ABSTRACT
Poxvirus prime-protein boost used in the RV144 trial remains the only immunization strategy shown to elicit a modest level of protection against HIV-1 acquisition in humans. Although neutralizing antibodies (NAb) were generated, they were against sensitive viruses, not the more resistant “tier 2” isolates that dominate circulating strains. Instead, risk reduction correlated with antibodies recognizing epitopes in the V1/V2 region of HIV-1 envelope glycoprotein (Env). Here, we examined whether tier 2 virus NAb and V1/V2-specific non-NAb could be elicited by a poxvirus prime-gp120 boost strategy in a rabbit model. We studied two clade B Envs that differ in multiple parameters, including tissue origin, neutralization sensitivity, and presence of the N197 (N7) glycan that was previously shown to modulate the exposure of conserved epitopes on Env. We demonstrate that immunized rabbits generated cross-reactive neutralizing activities against >50% of the tier 2 global HIV-1 isolates tested. Some of these activities were directed against the CD4 binding site (CD4bs). These rabbits also generated antibodies that recognized protein scaffolds bearing V1/V2 sequences from diverse HIV-1 isolates and mediated antibody-dependent cellular cytotoxicity. However, there are subtle differences in the specificities and the response rates of V1/V2-specific antibodies between animals immunized with different Envs, with or without the N7 glycan. These findings demonstrate that antibody responses that have been correlated with protection against HIV-1 acquisition in humans can be elicited in a preclinical model by a poxvirus prime-gp120 boost strategy and that improvements may be achievable by optimizing the nature of the priming and boosting immunogens.
IMPORTANCE The only vaccine approach shown to elicit any protective efficacy against HIV-1 acquisition is based on a poxvirus prime-protein boost regimen (RV144 Thai trial). Reduction of risk was associated with nonneutralizing antibodies targeting the V1/V2 loops of the envelope protein gp120. However, the modest efficacy (31.2%) achieved in this trial highlights the need to examine approaches and factors that may improve vaccine-induced responses, including cross-reactive neutralizing activities. We show here that rabbits immunized with a novel recombinant vaccinia virus prime-gp120 protein boost regimen generated antibodies that recognize protein scaffolds bearing V1/V2 sequences from diverse HIV-1 isolates and mediated antibody-dependent cellular cytotoxicity. Importantly, immunized rabbits also showed neutralizing activities against heterologous tier 2 HIV-1 isolates. These findings may inform the design of prime-boost immunization approaches and help improve the protective efficacy of candidate HIV-1 vaccines.
INTRODUCTION
While many vaccine approaches have been tested in the clinic, all but one have failed to protect against HIV-1 acquisition (1, 2). Only the RV144 trial achieved a modest efficacy of 31.2% using a prime-boost strategy with nonreplicative recombinant canarypox virus and bivalent gp120 protein (3). Antibodies against variable loops 1 and 2 (V1/V2) and high levels of antibody-dependent cellular cytotoxicity (ADCC) activities were found to inversely correlate with the risk of HIV-1 acquisition (4–6). Neutralizing antibodies (NAb) were generated but were primarily against tier 1 isolates, with little or no tier 2 neutralizing activity detected (7). Despite these limitations, results of the RV144 trial provide a starting point to examine factors in the prime-boost strategy that may improve vaccine efficacy, including the generation of antibodies that may neutralize tier 2 viruses.
Passively administered NAb have been shown to protect against primate lentivirus infection in animal models (1, 2, 8–11); therefore, it remains a major goal for HIV-1 vaccines to elicit these antibodies. Recent studies described vaccine-induced tier 2 virus NAb in immunized animals; however, these responses are limited, sporadic, and primarily against the autologous tier 2 isolates (12–14). Novel immunogens are being examined in the hope that they may elicit cross-reactive tier 2 NAb (1, 2, 15, 16). We previously reported that removal of a single N-linked glycan at amino acid N197 (N7) of gp120 enhanced the ability of Env to generate cross-reactive neutralizing responses (17). This study was based on a single isolate, 89.6. Since the N7 glycan and its effect on Env antigenicity are highly conserved (17–21), it is of interest to determine if the effects of the N7 glycan on Env immunogenicity can be observed in isolates other than 89.6.
In the present study, we sought to examine whether antibody responses that have been correlated with protection against HIV-1 acquisition in humans can be elicited in a preclinical model by a poxvirus prime-gp120 protein boost strategy. Specifically, we used a replication-competent vaccinia virus vector for priming and two clade B Envs (JR-FL or PVO.4) for boosting. These Envs differ in multiple parameters, including tissue origin, neutralization sensitivity, and presence of the N7 glycan, which modulates the exposure of variable loop 3 (V3) and CD4 binding sites (CD4bs) on Env (17, 21–23). Using this prime-boost immunization regimen, we were able to induce cross-reactive binding antibodies against V1/V2 fusion proteins and neutralizing responses against heterologous tier 2 isolates. However, in contrast to our previous finding with 89.6 Env (17), results from the present study showed that the absence of the N7 glycan had little impact or a negative one on Env immunogenicity, indicating the need for further improvements in immunization strategy by optimizing the nature of the priming and boosting immunogens.
MATERIALS AND METHODS
Envelope genes.
A plasmid encoding the Env of JR-FL (22) was a gift from Paul Clapham, while a plasmid encoding SF162 Env was a gift from Leonidas Stamatatos and Cecilia Cheng-Mayer (24). The following Env-encoding plasmids, including the standard clade B (23) and the global tier 2 (25) env panels, were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: TRO.11 (23, 25), 6535.3, SS1196, QH0692.42, SC422661.8, AC10.0.29, and PVO.4 from David Montefiori and Feng Gao (23); RHPA4259.7, REJO4541.67, and WITO4160.33 from B. H. Hahn and J. Salazar-Gonzalez (23); THRO4156.18 and CAAN5342.A2 from B. H. Hahn and D. L. Kothe (23); TRJO4551.58 from B. H. Hahn, X. Wei, and G. M. Shaw (23); Bal.26 (26) and DJ263.8 (27) from John Mascola; CRF02_AG clone 271 from Dennis Ellenberger, Bin Li, Margaret Callahan, and Salvatore Butera; CE1176_A3 from Ronald Swanstrom, Li-Hua Ping, Jeffrey Anderson, and David Montefiori (25); and murine leukemia virus (MuLV) from Nathaniel Landau and Dan Littman (28).
Construction of N-linked glycosylation site mutants.
Generation of N7 glycan mutants was described in previous publications (17, 21). Briefly, we used the QuikChange mutagenesis kit (Stratagene) to mutate or to restore the codon for the asparagine (N) residue in the potential N-linked glycan (PNLG) sequon (N-X-S/T). In the case of PVO.4, the asparagine (N) at amino acid residue 197 (HXB2 numbering) was substituted with glutamine (Q) to generate N7 deglycosylated PVO.4 (PVO.4 N7−) Env. In the case of JR-FL, the N7 PNLG sequon was restored by replacing the aspartic acid (D197) with N to generate N7 glycosylated JR-FL (JR-FL N7+) Env.
Cells.
TZM-bl cells (HeLa origin, catalog no. 8129, NIH AIDS Reagent Program), an African green monkey kidney cell line (BSC40; ATCC catalog no. CRL2761), 293T cells (ATCC catalog no. 11268), and a human osteosarcoma cell line that lacks a functional TK gene (TK− 143B; ATCC catalog no. CRL-8303) were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine (complete DMEM). TK− cells were cultured in complete DMEM containing 25 μg/ml 5-bromodeoxyuridine.
Recombinant vaccinia virus (rVV).
A plaque-purified, replication-competent vaccinia virus (VV) generated from the New York City Board of Health strain (v-NY) was used as the vector to express full-length Env gp160 or subunit gp120 protein. The latter was generated by introducing one or two stop codons at the primary cleavage site between gp120 and gp41. Recombinant vaccinia viruses expressing either N7+ or N7− versions of JR-FL or PVO.4 Env gp160 and gp120 were generated as previously described (29–31). In brief, Env-encoding transgenes were inserted into the thymidine kinase (TK) gene of the v-NY strain of VV by homologous recombination. Two rounds of plaque purification were performed under negative-selection conditions using medium containing 24 μg/ml 5-bromodeoxyuridine on TK− 143B cells before a third round of purification was performed under nonselective conditions on BSC40 cells. Plaques were screened for transgenes by PCR using a primer set specific to the TK gene and Env. Recombinant viruses were expanded and propagated on BSC40 cells to generate crude virus stocks. Virus stocks that expressed gp160 were subsequently concentrated by sucrose density sedimentation as previously described (30, 32). Subunit gp120s were purified from BSC40 cells infected with gp120-expressing recombinant vaccinia viruses as previously described (33).
Expression of the transgene, under the control of a synthetic early-late promoter of VV, was verified by Western blotting as previously described (33, 34). Briefly, monolayers of BSC40 cells were grown to confluence and subsequently infected with recombinant or v-NY parental virus at a multiplicity of infection of 3. At 48 h postinfection, cells were harvested and resuspended in PBSAM buffer (PBS, 10 mM MgCl2, 0.01% bovine serum albumin [BSA]). After two freeze-thaw cycles, the cells were sonicated, lysed with NuPage reducing agent (Invitrogen; catalog no. NP0004), and fractionated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After electrotransfer to a nitrocellulose membrane, immunoreactive proteins were detected with pooled HIV-positive serum (NABI).
Immunization.
Twenty-four female New Zealand White rabbits were purchased and maintained at Covance (Denver, PA). All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee. Rabbits were divided into four groups, sedated with ketamine (40 mg/kg) and xylazine (5 mg/kg), and then primed with ∼108 PFU of recombinant VV at weeks 0 and 8 by scarification. Virus was applied to two shaved sites on the back of each animal. Each area was pricked 20 times, penetrating the skin, with a bifurcated needle. Animals remained sedated until both scarification sites were dry. At weeks 35 and 48, animals were boosted intramuscularly at two sites (50 μg each) with 100 μg of the cognate gp120 formulated in alum. Blood was collected from each rabbit on the day of, and 2 weeks after, each immunization event. Blood samples were processed to serum according to standard operating procedures at Covance. Vaccine take was verified by visualization of vaccinia virus lesions, vaccinia neutralization assays, and seroconversion.
Antibody capture assays.
HIV-1 Env-specific antibody titers in serum were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (35). Wild-type (WT) JR-FL or WT PVO.4 gp120 produced from Chinese hamster ovary (CHO) cells (kind gift from Shan Lu) or WT SF162 gp120 (Immune-tech catalog no. 001-0028P) were used as the capture antigen (100 ng gp120 per well). The endpoint ELISA titer of binding antibodies is defined as the reciprocal of the serum dilution that resulted in optical density (OD) readings greater than the mean OD readings of the preimmune serum at a 1:100 dilution plus three times the standard deviation. The limit of detection was considered the starting dilution (1:100) of the test sera. All experiments were repeated three times in duplicate.
Bioplex assays against peptides were performed as previously described (36). Briefly, 15-mer peptides overlapping by 11-mers corresponding to the variable regions 1 to 3 (V1 to V3) or conserved region 1 or 5 (C1 or C5, respectively) were obtained through the HIV-1 Consensus Subtype B Env Peptide Set from the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH. Ten micrograms of each peptide was coupled to Bio-Plex COOH beads (Bio-Rad) by primary amine coupling according to the manufacturer's protocol. Immune and prebleed sera were diluted 1:20 in 1% Blotto-PBS, mixed with peptide-coupled beads (∼500 beads per peptide), and incubated at room temperature for 1.5 h with shaking. To avoid potential competition, only beads coupled to peptides with no overlapping segments were included in the same well. After washing, phycoerythrin-labeled secondary antibody diluted 1:100 in 1% Blotto was added for 1 h with shaking. Following washing, the beads were analyzed for binding on a Bioplex 100 system (Bio-Rad), and the net mean fluorescence intensity (MFI) against individual peptides from each pool, minus the net MFI of matched prebleed samples, is reported. The reactivity of 447-52D (from the NIH ARRRP and Susan Zolla-Pazner) to V3 peptide (37–42) and coupled beads with no antibody or serum added were used as positive and negative controls, respectively. All experiments were repeated at least twice in duplicate.
Serum antibodies to a panel of V1/V2 scaffolds were measured by a binding antibody multiplex assay (BAMA) similar to that previously described (4, 43), with the following modifications: secondary antibody was goat anti-rabbit IgG, biotinylated (Thermo Fisher Scientific, Rockford, IL). Antibody measurements were acquired on a Bio-Plex instrument (Bio-Rad, Hercules, CA) using software compliant with 21 CFR Part 11, with the primary readout as MFI. Samples with MFI values that were (i) at least 100, (ii) higher than the antigen-specific cutoffs (95%ile MFI of all preimmune sera for each V1/V2 scaffold), and (iii) >3-fold that of the preimmune serum from the same animal were considered positive.
Assays for ADCC activity.
ADCC activity in rabbit sera was determined by two methods: a flow-based assay using gp120-coated cell targets and a luciferase-based assay using virus-infected cell targets. These methods are based on the report of Pollara et al. (44).
Flow-based assay.
CEM.NKRCCR5 cells (45) coated with recombinant HIV-1 SF162 gp120 (Immune Technology Corp., New York, NY) were used as targets. The SF162 gp120s share the same subtype B assignment as the immunogen used in the vaccine but represent a heterologous antigen for all vaccine groups. Cryopreserved human peripheral blood mononuclear cells (PBMC) from an HIV-seronegative donor heterozygous for 158F/V polymorphic variants of Fcγ receptor 3A were used as the source of effector cells (46). Rabbit sera were tested at six 4-fold serial dilutions starting at 1:100. Effector and target cells (at a ratio of 30:1) were incubated in the presence of rabbit sera or assay controls for 1 h. ADCC activity was measured as the frequency of events recorded in the granzyme B (GzB)-positive gate, with the cutoff value for positivity at ≥8% GzB. Results are reported either as peak ADCC activity, representing the maximum percent GzB activity at any given dilution, or as ADCC titers, calculated as the dilution at which the responses were ≥8% GzB.
Luc-based assay.
CEM.NKRCCR5 cells were infected with an infectious molecular clone (IMC) of HIV-1 encoding the SF162 (accession number EU123924) env gene within an NL4-3 backbone that also expresses the Renilla luciferase (Luc) reporter gene (47). ADCC activity was determined as the change in the relative luciferase units (RLU) resulting from the loss of intact targets in test wells (containing effector cells, target cells, and the test serum) compared to RLU in control wells (containing only target cells and effector cells). ADCC activity was reported as percent specific killing, calculated as [(number of RLU in control well − number of RLU in test well)/(number of RLU in control well)] ×100. The results were considered positive if ADCC activity was ≥15% specific killing after subtracting the background observed in preimmune sera. ADCC activities are reported either as the maximum percent specific killing observed for each test serum at any dilution or as ADCC titers, defined as the serum dilution that intersects the positive cutoff at ≥15% specific killing.
Neutralization assays.
Stocks of Env-pseudotyped viruses were prepared by cotransfection of Env-encoding and backbone plasmids in 293T cells (23). pSG3ΔEnv backbone was used to generate clade B Env-pseudotyped virus, and Q23ΔEnv backbone was used to generate clade A and AG Env-pseudotyped virus. All assay stocks were titrated in TZM-bl cells as previously described (23). Infectivity of Env-pseudotyped virus was measured by luciferase reporter gene expression in TZM-bl cells. Neutralization as measured by the reduction of luciferase gene expression was performed as previously described (20, 48). Briefly, indicator virus containing 150 50% tissue culture infective doses (TCID50) was incubated with a single dilution or serial 3-fold dilutions of serum samples in duplicate in a total volume of 150 μl for 1.5 h at 37°C in 96-well flat-bottom culture plates before being added to TZM-bl cells (10,000 cells in 100 μl of growth medium containing DEAE dextran). One set of control wells received cells plus virus (virus control), and another set received cells only (background control) in addition to wells that received antibodies with known ID50 values (positive control). After a 72-h incubation, 100 μl of cells was transferred to a white 96-well solid plate (Costar) for assays of luciferase activities using BrightGlo substrate solution as described by the supplier (Promega). In peptide competition experiments, the same neutralization protocol described above was applied, with the exception that before the exposure of the sera to pseudovirus, the sera were incubated for 30 min at 37°C with 30 μg/ml of overlapping peptides spanning the V3 loop of a consensus B (ConB) HIV-1 subtype Env or scrambled V3 peptide. Percent neutralization was determined by measuring the reduction in number of RLU relative to that of wells that contain the same dilution of the corresponding preimmune sera.
Neutralization of the global tier 2 virus panel and mutant CE1176_A3 was performed as previously described (25, 49). The neutralization titer was expressed as the reciprocal serum dilution that resulted in 50% reduction of RLU (ID50).
Generation of pseudotyped viruses with mutant Env of CE1176_A3.
Mutations in an epitope(s) targeted by a panel of broadly neutralizing monoclonal antibodies (bNMAb) were introduced into the CE1176_A3 env gene using the QuikChange mutagenesis kit (Stratagene) (21). The epitopes targeted and the specific mutations introduced were as follows: (i) CD4bs epitopes, N280D and G458Y; (ii) V2 epitopes, N160K; (iii) V3 epitopes, N332A; (iv) membrane-proximal external region (MPER) epitopes, W672A; and (v) gp120/gp41 epitopes, N611A and N625A. Since pseudotyped viruses bearing a mutated epitope(s) showed increased resistance only to the bNMAb targeting these sites, this panel of mutants was used to characterize the nature of neutralizing activities against CE1176_A.
Statistical analysis.
Prism (GraphPad Software, Inc.) was employed for all statistical analyses, all using two-sided tests. The Wilcoxon matched-pairs signed-rank test was used to compare MFI binding and ID50 values against the global tier 2 panel. The Mann-Whitney test was used to compare pairwise reciprocal serum ID50 values and percent neutralization against tier 1 viruses. Differences between three or more groups were analyzed by the Kruskal-Wallis analysis of variance (ANOVA) test. The percentages of the global tier 2 panel neutralized were compared within the same groups by using McNemar's test for proportions for paired values.
RESULTS
Immunogens and immunization regimen.
To determine if antibody responses elicited by a poxvirus prime-gp120 protein boost strategy are affected by the choice of the Env immunogen used, we studied two clade B Envs (JR-FL and PVO.4) that differ in multiple parameters, including tissue origin, neutralization sensitivity, and presence of the N7 glycan previously shown to modulate the exposure of the CD4 and coreceptor binding sites on Env (17, 21–23). JR-FL is a brain isolate that lacks the N7 glycan sequon (22) and shows tier 2 neutralization sensitivity, whereas PVO.4 is a blood isolate that retains the highly conserved N7 glycan sequon and shows a tier 3 neutralization-resistant phenotype (21, 23, 50).
To increase the efficacy of priming with these Env immunogens, we took advantage of an attenuated but replication-competent vaccinia virus vector, v-NY, which was used in the first clinical trials of the poxvirus-protein boost strategy (29, 51). We generated recombinant vaccinia viruses that express full-length Envs with (N7+) or without (N7−) the sequon of the N197 glycan (Fig. 1). As reported previously, overexpression of full-length HIV-1 Env resulted in the accumulation of unprocessed gp160. However, these Envs are processed, albeit inefficiently, and are fully functional in engaging the CD4 receptor and mediating membrane fusion (34, 52).
FIG 1.
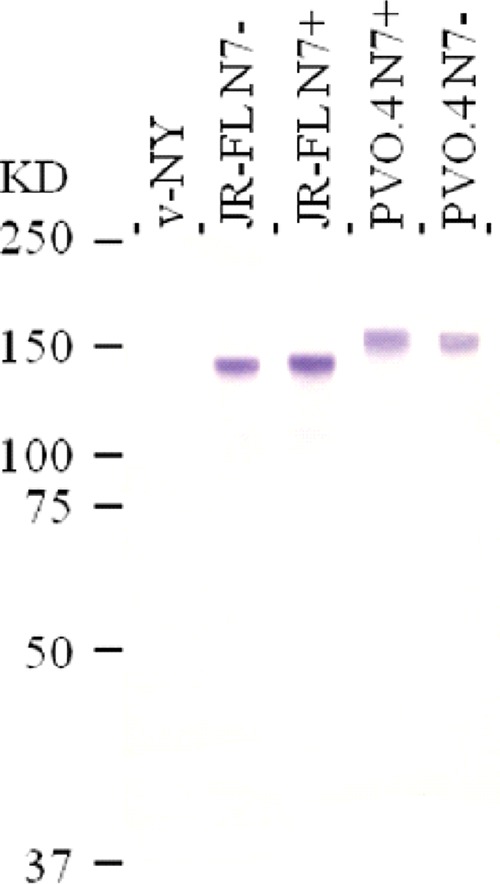
Western blot analysis of gp160 expressed by recombinant vaccinia virus. Lysates of African green monkey kidney cells (BSC-40) infected with parental vaccinia virus, v-NY, or recombinant viruses expressing the full-length Env of HIV-1 JR-FL or PVO.4 were resolved by SDS-PAGE, and immunoreactive protein gp160 was detected by HIV-positive human serum. N7− and N7+ denote the absence and presence, respectively, of the sequon for the N-linked glycan at amino acid 197.
New Zealand White rabbits were primed at weeks 0 and 8 with recombinant vaccinia virus expressing one of four full-length gp160s: JR-FL N7 deglycosylated (JR-FL N7−), JR-FL N7 glycosylated (JR-FL N7+), PVO.4 N7 glycosylated (PVO.4 N7+), or PVO.4 N7 deglycosylated (PVO.4 N7−) Env (Fig. 1). Animals were boosted with the cognate gp120 Env protein formulated in alum at weeks 35 and 48 (Fig. 2). Animal sera collected 2 weeks after the second vaccinia immunization (week 10) and after each gp120 boost (weeks 37 and 50) were tested for antibody binding, ADCC, and neutralizing capabilities in comparison to preimmune sera.
FIG 2.
Study design and immunization schedule. Rabbits were immunized with recombinant vaccinia virus expressing full-length gp160 at weeks 0 and 8, followed by a cognate gp120 protein boost formulated in alum at weeks 35 and 48 as described in Materials and Methods. Sera were collected before and 2 weeks after each immunization event. Time points at which sera were collected for the analyses described in this study are marked by open arrows (weeks 10, 37, and 50).
All immunized animals generated Env-specific antibodies.
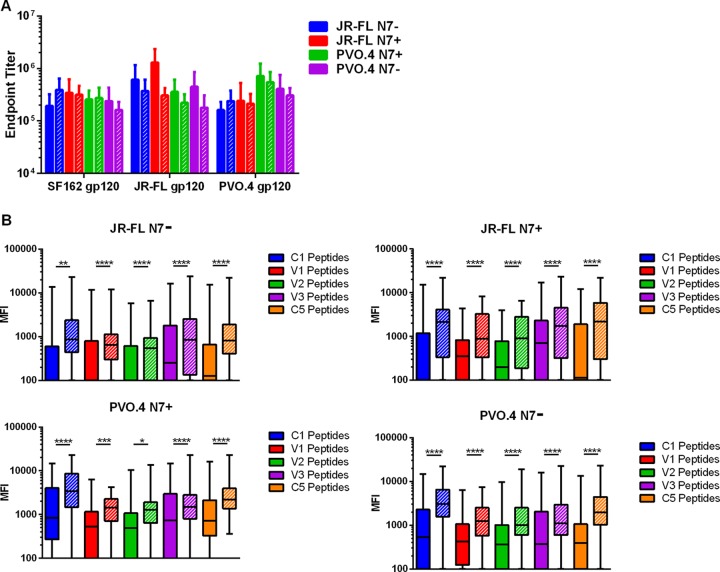
In order to determine the potency and cross-reactivity of the Env-specific antibodies, we tested the ability of week 37 and week 50 sera to bind homologous wild-type (WT) JR-FL and PVO.4 gp120 protein as well as heterologous SF162 gp120 protein in an ELISA (Fig. 3A). Immune sera showed endpoint binding titers of ∼105 against SF162 gp120 and titers up to ∼106 against homologous gp120. All sera demonstrate similar endpoint binding titers to heterologous gp120. Maximal endpoint titers were achieved after the first gp120 boost, with no further increase obtained after the second boost. This indicates that the prime-boost immunization regimen, regardless of the Env used in this study, was able to generate high titers of cross-reactive Env-specific antibodies.
FIG 3.
gp120-specific antibody response in immunized animals. (A) Endpoint titers of Env-specific antibodies in week 37 (solid boxes) or week 50 (hatched boxes) sera were determined by ELISA against a heterologous gp120 (WT SF162 gp120) or a homologous gp120 (WT JR-FL or PVO.4 gp120) as denoted on the x axis. Error bars indicate standard deviations within the group. (B) Reactivity of antibody responses targeting single, overlapping linear peptides corresponding to the variable (V1 to V3) or conserved (C1 and C5) regions of gp120 as indicated was reported as the mean fluorescence intensity (MFI) in the Bioplex assay. Solid boxes indicate week 37 serum, and hatched boxes represent week 50 serum. The boxplots indicate the median as well as the range of responses against individual peptides in each pool, including the maximum, minimum, and the 75% and 25% quartiles as indicated. P values (*, P ≤ 0.05; **, P ≤ 0.01, ***, P ≤ 0.001; ****, P ≤ 0.0001) were calculated by the Wilcoxon matched-pairs signed-rank test.
Effect of gp120 boost on the specificity of anti-Env responses.
Previous studies with subunit and prime-boost immunization have elicited higher antibody titers against V3, constant region 1 (C1), and constant region 5 (C5) than against V1 and V2 (53–55). To determine the epitope specificity of the antibody responses in immunized animals, we used a Bioplex assay to examine the ability of week 37 and week 50 sera to bind overlapping peptides derived from consensus B (ConB) Env spanning C1, C5, V1, V2, and V3 epitopes (Fig. 3B). Consistent with previous studies and regardless of which immunogen was used, high levels of serum reactivity were detected against C1, C5, and V3 peptides compared to V1 and V2 peptides. All animals generated antibodies that recognize peptides that span the midregion of the V2 loop (HXB2 numbering sequence 169 to 184; data not shown), a region recognized by antibodies generated by protected RV144 vaccinees (56). While there was no significant difference in the overall gp120-binding antibody titer between week 37 and week 50 sera (Fig. 3A), the second gp120 immunization significantly boosted antibody reactivity to all peptides tested (Fig. 3B), indicating a preferential expansion of antibodies that recognize linear epitopes resulting from the gp120 boosts.
Cross-reactive antibodies that recognize recombinant V1/V2 fusion proteins.
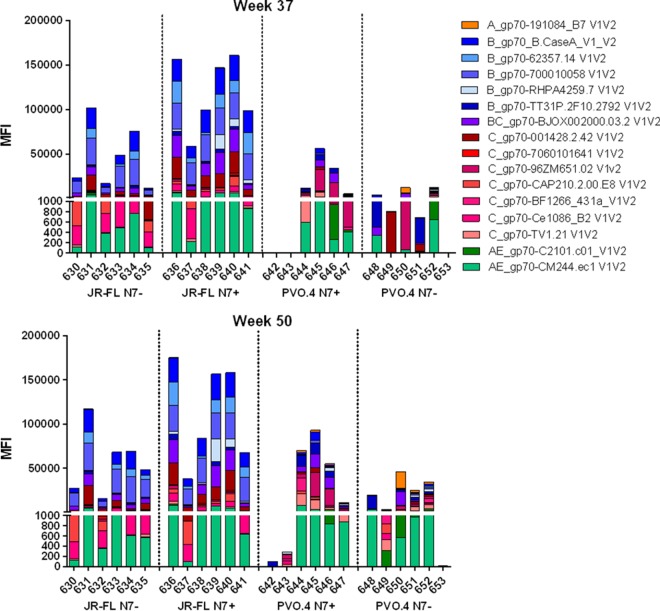
Antibodies against the V1/V2 loop, specifically those that recognize V1/V2-gp70 fusion proteins, have been shown to correlate with protection in the RV144 trial (4–6). Since all animals generated antibodies against peptides corresponding to V1 and V2 (Fig. 3B), we tested the rabbit serum's ability to recognize a cross-clade panel of V1/V2 fusion proteins derived from different Envs in a multiplex binding antibody assay (Fig. 4). By week 37, all 12 JR-FL-immunized animals and 9 of 12 PVO.4-immunized animals generated antibodies that recognize V1/V2 fusion proteins derived from multiple isolates of different clades. Reactivities to V1/V2 fusion proteins are comparable between week 37 and week 50 sera, indicating that the priming followed by one gp120 boost was sufficient to generate V1/V2 binding antibodies. The animals immunized with JR-FL N7+ generated some of the highest mean florescence intensity (MFI) values against clade B V1/V2 fusion proteins, compared to other groups. Regardless of the presence or absence of the N7 glycan, immunization with JR-FL Env consistently generated V1/V2 fusion binding antibodies capable of recognizing clade B, C, and AE Envs. This contrasts with the more sporadic reactivity observed with PVO.4 Env immunization. These results indicate that the prime-boost strategy effectively induced V1/V2-directed antibodies, but the profile of their V1/V2 reactivity differed between animals that received the JR-FL or the PVO.4 immunogens.
FIG 4.
Cross-reactivity of anti-V1/V2 antibodies in immunized animals. V1/V2-specific antibodies in week 37 and week 50 sera at a 1:500 dilution were measured against a cross-clade panel (color coded by clade) of gp70-V1/V2 MuLV scaffold proteins in a binding antibody multiplex assay (BAMA). The animal number and immunization group are indicated on the x axis. Dotted lines separate the groups.
Antibody-dependent cellular cytotoxicity in immune sera.
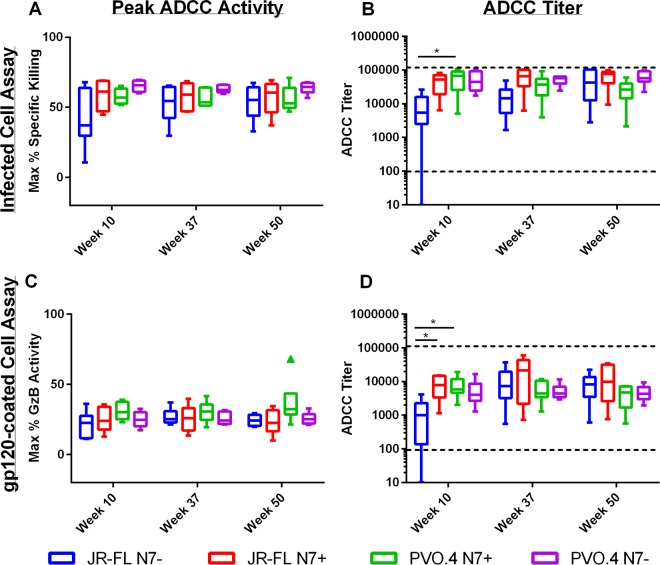
Prime-boost immunization in the RV144 trial induced robust HIV-1-specific ADCC responses (4), some of which were linked to the protective V1/V2 Ab response (6, 57). Since Env-specific ADCC activity can be elicited in rabbits (58), we examined if antisera from the present study were able to mediate ADCC. As shown in Fig. 5, immune sera from all experimental groups showed robust ADCC activities against target cells expressing a heterologous clade B (SF162) Env in the infected cell assay (Fig. 5A and B) and target cells coated with SF162 gp120 (Fig. 5C and D). It is notable that with the exception of JR-FL N7− Env-immunized animals, close to maximal levels of ADCC activities were elicited in animals from all immunization groups 2 weeks after recombinant vaccinia virus immunization alone (week 10, Fig. 5). ADCC titers were significantly lower in JR-FL N7− Env-immunized animals than in JF-FL N7+ and/or PVO.4 N7+ Env-immunized groups after vaccinia priming (P ≤ 0.05, Kruskal-Wallis test) (Fig. 5B and D, week 10). However, after gp120 boosts, comparable ADCC titers were achieved in all immunization groups by the end of the study (Fig. 5B and D, week 50).
FIG 5.
ADCC activity in rabbit sera. ADCC activity was measured by two methods: a luciferase-based assay using CEM.NKRCCR5 cells infected with HIV-1 SF162 infectious molecular clone as targets (A and B), or a flow-based assay using the same target cells but coated with SF162 gp120 (C and D). Results are expressed in the left panels as the peak ADCC activity, which is the maximum percent specific killing (A) or the maximum percent GzB activity (C) observed for each serum tested at any dilution. Alternatively, activities are reported in the right panels as the ADCC titer, which is the serum dilution at which the last positive ADCC response was observed (≥15% specific killing for panel B; ≥8% GzB activity for panel D). Activities in preimmune sera were subtracted from all values reported for immune sera collected at weeks 10, 37, and 50. The median, range, and upper and lower quartile of values within each group are indicated by the boxplots, and statistically significant differences between groups are indicated by horizontal bars above the respective boxes (*, P ≤ 0.05, Kruskal-Wallis test).
Neutralization of standard tier 1B and tier 2 clade B isolates.
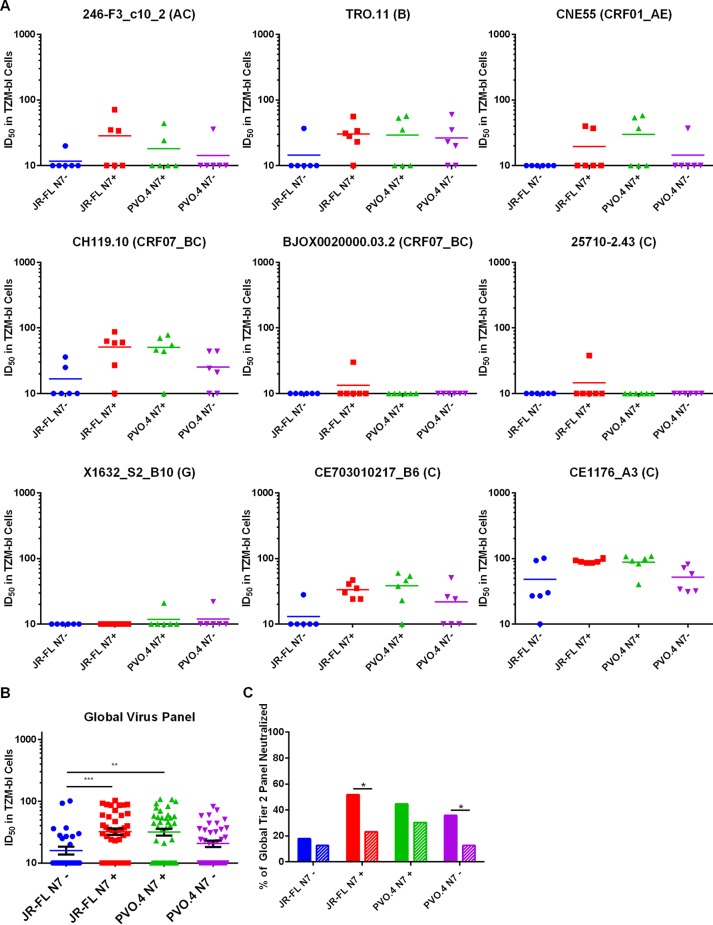
In order to determine their neutralization capabilities, we tested immune sera against a number of tier 1 HIV-1 isolates and a standard panel of primary clade B isolates (Table 1). At a 1:15 dilution, all sera neutralized SF162, a tier 1A isolate, and most sera were capable of neutralizing some tier 1B isolates, including DJ263.8, a clade A isolate (27), indicating the presence of cross-neutralizing activities. However, CRF02_AG_271, a clade AG isolate, was not as readily neutralized by the sera. Sera from 4 of 6 animals immunized with JR-FL N7+ and from 3 of 6 animals immunized with PVO.4 N7+ neutralized one or more of the tier 2 and tier 3 isolates on the standard clade B panel, whereas those from N7− Env-immunized animals failed to do so. Notably, two animals in the PVO.4 N7+ group, animals 643 and 645, showed neutralizing activities against nearly the entire panel of tier 1, 2, and 3 isolates tested.
TABLE 1.
Neutralization of tier 1 and tier 2 viruses
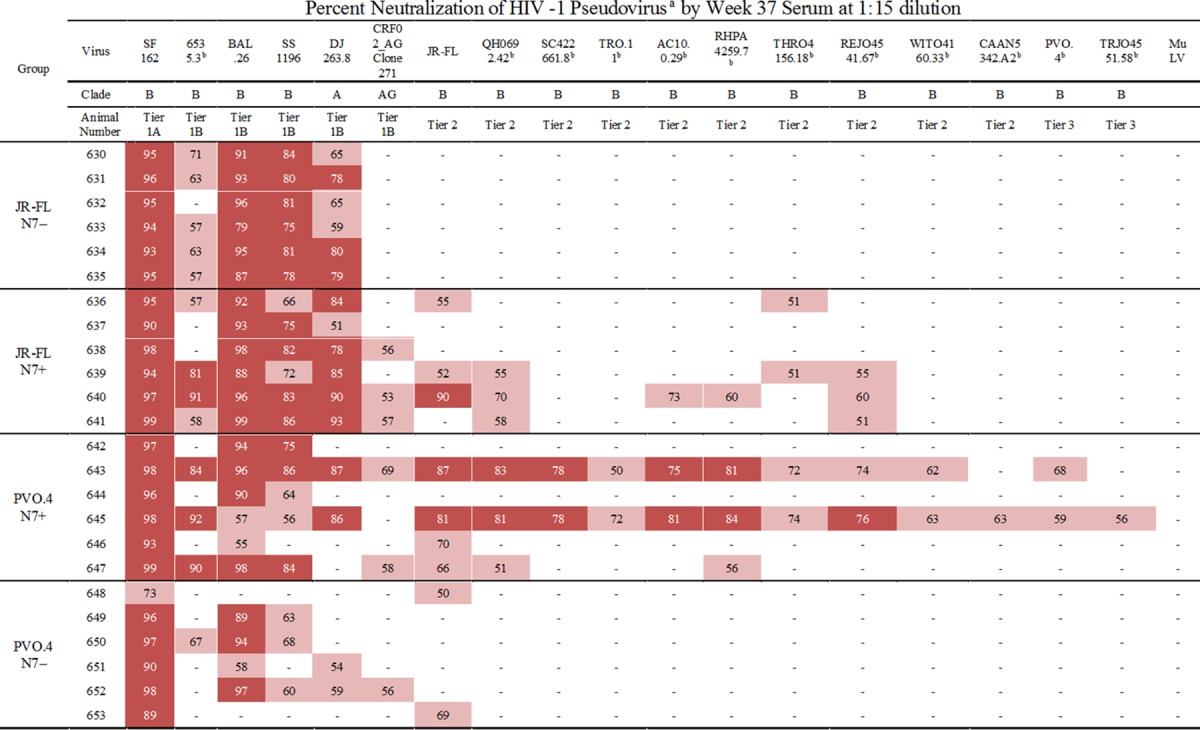
Input virus was normalized by infectivity at 150 TCID50 in TZM-bl cells. The column subheadings specify the indicator viruses and their respective clades. Percent neutralization was determined in TZM-bl cells by measuring the reduction in number of RLU relative to that of wells that contain the same dilution of corresponding preimmune sera. Pink highlighting, 50% to 74% neutralization; red highlighting, ≥75% neutralization. Results are the average of two or more experiments, each performed in duplicate.
Isolates included in the standard clade B panel are indicated (23).
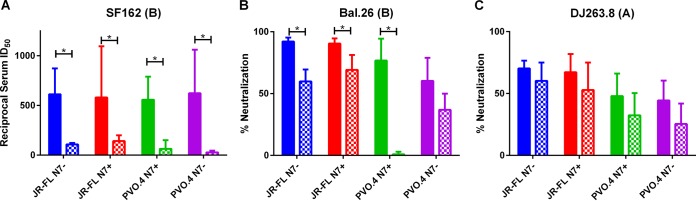
Previous studies have shown that sera neutralizing tier 1 isolates are primarily directed against the V3 loop. Therefore, we tested whether linear ConB V3 peptides would compete with the ability of immune sera to neutralize tier 1 viruses (Fig. 6). Regardless of the immunogen used, neutralizing activities of immune sera against SF162 and Bal.26 were fully or partially competed out with the addition of V3 peptides (Fig. 6A and B), indicating that V3-directed antibodies contributed to the neutralizing activities in the sera. However, neutralization of DJ263.8 was unchanged with the addition of V3 peptides (Fig. 6C), indicating that the neutralizing activities could not be competed with the V3 peptides used, or the NAb may recognize epitopes other than V3 on the clade A Env.
FIG 6.
Competition of tier 1 virus-neutralizing activities by V3 peptides. The effect of V3 peptide on the neutralizing activity of week 37 sera against tier 1A (SF162) and tier 1B (Bal.26 and DJ263.8) viruses was evaluated. The clade designation of the indicator viruses is shown in parentheses. Neutralizing activities are shown either as the reciprocal serum dilution that resulted in 50% reduction of infectivity (ID50) (A) or as the percent neutralization of virus infectivity by test sera at a 1:15 dilution (B and C). Neutralizing activities detected in the presence of a scrambled V3 peptide are represented by solid boxes, and activities detected in the presence of overlapping peptides corresponding to the consensus clade B V3 sequence are represented by checkered boxes. Error bars indicate standard deviations of data from at least two independent experiments performed in duplicate. P values (*, P ≤ 0.05) were calculated by the Wilcoxon matched-pairs signed-rank test.
Cross-clade neutralization of a panel of global tier 2 isolates.
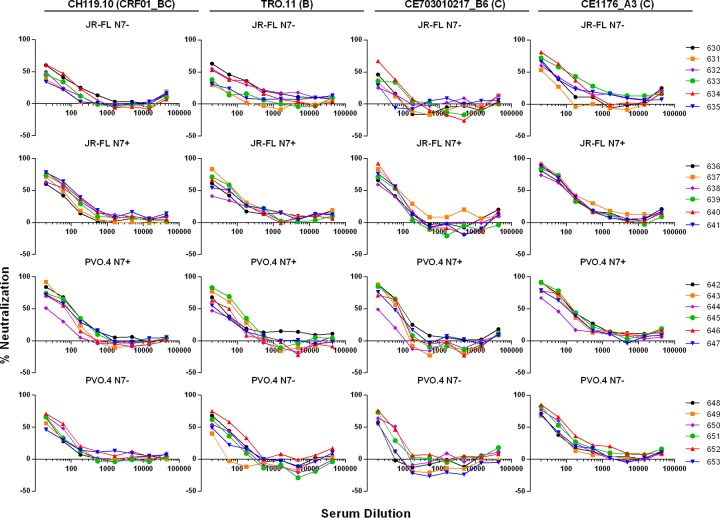
To further test the breadth of the neutralizing reactivity of the immune sera, we tested the ability of week 37 sera to neutralize a standard panel of global tier 2 viruses (Fig. 7). Interestingly, each virus on the panel was neutralized by at least one animal sera tested. All animals, except one in the JR-FL N7− group (animal 631) (Fig. 7A, CE1176_A3 panel), generated sera capable of neutralizing at least one virus on this global panel. Notably, a tier 2 clade C isolate, CE1176_A3, was neutralized by sera from all but one animal, with titers reaching ∼1:100 for some sera (Fig. 7A). JR-FL N7+ and PVO.4+ Env-immunized animals generated higher ID50 titers against the global tier 2 virus panel than the JR-FL N7− Env-immunized animals, while PVO.4 N7+ Env-immunized animals showed a trend (P = 0.08) toward higher ID50 titers against the same panel of viruses in comparison to PVO.4 N7− Env-immunized animals (Fig. 7B). Dose dependence of the neutralizing activities of week 37 sera against a subset of the tier 2 viruses is shown in Fig. 8. To demonstrate the specificity of viral inhibition activities in the immune sera, we summarize in Table 2 the ID50 values for all the virus and serum combinations (including MuLV as the negative control and sera from weeks 37 and 50) without background subtraction. Notably, the percent neutralization against the global panel diminished after a second gp120 boost for animals in the JR-FL N7+ and PVO.4 N7− groups (Table 2 and Fig. 7C), consistent with the notion that repeated immunization with gp120 did little to boost the neutralizing antibody response against heterologous tier 2 isolates tested.
FIG 7.
Neutralization of global tier 2 viruses. (A) Each graph shows the reciprocal dilution of week 37 serum from each immunized animal that resulted in 50% reduction of infectivity (ID50) against the indicator virus shown at the top of each graph. The clade designation of the indicator viruses is shown in parentheses. Values above 10 were considered positive neutralization, as nonspecific activities measured in the same assay against virus pseudotyped with the MuLV envelope were subtracted as background for each serum sample before the ID50 values were reported here. See Table 2 for ID50 values before background subtraction. (B) The neutralization titer (ID50) for each animal serum from each immunization group collected at week 37 was determined against the entire global tier 2 virus panel. The means are indicated by colored bars, and the standard errors are indicated by black error bars. P values (**, P ≤ 0.01; ***, P ≤ 0.001) were calculated by the Mann-Whitney test for paired values. (C) Percent positive neutralization is defined as the percentage of the total number of serum samples and indicator virus combinations that resulted in positive neutralization in the TZM-bl assay. This value was determined for all the animals in each immunization group after the first (week 37) and second (week 50) gp120 boost as indicated. The difference in results between week 37 and week 50 samples was determined by McNemar's test for proportions for paired values (*, P ≤ 0.05).
FIG 8.
Dose-dependent neutralization of tier 2 viruses by week 37 sera. The x axis indicates the reciprocal dilutions of the immune sera tested (3-fold serial dilution starting at 1:20). The y axis indicates the percentage of viral infectivity neutralized by a given serum dilution against the indicator viruses. The four groups of experimental animals are aligned horizontally and designated as indicated on each panel. The four indicator viruses are aligned vertically, and their designations and clade origin (in parentheses) are given at the top of each column. Please note that the same color scheme denotes different animals in the four immunization groups (see legends on the right).
TABLE 2.
Neutralization against global tier 2 viruses
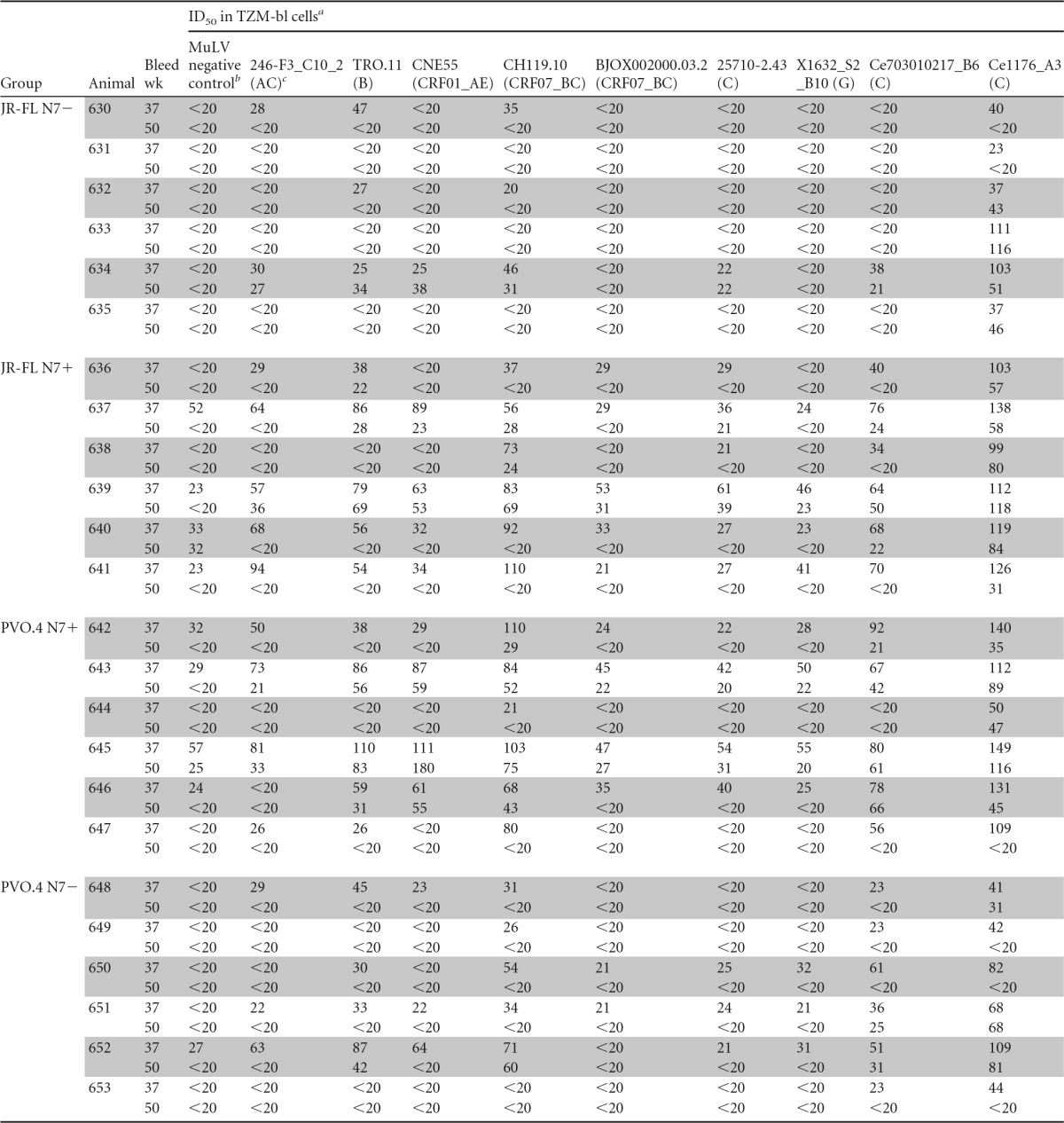
ID50 is defined as the serum dilution that resulted in 50% reduction of infectivity as measured by RLU. The starting dilution was 1:20.
MuLV was used as the negative control; nonspecific activities shown in the column were not subtracted from other values in the table.
Clade designations of the indicator viruses used in the neutralization assay are indicated in parentheses.
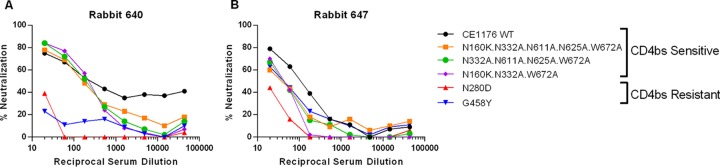
Characterization of neutralizing activities in immune sera against CE1176_A3.
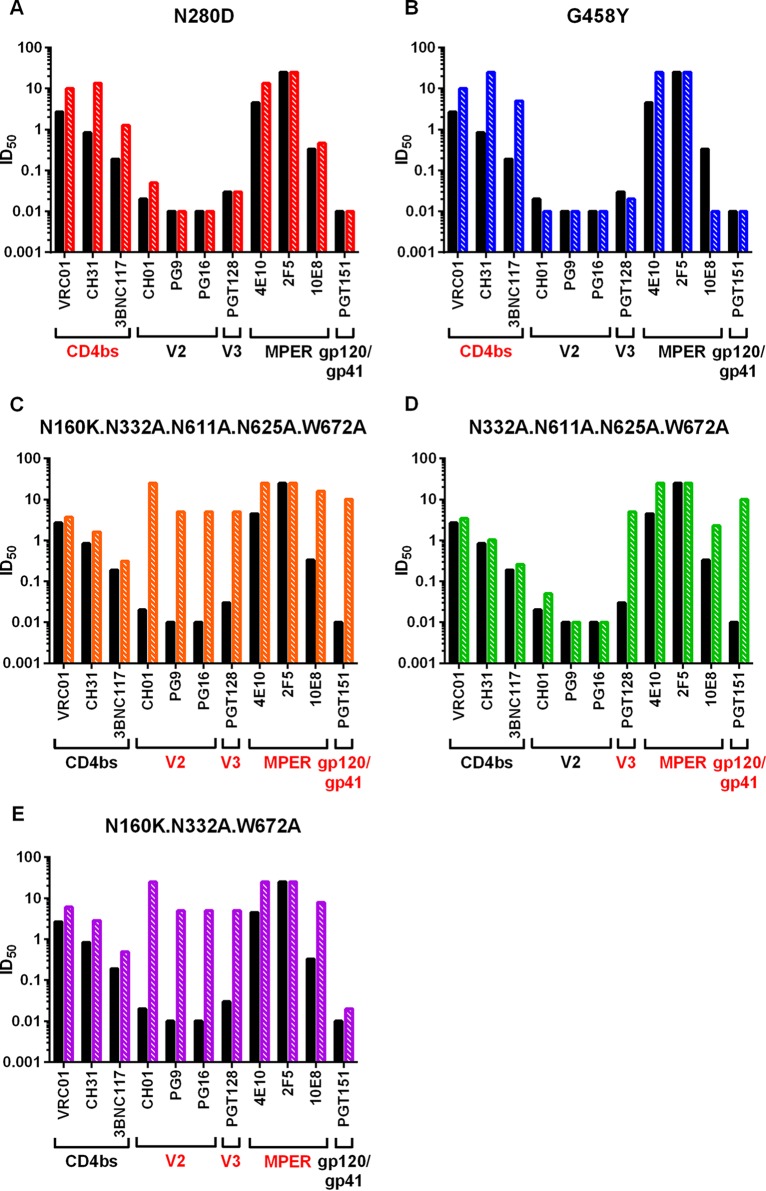
To determine the epitope specificity of the tier 2 neutralizing activities in the immune sera, we generated a panel of site-specific mutants of CE1176_A3 that are resistant to one or more broadly neutralizing monoclonal antibodies (bNMAb) of known specificities. CE1176_A3 env was used to generate these mutants because all animals but one could neutralize this isolate better than other isolates on the global panel (Fig. 7A). Previous work has defined the epitopes targeted by bNMAb by mutational analysis: the N280D and G458Y mutants are resistant to CD4bs-directed antibodies VRC01, CH31, and 3BNC117 (59), the N160K mutant is resistant to V2-directed antibodies CH01, PG9, and PG16 (60–62), the N332A mutant is resistant to V3 glycan-directed antibody PGT128 (63), the W672A mutant is resistant to MPER-directed antibody 4E10, 2F5, or 10E8 (64, 65), and the N611A N625A double mutant is resistant to antibody PGT151 directed to gp120/gp41 interface epitopes (66, 67). Based on this information, a panel of single-site and multiple-site mutants was generated and their sensitivity profile was tested against a panel of bNMAb listed above. Results in Fig. 9 confirm the expected neutralization sensitivity profiles of the following mutants: (i) N280D and G458Y single-site mutants were resistant to CD4bs-directed monoclonal antibodies (MAb) VRC01, CH31, and 3BNC117 but sensitive to MAb directed to all other sites (Fig. 9A and B); (ii) a quintuple mutant (N160K N332A N611A N625A W672A) retained sensitivity to CD4bs antibodies but was resistant to antibodies directed to all other sites (Fig. 9C); (iii) a quadruple mutant (N332A N611A N625A W672A) was sensitive to CD4bs- and V2-directed antibodies but resistant to V3, MPER, and gp120/gp41 interface antibodies (Fig. 9D); and (iv) a triple mutant (N160K N332A W672A) was sensitive to CD4bs- and gp120/gp41 interface-directed antibodies but resistant to V2-, V3-, and MPER-directed antibodies (Fig. 9E). Thus, these mutants demonstrated the expected neutralization sensitivity profile against the panel of bNMAb tested.
FIG 9.
Neutralization sensitivity profile of CE1176_A3 mutants determined by a panel of broadly neutralizing monoclonal antibodies. The ID50 values for a given neutralizing antibody are shown against the WT CE1176_A3 (solid black bar) and the indicated mutants (hatched, colored bar). The epitope recognized by each antibody is indicated at the bottom of the panel. The maximum antibody concentrations used were as follows: 10 μg/ml for VRC01 or PGT151; 25 μg/ml for CH31, CH01, 4E10, 2F5, or 10E8; and 5 μg/ml for 3BNC117, PG9, PG16, or PGT128. The lower limit of detection is 0.01 μg/ml of antibody. A greater height of the hatched, colored bar than of the black bar indicates increased resistance of the mutant virus to the antibody tested compared to that of the WT virus. Resistance of the mutant to any MAb tested is indicated by the epitopes in red letters at the bottom of the panel.
Having characterized the neutralization sensitivity profile of these mutants, we examined the epitope specificity of the neutralizing activity in a subset of immune sera against WT and mutant CE1176_A3. Most sera had similar neutralizing potencies against WT CE1176_A3 and mutant viruses, thus showing no identifiable specificity but confirming their neutralizing activities against this tier 2 virus. Notably, two animals, 640 and 647, showed clearly reduced neutralization potency when tested against CD4bs-resistant mutants (Fig. 10). Serum from animal 640 was unable to neutralize the CE1176_A3 N280D and G458Y mutants, both of which are resistant to CD4bs-directed antibodies, while retaining neutralizing activities against all other mutants tested (Fig. 10A). Serum from animal 647 also showed reduced neutralization potency against the N280D mutant, but not the G458Y mutant, consistent with the idea that the antibodies in this animal's serum targeted an epitope(s) dependent on N280 but not G458 (Fig. 10B). Together, these data indicate that CD4bs-directed antibodies may contribute to the neutralizing activities in the immune sera from these rabbits against a heterologous tier 2 virus, CE1176_A3. Interestingly, animal 647 serum also showed reduced potency against three multiple-site mutants tested (Fig. 10B), indicating neutralizing activities directed against multiple targets.
FIG 10.
Specificity of neutralizing activities in immune sera determined by sensitivity to epitope-specific mutants of CE1176_A3. Week 37 sera from two immunized rabbits were tested for neutralizing activities against WT CE1176_A3 and a panel of epitope-specific mutants. The three multiple-site mutants shown retain their sensitivity to CD4bs-directed antibodies, whereas the two single-site mutants are resistant. The x axis indicates the reciprocal dilutions of the immune sera tested (3-fold serial dilution starting at 1:20). The y axis indicates the percentage of viral infectivity neutralized by a given serum dilution against the WT or mutant viruses. A decreased percent neutralization against a mutant virus compared to that of the WT indicates a loss of neutralizing activities due to the mutation(s) introduced into a specific epitope(s) in the indicator virus.
DISCUSSION
We demonstrate here a poxvirus prime-gp120 boost immunization regimen that elicited, in a rabbit model, immune responses that have been shown to correlate with protection against HIV-1 acquisition in humans or other preclinical animal models. Specifically, we showed that immunized rabbits generated cross-clade V1/V2-specific antibodies (Fig. 4), as well as those that mediate ADCC activities (Fig. 5), both responses found to correlate with risk reduction of HIV-1 acquisition in the RV144 trial (4, 5, 56). In addition, all the immunized rabbits in this study showed neutralizing activities against one or more of the HIV-1 isolates tested, including a number of heterologous tier 2 isolates in the standard TZM-bl assay (Tables 1 and 2; Fig. 7 and 8), albeit at low titers (1:20 to ∼1:100). These observations, if confirmed in parallel studies in humans or nonhuman primates, may provide a starting point to examine factors in the prime-boost immunization strategy that may improve upon the modest efficacy achieved by the RV144 trial.
The profiles of V1/V2-specific responses differed between animals immunized with JR-FL or PVO.4 Env (Fig. 4). All JR-FL-immunized animals generated antibodies that recognized V1/V2 fusion proteins from diverse isolates, whereas a number of PVO.4-immunized animals failed to show any detectable V1/V2 reactivity. The V1/V2 loops of different HIV-1 isolates differ significantly in their amino acid lengths and the numbers of potential N-linked glycan sites (68–70). Whether the differential immunogenicity in V1/V2 responses observed in this study reflects the accessibility or structural stability of the V1/V2 loop in these Envs is yet to be determined. Nevertheless, our finding indicates that Envs from some HIV-1 isolates may be better than others at inducing antibodies that recognize the conformation-dependent epitopes in the V1/V2 loop. Our findings also indicate the importance of examining diverse Env designs, including polyvalent Envs and Envs with judiciously chosen modifications (e.g., glycans, stabilized trimers, etc.) that may provide consistent and sufficient coverage of V1/V2 responses and other desired immunogenicity profiles.
While we have yet to demonstrate any functional role of the V1/V2-specific antibodies generated in this study, sera from all animals mediated ADCC against gp120-coated or HIV-1-infected target cells (Fig. 5). It is of interest to note that even animals that failed to generate V1/V2 antibodies against the panel of gp70 fusion proteins tested (e.g., animals 642 and 643 in the PVO.4 N7+ immunization group) (Fig. 4) showed robust ADCC activities against SF162 Env (Fig. 5 and data not shown). This might indicate differential specificity of their V1/V2 responses or the presence of ADCC activities against Env-specific targets outside V1/V2. This is also consistent with the differential ADCC activities measured by two different assay formats, as shown in Fig. 5 (panels A and B versus panels C and D). We used two different assay formats because, by virtue of the nature of the antigens expressed on the target cells, they display different epitopes. For instance, use of gp120-coated target cells allows detection of ADCC activities against CD4-induced epitopes but not activities mediated by CD4bs antibodies, since the latter is engaged by the cellular CD4 receptor. In contrast, the infected target cells provide a wide range of epitopes exposed at the time of virus budding, including the CD4bs. Therefore, not surprisingly, the two assays yielded different results, as the target cells are recognized by polyclonal Ab responses with different ADCC epitope specificities. Regardless, these findings indicate that the recombinant vaccinia prime-gp120 protein boost regimen described here was able to elicit, in a preclinical animal model, antibodies with epitope specificity (reactive to V1/V2 fusion protein) and functional activities (ADCC) that have been correlated with protection in the RV144 trial (4, 56).
Importantly, the immunized rabbits described here also generated cross-reactive neutralizing activities, not only against tier 1A and some tier 1B viruses but also against heterologous tier 2 viruses, with up to 50% neutralization of the global tier 2 panel tested (Table 1 and Fig. 7). This neutralizing activity was dose dependent (Fig. 8), reproducible (Fig. 10 and data not shown), and specific, as indicated by the minimal or undetectable activities against MuLV Env-pseudotyped virus (Tables 1 and 2). However, the nature and specificity of the neutralizing responses observed in these animals remain to be defined. Some, but not all, of the tier 1 neutralizing activities can be competed out by V3 peptides (Fig. 6), indicating that antibodies directed to non-V3 or nonlinear epitopes in V3 may contribute to the neutralization of these easy-to-neutralize isolates. Analysis with epitope-specific mutants of CE1176_A3 indicates that some animals generated CD4bs-directed antibodies, albeit with differential and overlapping specificities, that contributed to the neutralization against this tier 2 virus (Fig. 10). This is highly surprising, since cross-reactive neutralizing antibodies directed against the CD4bs typically take 2 to 4 years after seroconversion to develop in infected individuals (2, 7, 9, 48). Without further substantiation, we cannot attribute the CD4bs-directed neutralizing activities in the immunized rabbits to bNAb as defined by MAb such as VRC01. Nevertheless, our findings support the notion that the prime-boost immunization approach as described here can induce NAb responses against heterologous tier 2 isolates, albeit with limited breadth and modest titers. It is noteworthy in this context that the pattern of NAb response reported here is different from those reported by others. For instance, using SOSIP Env trimers as immunogens, Sanders et al. (14) were able to elicit high titers of autologous NAb (against BG505, a tier 2 virus) in rabbits and macaques but little or no NAb against heterologous tier 2 viruses, such as CE1176_A3. In the present study, despite little or no NAb response against the autologous viruses (JR-FL and PVO.4) (Table 1), the majority of immunized rabbits generated NAb against heterologous tier 2 viruses in the global virus panel (Fig. 7 and 8; Table 2). It should also be noted that the study described by Sanders et al. (14) was done at the same contract facility (Covance) as ours and the neutralization assays were performed by the same investigators, using the same assay (pseudotyped virus assay in TZM-bl cells) and many of the same indicator viruses, including CE1176_A3 in the global tier 2 virus panel. These findings suggest that the immunization approach described here may have elicited a profile of anti-gp120 responses distinct from those described by Sanders et al. (14). Future studies will be needed to better define the nature of these responses and to determine if and how they may be improved and translated in nonhuman primates or humans.
It is interesting to note that repeated gp120 boosting increased the ability of immune sera to bind certain peptides, including those that were also recognized by RV144 vaccinees (Fig. 3B) (4–6). However, the increase in peptide-specific responses was not accompanied by increased titers to gp120 (Fig. 3A). Therefore, the second boost may preferentially increase responses to linear peptides presented by the gp120 proteins. Additionally, repeated gp120 immunization failed to boost and, in some cases, resulted in diminished neutralization responses (Fig. 7C). This observation is consistent with the idea that the first subunit protein immunization may boost antibodies that were generated after the priming event, while the second subunit protein boost preferentially expanded antibodies induced by the first protein immunization that were limited in their neutralizing activities (71). If so, gp120 may be a suboptimal immunogen for a prime-boost immunization regimen aimed at generating neutralizing antibody responses, in line with the observation of Sanders et al. (14).
While the N7 glycan has been shown to increase the stability or exposure of the CD4bs and V3 epitopes in a conserved manner (17, 21), results reported here suggest that the N7 glycan may not play a conserved role in modulating Env immunogenicity, at least in the context of the vaccinia prime-gp120 boost regimen. As shown in Table 1 and Fig. 7, animals immunized with N7+ Env showed similar or slightly broader neutralization and higher titers of neutralizing activity than those immunized with their N7− Env counterparts. The failure to observe an improved NAb response in N7− Env-immunized animals is not due to suboptimal immunization in these animals, since sera from all experimental groups have similar vaccinia virus neutralizing (data not shown) and gp120 binding titers (Fig. 3), although we cannot exclude the possibility that any putative effect of the N7 glycan on Env immunogenicity may have been masked by responses not detected by these assays. This is in direct contrast to our previous study in macaques demonstrating that N7− 89.6 Env induced broader and more potent neutralizing serum than its glycosylated counterpart (17). While these results may be related to the Env immunogen (89.6 versus JR-FL or PVO.4) or the animal species (rabbits versus macaques) used, the potential effect of the N7 glycan on Env immunogenicity may also depend on a number of other factors. For example, while the removal of the N7 glycan enhanced the ability of 89.6 Env to mediate CD4-independent entry (17), no such effect was observed for N7− versions of JR-FL or PVO.4 Env (21). Additionally, our earlier study in macaques used 89.6 gp140 as the boosting immunogen, whereas the current study used monomeric gp120, in an attempt to better simulate the RV144 regimen. Since the N7 glycan is predicted to shield the adjacent protomer's V3 loop (72, 73), the biological property of the N7 glycan may depend on the trimeric structure of Env. In fact, our V3 peptide competition data demonstrate no difference between the ability of N7+ or N7− Env to generate V3-neutralizing antibodies in the context of gp120 boost (Fig. 6). Therefore, gp120 may not be the appropriate immunogen to determine if the N7 glycan has any impact on Env immunogenicity. Indeed, other studies have demonstrated improved neutralization responses in animals immunized with trimeric gp140 compared to those of animals immunized with gp120 monomers (74–77). Our finding that repeated gp120 boosting skewed antibody responses to linear epitopes (Fig. 3B) may also contribute to the lack of improved neutralization responses observed in N7− Env-immunized animals (Fig. 7C). It is therefore of interest to explore immunogens that can better preserve the trimer structure, such as SOSIP trimers, to test the potential role of the N7 glycan in modulating Env immunogenicity.
Despite many attempts (12–14, 78–80), generation of bNAb against tier 2 HIV-1 primary isolates by vaccination remains an unmet goal. Several recent studies have succeeded in demonstrating vaccine-induced NAb against autologous tier 2 virus or a panel of mutated tier 2 isolates with increased CD4 or V3 epitope exposure (12–14, 21). Our finding of neutralizing responses against heterologous tier 2 global isolates, albeit of modest potency and breadth, point to the possibility of generating such responses through the prime-boost immunization platform. The basis for the apparently improved response reported here is not clear. Several factors may contribute to our findings, including the possible role of a replication-competent virus for priming (29, 51). Replication-competent viruses have been shown to be more effective in protecting against certain viral infections and disease than immunization by replication-incompetent viruses or subunit vaccines (81–83). Alternatively, differences in vaccine responses may also result from the specific Env, the nature of the boosting immunogens, and the immunization regimen used. Further studies will be needed to determine the potential contribution of these factors, including the role of specific glycans, to improve the protective efficacy achievable by the prime-boost immunization regimen.
ACKNOWLEDGMENTS
We thank Patricia Firpo, Lifei Yang, Rajesh Thippeshappa, Thaddeus Davenport, and Miklos Guttman for expert advice; Taryn Urion and LaRene Kuller for technical assistance; Cecilia Cheng-Mayer, Paul Clapham, Joseph Sodroski, Feng Gao, Beatrice Hahn, Jesus Salazar-Gonzalez, Denise Kothe, Xiping Wei, George Shaw, John Mascola, Dennis Ellenberger, Bin Li, Margaret Callahan, Salvatore Butera, Nathaniel Landau, Dan Littman, Susan Zolla-Pazner, Shan Lu, and the NIH AIDS Research and Reference Reagent Program for providing monoclonal antibodies and reagents; and Shan Lu, James Hoxie, Drew Weisman, and Kelly Lee for helpful discussions.
This study was supported in part by NIH grants R01 AI076170, P01 AI082274, P51 OD010425, 1R01AI102718, and HHSN27201100016C and the Bill and Melinda Gates Foundation award OPP1033102.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Saunders KO, Rudicell RS, Nabel GJ. 2012. The design and evaluation of HIV-1 vaccines. AIDS 26:1293–1302. doi: 10.1097/QAD.0b013e32835474d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascola JR, Montefiori DC. 2010. The role of antibodies in HIV vaccines. Annu Rev Immunol 28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 3.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 4.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zolla-Pazner S, deCamp A, Gilbert PB, Williams C, Yates NL, Williams WT, Howington R, Fong Y, Morris DE, Soderberg KA, Irene C, Reichman C, Pinter A, Parks R, Pitisuttithum P, Kaewkungwal J, Rerks-Ngarm S, Nitayaphan S, Andrews C, O'Connell RJ, Yang ZY, Nabel GJ, Kim JH, Michael NL, Montefiori DC, Liao HX, Haynes BF, Tomaras GD. 2014. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 9:e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 6:228ra39. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. 2012. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol 30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog 7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med 5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 11.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 12.Crooks ET, Tong T, Chakrabarti B, Narayan K, Georgiev IS, Menis S, Huang X, Kulp D, Osawa K, Muranaka J, Stewart-Jones G, Destefano J, O'Dell S, LaBranche C, Robinson JE, Montefiori DC, McKee K, Du SX, Doria-Rose N, Kwong PD, Mascola JR, Zhu P, Schief WR, Wyatt RT, Whalen RG, Binley JM. 2015. Vaccine-elicited tier 2 HIV-1 neutralizing antibodies bind to quaternary epitopes involving glycan-deficient patches proximal to the CD4 binding site. PLoS Pathog 11:e1004932. doi: 10.1371/journal.ppat.1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley T, Fera D, Bhiman J, Eslamizar L, Lu X, Anasti K, Zhang R, Sutherland LL, Scearce RM, Bowman CM, Stolarchuk C, Lloyd KE, Parks R, Eaton A, Foulger A, Nie X, Karim SS, Barnett S, Kelsoe G, Kepler TB, Alam SM, Montefiori DC, Moody MA, Liao HX, Morris L, Santra S, Harrison SC, Haynes BF. 2016. Structural constraints of vaccine-induced tier-2 autologous HIV neutralizing antibodies targeting the receptor-binding site. Cell Rep 14:43–54. doi: 10.1016/j.celrep.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. 2015. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamatatos L, Morris L, Burton DR, Mascola JR. 2009. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med 15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 16.Day TA, Kublin JG. 2013. Lessons learned from HIV vaccine clinical efficacy trials. Curr HIV Res 11:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Cleveland B, Klots I, Travis B, Richardson BA, Anderson D, Montefiori D, Polacino P, Hu SL. 2008. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J Virol 82:638–651. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Jin W, Hu K, Luo S, Du T, Griffin GE, Shattock RJ, Hu Q. 2012. Highly conserved HIV-1 gp120 glycans proximal to CD4-binding region affect viral infectivity and neutralizing antibody induction. Virology 423:97–106. doi: 10.1016/j.virol.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Kolchinsky P, Kiprilov E, Bartley P, Rubinstein R, Sodroski J. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J Virol 75:3435–3443. doi: 10.1128/JVI.75.7.3435-3443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolchinsky P, Kiprilov E, Sodroski J. 2001. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J Virol 75:2041–2050. doi: 10.1128/JVI.75.5.2041-2050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Townsley S, Li Y, Kozyrev Y, Cleveland B, Hu SL. 2016. Conserved role of an N-linked glycan on the surface antigen of human immunodeficiency virus type 1 modulating virus sensitivity to broadly neutralizing antibodies against the receptor and coreceptor binding sites. J Virol 90:829–841. doi: 10.1128/JVI.02321-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng-Mayer C, Liu R, Landau NR, Stamatatos L. 1997. Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol 71:1657–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.deCamp A, Hraber P, Bailer RT, Seaman MS, Ochsenbauer C, Kappes J, Gottardo R, Edlefsen P, Self S, Tang H, Greene K, Gao H, Daniell X, Sarzotti-Kelsoe M, Gorny MK, Zolla-Pazner S, LaBranche CC, Mascola JR, Korber BT, Montefiori DC. 2014. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 88:2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol 80:1414–1426. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, Wu X, Shaw GM, Connors M, Wyatt RT, Mascola JR. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med 13:1032–1034. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landau NR, Page KA, Littman DR. 1991. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol 65:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooney EL, McElrath MJ, Corey L, Hu SL, Collier AC, Arditti D, Hoffman M, Coombs RW, Smith GE, Greenberg PD. 1993. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci U S A 90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thippeshappa R, Tian B, Cleveland B, Guo W, Polacino P, Hu SL. 2015. Oral immunization with recombinant vaccinia virus prime and intramuscular protein boost provides protection against intra-rectal SHIV challenge in macaques. Clin Vaccine Immunol 23:204–212. doi: 10.1128/CVI.00597-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham BS, Matthews TJ, Belshe RB, Clements ML, Dolin R, Wright PF, Gorse GJ, Schwartz DH, Keefer MC, Bolognesi DP, Corey L, Stablein DM, Esterlitz JR, Hu SL, Smith GE, Fast PE, Koff WC, the NIAID AIDS Vaccine Clinical Trial Network. 1993. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. J Infect Dis 167:533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 32.Joklik WK. 1962. The purification of four strains of poxvirus. Virology 18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- 33.Guo W, Cleveland B, Davenport TM, Lee KK, Hu SL. 2013. Purification of recombinant vaccinia virus-expressed monomeric HIV-1 gp120 to apparent homogeneity. Protein Expr Purif 90:34–39. doi: 10.1016/j.pep.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu SL, Kosowski SG, Dalrymple JM. 1986. Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature 320:537–540. doi: 10.1038/320537a0. [DOI] [PubMed] [Google Scholar]
- 35.Hu SL, Zarling JM, Chinn J, Travis BM, Moran PA, Sias J, Kuller L, Morton WR, Heidecker G, Benveniste RE. 1989. Protection of macaques against simian AIDS by immunization with a recombinant vaccinia virus expressing the envelope glycoproteins of simian type D retrovirus. Proc Natl Acad Sci U S A 86:7213–7217. doi: 10.1073/pnas.86.18.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carbonetti S, Oliver BG, Glenn J, Stamatatos L, Sather DN. 2014. Soluble HIV-1 envelope immunogens derived from an elite neutralizer elicit cross-reactive V1V2 antibodies and low potency neutralizing antibodies. PLoS One 9:e86905. doi: 10.1371/journal.pone.0086905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook RF, Berger SL, Rushlow KE, McManus JM, Cook SJ, Harrold S, Raabe ML, Montelaro RC, Issel CJ. 1995. Enhanced sensitivity to neutralizing antibodies in a variant of equine infectious anemia virus is linked to amino acid substitutions in the surface unit envelope glycoprotein. J Virol 69:1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorny MK, Conley AJ, Karwowska S, Buchbinder A, Xu JY, Emini EA, Koenig S, Zolla-Pazner S. 1992. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol 66:7538–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorny MK, VanCott TC, Hioe C, Israel ZR, Michael NL, Conley AJ, Williams C, Kessler JA II, Chigurupati P, Burda S, Zolla-Pazner S. 1997. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and interclade cross-reactivity. J Immunol 159:5114–5122. [PubMed] [Google Scholar]
- 40.Gorny MK, Xu JY, Karwowska S, Buchbinder A, Zolla-Pazner S. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol 150:635–643. [PubMed] [Google Scholar]
- 41.Nyambi PN, Gorny MK, Bastiani L, van der Groen G, Williams C, Zolla-Pazner S. 1998. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J Virol 72:9384–9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zolla-Pazner S, O'Leary J, Burda S, Gorny MK, Kim M, Mascola J, McCutchan F. 1995. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J Virol 69:3807–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. 1999. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J Virol 73:8966–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. 1997. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 90:1109–1114. [PubMed] [Google Scholar]
- 47.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. 2010. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montefiori DC. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol Chapter 12:Unit 12.11. [DOI] [PubMed] [Google Scholar]
- 49.Montefiori DC. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 50.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham BS, Belshe RB, Clements ML, Dolin R, Corey L, Wright PF, Gorse GJ, Midthun K, Keefer MC, Roberts NJ Jr, Schwartz DH, Agosti JM, Fernie BF, Stablein DM, Montefiori DC, Lambert JS, Hu SL, Esterlitz JR, Lawrence DN, Koff WC, the NIAID AIDS Vaccine Clinical Trial Network. 1992. Vaccination of vaccinia-naive adults with human immunodeficiency virus type 1 gp160 recombinant vaccinia virus in a blinded, controlled, randomized clinical trial. J Infect Dis 166:244–252. doi: 10.1093/infdis/166.2.244. [DOI] [PubMed] [Google Scholar]
- 52.Lifson JD, Feinberg MB, Reyes GR, Rabin L, Banapour B, Chakrabarti S, Moss B, Wong-Staal F, Steimer KS, Engleman EG. 1986. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature 323:725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 53.Kim YB, Han DP, Cao C, Cho MW. 2003. Immunogenicity and ability of variable loop-deleted human immunodeficiency virus type 1 envelope glycoproteins to elicit neutralizing antibodies. Virology 305:124–137. doi: 10.1006/viro.2002.1727. [DOI] [PubMed] [Google Scholar]
- 54.Qin Y, Banasik M, Kim S, Penn-Nicholson A, Habte HH, LaBranche C, Montefiori DC, Wang C, Cho MW. 2014. Eliciting neutralizing antibodies with gp120 outer domain constructs based on M-group consensus sequence. Virology 462-463:363–376. doi: 10.1016/j.virol.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin Y, Shi H, Banerjee S, Agrawal A, Banasik M, Cho MW. 2014. Detailed characterization of antibody responses against HIV-1 group M consensus gp120 in rabbits. Retrovirology 11:125. doi: 10.1186/s12977-014-0125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Arworn D, Shen X, Tomaras GD, Currier JR, Jiang M, Magaret C, Andrews C, Gottardo R, Gilbert P, Cardozo TJ, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Paris R, Greene K, Gao H, Gurunathan S, Tartaglia J, Sinangil F, Korber BT, Montefiori DC, Mascola JR, Robb ML, Haynes BF, Ngauy V, Michael NL, Kim JH, de Souza MS. 2012. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses 28:1444–1457. doi: 10.1089/aid.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evans LA, Thomson-Honnebier G, Steimer K, Paoletti E, Perkus ME, Hollander H, Levy JA. 1989. Antibody-dependent cellular cytotoxicity is directed against both the gp120 and gp41 envelope proteins of HIV. AIDS 3:273–276. doi: 10.1097/00002030-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Lynch RM, Wong P, Tran L, O'Dell S, Nason MC, Li Y, Wu X, Mascola JR. 2015. HIV-1 fitness cost associated with escape from the VRC01 class of CD4 binding site neutralizing antibodies. J Virol 89:4201–4213. doi: 10.1128/JVI.03608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X, Changela A, O'Dell S, Schmidt SD, Pancera M, Yang Y, Zhang B, Gorny MK, Phogat S, Robinson JE, Stamatatos L, Zolla-Pazner S, Kwong PD, Mascola JR. 2011. Immunotypes of a quaternary site of HIV-1 vulnerability and their recognition by antibodies. J Virol 85:4578–4585. doi: 10.1128/JVI.02585-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honnen WJ, Krachmarov C, Kayman SC, Gorny MK, Zolla-Pazner S, Pinter A. 2007. Type-specific epitopes targeted by monoclonal antibodies with exceptionally potent neutralizing activities for selected strains of human immunodeficiency virus type 1 map to a common region of the V2 domain of gp120 and differ only at single positions from the clade B consensus sequence. J Virol 81:1424–1432. doi: 10.1128/JVI.02054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunel FM, Zwick MB, Cardoso RM, Nelson JD, Wilson IA, Burton DR, Dawson PE. 2006. Structure-function analysis of the epitope for 4E10, a broadly neutralizing human immunodeficiency virus type 1 antibody. J Virol 80:1680–1687. doi: 10.1128/JVI.80.4.1680-1687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim AS, Leaman DP, Zwick MB. 2014. Antibody to gp41 MPER alters functional properties of HIV-1 Env without complete neutralization. PLoS Pathog 10:e1004271. doi: 10.1371/journal.ppat.1004271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falkowska E, Le KM, Ramos A, Doores KJ, Lee JH, Blattner C, Ramirez A, Derking R, van Gils MJ, Liang CH, McBride R, von Bredow B, Shivatare SS, Wu CY, Chan-Hui PY, Liu Y, Feizi T, Zwick MB, Koff WC, Seaman MS, Swiderek K, Moore JP, Evans D, Paulson JC, Wong CH, Ward AB, Wilson IA, Sanders RW, Poignard P, Burton DR. 2014. Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity 40:657–668. doi: 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Imamichi H, Georgiev IS, Chuang GY, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, de la Pena AT, Derking R, Klasse PJ, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD, Connors M. 2014. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chohan B, Lang D, Sagar M, Korber B, Lavreys L, Richardson B, Overbaugh J. 2005. Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J Virol 79:6528–6531. doi: 10.1128/JVI.79.10.6528-6531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Curlin ME, Zioni R, Hawes SE, Liu Y, Deng W, Gottlieb GS, Zhu T, Mullins JI. 2010. HIV-1 envelope subregion length variation during disease progression. PLoS Pathog 6:e1001228. doi: 10.1371/journal.ppat.1001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sagar M, Wu X, Lee S, Overbaugh J. 2006. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J Virol 80:9586–9598. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. 2004. HIV vaccine design and the neutralizing antibody problem. Nat Immunol 5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 72.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. 2013. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovacs JM, Nkolola JP, Peng H, Cheung A, Perry J, Miller CA, Seaman MS, Barouch DH, Chen B. 2012. HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc Natl Acad Sci U S A 109:12111–12116. doi: 10.1073/pnas.1204533109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grundner C, Li Y, Louder M, Mascola J, Yang X, Sodroski J, Wyatt R. 2005. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology 331:33–46. doi: 10.1016/j.virol.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 76.Yang X, Wyatt R, Sodroski J. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J Virol 75:1165–1171. doi: 10.1128/JVI.75.3.1165-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim M, Qiao ZS, Montefiori DC, Haynes BF, Reinherz EL, Liao HX. 2005. Comparison of HIV type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies. AIDS Res Hum Retroviruses 21:58–67. doi: 10.1089/aid.2005.21.58. [DOI] [PubMed] [Google Scholar]
- 78.Bowles EJ, Schiffner T, Rosario M, Needham GA, Ramaswamy M, McGouran J, Kessler B, LaBranche C, McMichael AJ, Montefiori D, Sattentau QJ, Hanke T, Stewart-Jones GB. 2014. Comparison of neutralizing antibody responses elicited from highly diverse polyvalent heterotrimeric HIV-1 gp140 cocktail immunogens versus a monovalent counterpart in rhesus macaques. PLoS One 9:e114709. doi: 10.1371/journal.pone.0114709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaine M, Wang S, Crooks ET, Jiang P, Montefiori DC, Binley J, Lu S. 2008. Improved induction of antibodies against key neutralizing epitopes by human immunodeficiency virus type 1 gp120 DNA prime-protein boost vaccination compared to gp120 protein-only vaccination. J Virol 82:7369–7378. doi: 10.1128/JVI.00562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaine M, Wang S, Hackett A, Arthos J, Lu S. 2010. Antibody responses elicited through homologous or heterologous prime-boost DNA and protein vaccinations differ in functional activity and avidity. Vaccine 28:2999–3007. doi: 10.1016/j.vaccine.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR III, Castro E, Akondy R, Rinfret A, Yassine-Diab B, Said EA, Chouikh Y, Cameron MJ, Clum R, Kelvin D, Somogyi R, Greller LD, Balderas RS, Wilkinson P, Pantaleo G, Tartaglia J, Haddad EK, Sekaly RP. 2008. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakaya HI, Pulendran B. 2012. Systems vaccinology: its promise and challenge for HIV vaccine development. Curr Opin HIV AIDS 7:24–31. doi: 10.1097/COH.0b013e32834dc37b. [DOI] [PMC free article] [PubMed] [Google Scholar]