., Päbo S., Villablanca F. X., Wilson A. C. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D. A., Dickel C. D., Hauswirth W. W., Parham P. Ancient HLA genes from 7,500-year-old archaeological remains. Nature. 1991 Feb 28;349(6312):785–788. doi: 10.1038/349785a0. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Ward F. E., Ennis P. D., Jackson A. P., Parham P. HLA-A and B polymorphisms predate the divergence of humans and chimpanzees. Nature. 1988 Sep 15;335(6187):268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S. J., Roelke M. E., Marker L., Newman A., Winkler C. A., Meltzer D., Colly L., Evermann J. F., Bush M., Wildt D. E. Genetic basis for species vulnerability in the cheetah. Science. 1985 Mar 22;227(4693):1428–1434. doi: 10.1126/science.2983425. [DOI] [PubMed] [Google Scholar]
- Parham P., Lawlor D. A., Lomen C. E., Ennis P. D. Diversity and diversification of HLA-A,B,C alleles. J Immunol. 1989 Jun 1;142(11):3937–3950. [PubMed] [Google Scholar]
- Persing D. H., Telford S. R., 3rd, Rys P. N., Dodge D. E., White T. J., Malawista S. E., Spielman A. Detection of Borrelia burgdorferi DNA in museum specimens of Ixodes dammini ticks. Science. 1990 Sep 21;249(4975):1420–1423. doi: 10.1126/science.2402635. [DOI] [PubMed] [Google Scholar]
- Päbo S. Ancient DNA: extraction, characterization, molecular cloning, and enzymatic amplification. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1939–1943. doi: 10.1073/pnas.86.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Gifford J. A., Wilson A. C. Mitochondrial DNA sequences from a 7000-year old brain. Nucleic Acids Res. 1988 Oct 25;16(20):9775–9787. doi: 10.1093/nar/16.20.9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Irwin D. M., Wilson A. C. DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem. 1990 Mar 15;265(8):4718–4721. [PubMed] [Google Scholar]
- Päbo S. Molecular cloning of Ancient Egyptian mummy DNA. Nature. 1985 Apr 18;314(6012):644–645. doi: 10.1038/314644a0. [DOI] [PubMed] [Google Scholar]
- Päbo S., Wilson A. C. Miocene DNA sequences - a dream come true? Curr Biol. 1991 Feb;1(1):45–46. doi: 10.1016/0960-9822(91)90125-g. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Shedding light on PCR contamination. Nature. 1990 Jan 4;343(6253):27–27. doi: 10.1038/343027a0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. K., Päbo S., Villablanca F. X., Wilson A. C. Spatial and temporal continuity of kangaroo rat populations shown by sequencing mitochondrial DNA from museum specimens. J Mol Evol. 1990 Aug;31(2):101–112. doi: 10.1007/BF02109479. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wayne R. K., Van Valkenburgh B., O'Brien S. J. Molecular distance and divergence time in carnivores and primates. Mol Biol Evol. 1991 May;8(3):297–319. doi: 10.1093/oxfordjournals.molbev.a040651. [DOI] [PubMed] [Google Scholar]
- Yuhki N., Heidecker G. F., O'Brien S. J. Characterization of MHC cDNA clones in the domestic cat. Diversity and evolution of class I genes. J Immunol. 1989 May 15;142(10):3676–3682. [PubMed] [Google Scholar]
- Yuhki N., O'Brien S. J. DNA recombination and natural selection pressure sustain genetic sequence diversity of the feline MHC class I genes. J Exp Med. 1990 Aug 1;172(2):621–630. doi: 10.1084/jem.172.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]



