Abstract
Background
Small renal arteries have a significant role in regulation of renal hemodynamics and blood pressure (BP). To study potential changes in regulation of vascular function in hypertension, we examined renal vasodilatory responses of small arteries from nonclipped kidneys of the two-kidney, one-clip (2K1C) Goldblatt hypertensive rats to native epoxyeicosatrienoic acids (EETs) which are believed to be involved in regulation of renal vascular function and BP. Two newly synthesized EET analogs were also examined.
Methods
Renal interlobular arteries isolated from the nonclipped kidneys on day 28 after clipping were preconstricted with phenylephrine (PE), pressurized, and the effects of a 14,15-EET analog, native 14,15-EET, and 11,12-ether-EET-8ZE, an analog of 11,12-EET, on the vascular diameter were determined and compared to the responses of arteries from the kidneys of sham-operated rats.
Results
In the arteries from non-clipped kidneys isolated in the maintenance phase of Goldblatt hypertension the maximal vasodilatory response to 14,15-EET analog was 30.1 ± 2.8% versus 49.8 ± 7.2% in sham-operated rats; the respective values for 11,12-ther-EET-8ZE were 31.4± 6.4% versus 80.4±6%, and for native EETs they were 41.7 ± 6.6 % versus 62.8 ± 4.4 % (P ≤ 0.05 for each difference).
Conclusions
We propose that reduced vasodilatory action and decreased intrarenal bioavailability of EETs combined with intrarenal ANG II levels that are inappropriately high for hypertensive rats underlie functional derangements of the nonclipped kidneys of 2K1C Goldblatt hypertensive rats. These derangements could play an important role in pathophysiology of sustained BP elevation observed in this animal model of human renovascular hypertension.
Keywords: renovascular hypertension, two-kidney, one-clip Goldblatt hypertension, epoxyeicosatrienoic acids, vasodilatory responses
Introduction
The two-kidney, one-clip (2K1C) Goldblatt model of hypertension is an experimental paradigm for human renovascular hypertension. Previous studies demonstrated that increased activity of the renin-angiotensin system (RAS) plays a crucial role in the development of hypertension in this model [1–4]. However, recent studies have shown that while plasma angiotensin II (ANG II) levels are elevated during the developmental phase of hypertension, later they normalize [2,5–7]. These findings indicate that enhancement of systemic RAS activity cannot be the exclusive causative factor of hypertension, especially during its maintenance phase. It has been suggested that in the stage of advanced 2K1C hypertension an interaction between RAS and other vasoactive system(s) in the nonclipped kidney must play an important pathophysiological role [8–11].
Particular attention in cardiovascular and renal research was focused on epoxyeicosatrienoic acids (EETs), the metabolites of cytochrome 450 (CYP) dependent epoxygenase pathway of arachidonic acid metabolism. It has been proposed that intrarenal EETs operate as an endogenous compensatory system with protective actions against increased RAS activity [7,10,17,19]. Recent studies have shown that during the maintenance phase of hypertension, the nonclipped kidney of 2K1C Goldblatt rats shows reduced availability of biologically active EETs as the result of increased conversion of EETs to biologically inactive dihydroxyeicosatrienoic acids (DHETs), an effect dependent on enhanced activity of soluble epoxide hydrolase (sEH) [10,11]. Based on these studies, it has been postulated that reduced intrarenal EETs bioavailability contributes to the derangement of the pressure-natriuresis relationship in the nonclipped kidney of 2K1C Goldblatt hypertension and, during the sustained phase of hypertension, plays an important pathophysiological role [7,10].
While the natriuretic action of EETs plays a major role in their antihypertensive properties, a direct vasodilatory effect of EETs on the renal circulation might be equally important, particularly in hypertension characterized by an imbalance between vasoconstrictor and vasodilatory influences [12–14]. EETs have been shown to counteract the usual renal arteriolar constriction in response to BP elevation as well as to attenuate the vasoconstrictor effects of endothelin-1 [20,21]. In addition, EETs oppose renal vasoconstrictor actions of ANG II [22–25] and this effect might also occur in the arteries of the nonclipped kidneys of 2K1C hypertensive rats.
While beneficial hemodynamic effects of EETs were further emphasized in studies showing that pharmacological blockade of sEH is associated with improvement of renal blood flow [10], the responsiveness of hypertensive small renal arteries to EETs has never been studied. Considering all the evidence available, we hypothesized that, in addition to the reduced intrarenal availability of EETs, the vasculature of the nonclipped kidney of 2K1C Goldblatt hypertensive rats exhibits impaired vasodilatory responsiveness to EETs. Therefore, in the present study, we examined vasodilatory responses displayed by small arteries from the nonclipped kidneys of 2K1C hypertensive and sham-operated rats to native EETs and to two newly developed EET analogs that are resistant to oxidation and therefore exhibit a greater stability compared to native EETs.
Materials and Methods
The studies were performed in accordance with the guidelines and practices established by the Animal Care and Use Committees of the Institute for Clinical and Experimental Medicine and are in accordance with appropriate regulations in the Czech Republic and accord with the American Physiological Society Code of Practice for the Care and Use of Animals for Scientific Purposes. Hannover Sprague-Dawley (HanSD) rats were bred at the Center for Experimental Medicine.
Preparation of 2K1C Goldblatt Hypertensive Rats
2K1C Goldblatt hypertension was induced as described previously [5,7]. Briefly, male HanSD rats (initial body weight 100 – 120 g) were anesthetized with a combination of tiletamine, zolazepam (Zoletil, 8 mg/kg), and xylasine (4 mg/kg, Rometar, Spofa, Czech Republic) which were administrated intramuscularly. The right renal artery was isolated through a flank incision and a clip (0.25 mm internal diameter) was placed on the renal artery. Sham-operated rats underwent the same surgical procedure except placement of the arterial clip. Throughout the experiment, the animals were housed under conditions of constant temperature and humidity, maintained on a 12:12-h light-dark cycle, and fed standard chow, with tap water ad libitum. Arteries were isolated from the nonclipped kidney of 2K1C rats and from the left kidney of sham-operated rats. Only one well-reacting artery from one animal was used in each experiment. The average systolic blood pressure of our 2K1C rats was 163 ± 3 mmHg and of sham-operated rats 113 ± 2 mm Hg.
In vitro effects of 14,15-EET analog [disodium (S)-2-(13-(3-pentyl)ureido)-tridec-8(Z)-enamido)succinate] (EET-A); native 14,15-EET and 11,12-EET, and 11-nonyloxy-undec-8(Z)-enoic acid analog (11,12-ether-EET-8ZE) on vascular diameter of small renal arteries.
On or soon after day 28 post-surgery, when hypertension was established, animals were killed by an overdose of thiopental sodium. The non-clipped kidney was flushed, removed and placed in ice-cold physiological solution. Subsequently, the vessels were prepared as described in detail previously [26]. Briefly, the kidney was cut longitudinally into two to three sections from which interlobular arteries were dissected, isolated and cleared of adhering tubules and connective tissue. The diameter of the vessels was approximately 250 μm. After dissection, the arteries were cannulated with glass micropipettes in a pressure myograph chamber (Danish Myograph Company). Slowly, the intravascular pressure was raised at 10 mm Hg/min till 80 mm Hg while the vessels were perfused with oxygenated Krebs solution of the following composition (in mM): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3 and 11 glucose, at 37°C at a flow rate of about 2 ml.min−1. Subsequently, the mounted arteries were slowly pressurized to reach the intravascular pressure of 80 mmHg and equilibrated. The output of a black-and-white video camera attached to the microscope was fed to a frame grabber card mounted in a personal computer. Video images were analyzed using MioVIEW data acquisition software that continuously acquires the diameter measurements of the blood vessel. Drugs were added externally via superfusing Krebs solution. The effects of 14,15-EET analog, native 14,15-EET, and 11,12-ether-EET-8ZE, an analog of 11,12-EET, on the vascular diameter were determined after the vessels had been preconstricted by submaximal concentration of phenylephrine (PE) (0.3–1.0 μM). In preliminary studies we found that the contraction produced by phenylephrine was more stable and reproducible compared to constriction produced by ANG II.
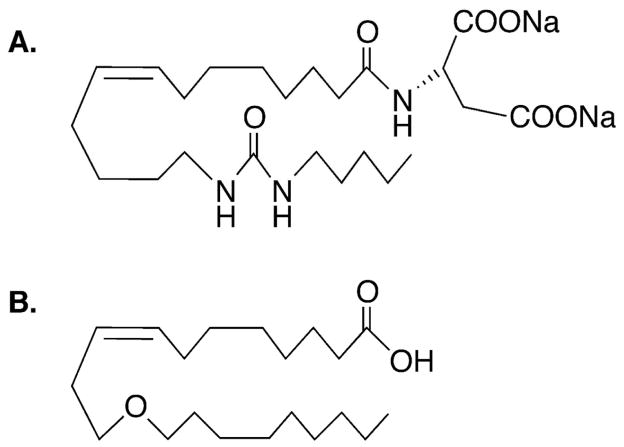
The chemical structure of 14,15-EET analog and 11,12-EET analog are depicted in Figure 1. These analogs are resistant to oxidation and degradation by sEH to DHETE [27,28]. 14,15-EET was purchased from Cayman Pharma (Neratovice, Czech Republic). EET-A and 11,12-ether-EET-8ZE were synthetized by Professor Falck’s group [27,28].
Figure 1.
(A) Chemical structure of sodium (S)-2-(Z)-(13-(3-pentyl)ureido)-tridec-8(Z)-enamido)succinate, an agonistic orally-active analog of 14,15-epoxyeicosatrienoic acid. (B) Chemical structure of 11-nonyloxy-undec-8(Z)-enoic acid (11,12-ether-EET-8ZE), an analog of 11,12-epoxyeicosatrienoic acid.
Artery vasodilator responses are expressed as percent dilation of PE-preconstricted vessels. Half-maximal effective agonist concentration (EC50) and maximal dilatory response (Emax) were calculated from least squares fit of the individual agonist concentration-response curves using the following logistic function from Origin 8.5:
where Emin is the minimum response and was constrained to zero and n is the slope factor.
Statistical Analysis
All values are expressed as means ± SEM. Graph-Pad Prism software (Graph Pad Software, San Diego, CA, USA) was used for statistical calculations. The differences between groups for Emax and EC50 were assessed by Student’s two-tailed unpaired t-test. Values exceeding the 95% probability limits (P<0.05) were considered statistically significant.
Results
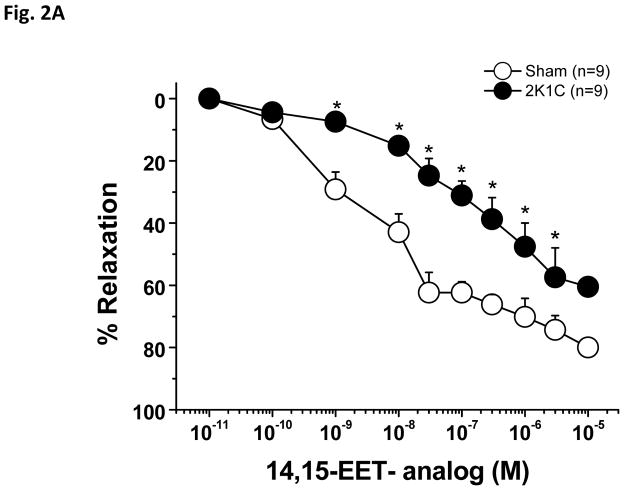
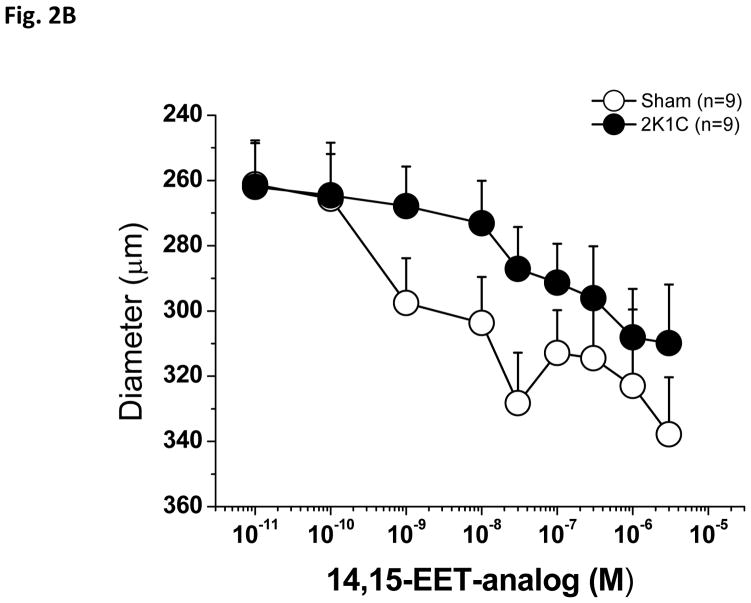
Phenylephrine produced a stable constriction of interlobular renal arteries. As tested in the first experimental group, application of 1.0 μM Phenylephrine produced 28.5±5% constriction in sham arteries and 29 ± 5 % constriction in 2K1C arteries. As shown in Figure 2A, PE-preconstricted renal interlobular arteries from sham-operated and from 2K1C rats responded to application of the 14,15-EET analog with dose-dependent vasodilatation, however, in 2K1C rats, the dose-response curve to 14,15-EET was slightly shifted to the right. Moreover, Emax was significantly reduced in the arteries of the 2K1C rats as compared to sham-operated rats (Table 1). The absolute vessel diameter changes are shown in Figure 2B.
Figure 2.
Vasodilator effects of 14,15-epoxyeicosatrienoic acid analog (14,15-EET analog) (A) in phenylephrine-preconstricted renal interlobular arteries isolated from nonclipped kidneys of two-kidney, one-clip (2K1C) rats or from kidneys of sham-operated rats. *P<0.05 versus sham-operated rats at the same concentration. Figure B depicts the absolute changes of the diameter.
Table 1.
Maximal dilatory responses (Emax) and half maximal effective concentration (EC50) to produce vascular dilation of phenylephrine-preconstricted renal interlobular arteries for 14,15-EET and the two analogs.
| 14,15-EETs | Shams, n=7 | 2K1C, n=6 |
|---|---|---|
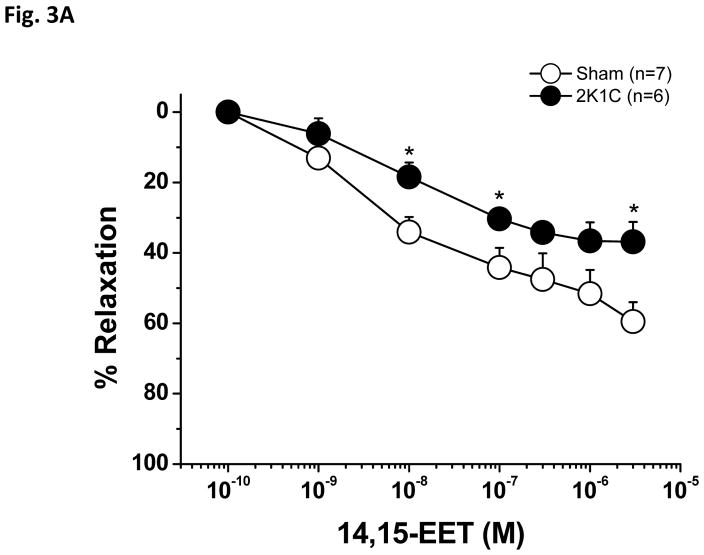
| Emax (%) | 62.8 ± 4.4 | 41.7 ± 6.6* |
| EC50 (nM) | 16.8 ± 10 | 16.9 ± 9 |
| 14,15-EET analog | Shams n=9 | 2K1C n=9 |
| Emax | 49.8 ± 7.2 | 30.1 ± 2.8* |
| EC50 (nM) | 18 ± 10 | 55 ± 17 |
| 11,12-ether-EET-8ZE | Shams n=6 | 2K1C n=7 |
| Emax | 80.8 ± 6.5 | 31.4 ± 6.4 * |
| EC50 (μM) | 0.26 ± 0.22 | 0.25 ± 0.24 |
14,15-EET analog: sodium (S)-2-(Z)-(13-(3-pentyl)ureido)-tridec-8(Z)-enamido)succinate, an agonistic orally-active analog of 14,15-epoxyeicosatrienoic acid, 11,12-ether-EET-8ZE: 11-nonyloxy-undec-8(Z)-enoic acid, an analog of 11,12-epoxyeicosatrienoic acid, Emax – maximal response, EC50 – concentration of drug which produces 50% response
P<0.05 versus sham-operated rats.
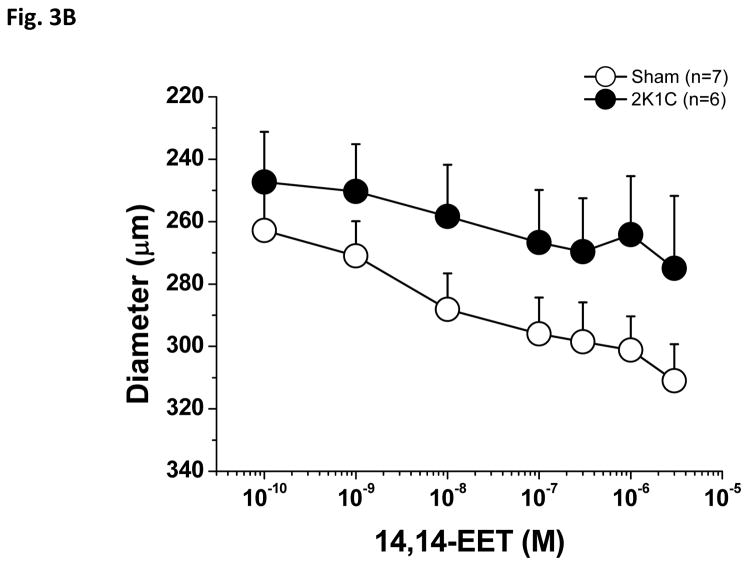
As shown in Figure 3A, PE-preconstricted renal interlobular arteries from 2K1C rats exhibited a significantly smaller vasodilatory response to native endogenous 14,15-EET as compared with those of sham-operated rats; Emax was significantly lower in the arteries of the 2K1C rats as compared to sham-operated rats (Table 1). In this experimental group, application of 1.0 μM Phenylephrine induced 21.5±7% constriction in the arteries of sham-operated rats and 22±7.2% constriction in 2K1C arteries. Figure 3B shows the absolute diameter changes.
Figure 3.
Vasodilator effects of 14,15-epoxyeicosatrienoic acid (14,15-EET) in phenylephrine-preconstricted renal interlobular arteries isolated from nonclipped kidneys of two-kidney, one-clip (2K1C) rats or from kidneys of sham-operated rats (A). *P<0.05 versus sham-operated rats at the same concentration. Figure B depicts the absolute changes of the diameter.
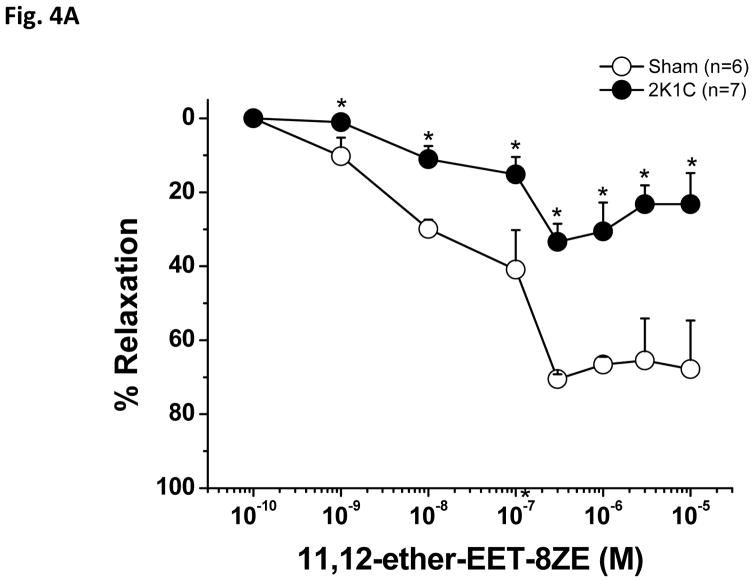
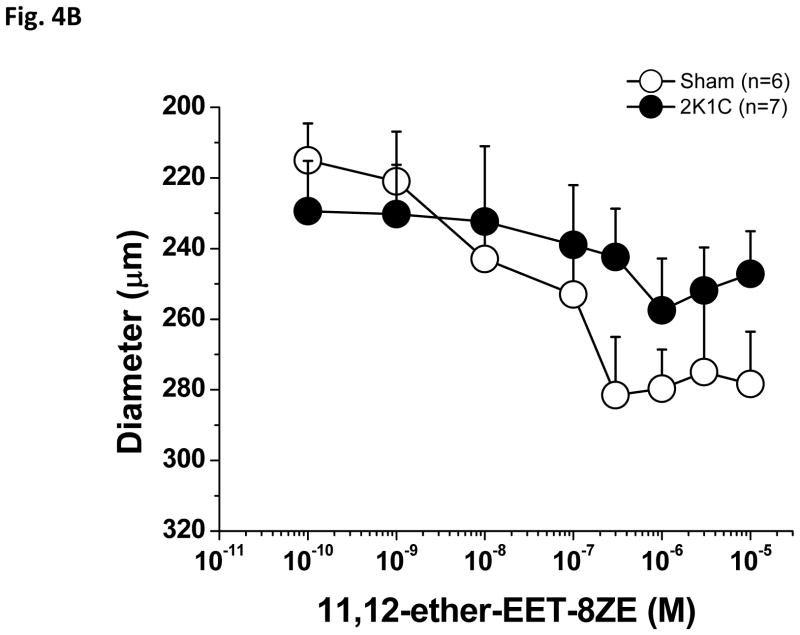
As shown in Figure 4A, PE-preconstricted renal interlobular arteries from 2K1C rats showed substantially reduced vasodilatory responses to 11,12-ether-EET-8ZE, the 11,12-EET analog compared to arteries of sham-operated rats, and, again, Emax was substantially lower in 2K1C rats as compared to sham-operated rats (Table 1). In this experimental group, application of 1.0 μM Phenylephrine induced 33 ± 5% constriction in sham-operated rat arteries and 27±7 % constriction in 2K1C arteries. Figure 4B shows the absolute diameter changes.
Figure 4.
Vasodilator effects of 11,12-epoxyeicosatrienoic acid analog (11,12-ether-EET-8ZE) in phenylephrine-preconstricted renal interlobular arteries isolated from nonclipped kidneys of two-kidney, one-clip (2K1C) rats or from kidneys of sham-operated rats (A). *P<0.05 versus sham-operated rats at the same concentration. The figure 4B depicts the absolute changes of the diameter.
Discussion
The present study demonstrates that renal interlobular arteries isolated from the nonclipped kidneys of 2K1C Goldblatt hypertensive rats during the maintenance phase of hypertension exhibit markedly reduced vasodilator responses to both EET analogs and native EETs compared to arteries from kidneys of sham-operated control rats.
While the dose-response curve to the 14,15-EET analog was only slightly shifted to the right in the arteries of 2K1C hypertensive rats, the maximal dilatory response was in these arteries significantly reduced. Vasodilatory responses to native 14,15-EET were also reduced. Of special interest is also the observation that renal vasodilatation induced by the 14,15-EET analog was significantly greater compared to native 14,15-EET, both in arteries isolated from kidneys of sham-operated and 2K1C rats. This observation strengthens the notion that the appropriate structural modifications of EETs can result in generation of EET analogs that, being more stable since they are not a substrate for sEH enzyme, exhibit more pronounced vascular actions compared to native EETs. Such observations enhance the analogs’ potential therapeutic value [27,28].
Similarly to 14,15-EET, in the arteries isolated from the nonclipped kidneys of 2K1C rats we found attenuated vasodilator responses to the 11,12-EET analog. On the whole, these findings indicate that during the maintenance phase of hypertension renal arteries of the nonclipped kidneys of 2K1C rats are characterized by reduced vasodilator responsiveness to EETs.
While our data indicate that the vessels from 2K1C hypertensive rats exhibit reduced responsiveness to EETs, previous studies have also demonstrated impairment of the vasodilatory function of 2K1C arteries. Dilation to an NO donor or acetylcholine as well as β-adrenoreceptor-mediated vasorelaxation were found to be reduced in 2K1C hypertensive arteries [29,30]. this indicates that some general dysregulation of the mechanisms contributing to the attenuated hyperpolarization of VSMC might occur in 2K1C hypertensive vessels, resulting in decreased vasorelaxation in response to various dilatory agents.
Since during the maintenance phase of 2K1C hypertension the rats exhibit decreased intrarenal EET bioavailability in the nonclipped kidneys [7,10,11], it is likely that both decreased intrarenal EET and reduced vasodilatory responsiveness to EET contribute to the derangement of the pressure-natriuresis relationship in the nonclipped kidney of 2K1C Goldblatt hypertension; such combined effect could play an important pathophysiological role during the sustained phase of hypertension [7,10]. This hypothesis offers a plausible explanation to the major mystery of 2K1C Goldblatt hypertension, i.e., the reason why the presumably normal nonclipped kidney does not, as postulated by Guyton, prevent development of hypertension in its sustained phase [1,2].
What are the mechanism(s) responsible for the reduced vasodilator responsiveness of the arteries isolated from the nonclipped kidneys of 2K1C rats to EETs?
It is well documented that EETs, in particular 11,12-EET and 14,15-EET isoforms, induce a direct renal arterial dilation through activation of large-conductance calcium-activated K+ channels (BKca) and hyperpolarization of vascular smooth muscle cells (VSMC) [12–14]. Thus, by regulating the activity of BKCa, EETs can directly control the membrane potential of VSMC and the vascular tone [12–14,17,19]. Therefore, one can hypothesize that a functional impairment of BKCa in the nonclipped kidneys of 2K1C rats is responsible for the reduction of vasodilatory responses to EETs. Indeed, it was reported that deficient activity of BKCa in the aorta of 2K1C rats contributed to the impaired acetylcholine-induced and nitric oxide (NO)-mediated vasorelaxation [26,27]. Changes in the expression of BK channel subunits have been suggested to play a significant role in alteration of vascular responses in a number of physiological and pathological conditions. Exploration of the involvement of BK channels in vasodilation and possible alterations of their function in hypertension would be a fascinating subject of a mechanistic study that is beyond the scope of our present work.
Another established mechanism by which EETs produce vasodilation is by activating small and intermediate KCa channels in endothelial cells through transient receptor potential canonical channels types 3 and 6 (TRPC3 and TRPC6) which promote VSMC hyperpolarization [15,16]. Changes in the function of these receptor channels in hypertension might also contribute to diminished vasorelaxation to EETs. These receptor channels would also be an interesting target to look at in a mechanistic study.
It is evident that the mechanisms whereby EETs elicit vasodilatation are complex and an impairment of one or more of these mechanisms might be responsible for the reduced vasodilator responses in the nonclipped kidneys of 2K1C rats. In this regard, it is important to recognize that also the metabolites of CYP-dependent ω-hydroxylase pathway of arachidonic acid, such as 20-hydroxyeicostetraenoic acid (20-HETE), play an important role in the regulation of renal and systemic vascular tone, sodium excretion and pathophysiology of hypertension [17,26, 31]. In our previous study, we found that the nonclipped kidney of 2K1C rats in the phase of sustained hypertension simultaneously exhibits decreased intrarenal EETs and increased 20-HETE levels as compared with the kidney of sham-operated animals [7]. Since it is well known that 20-HETE itself is a potent vasoconstrictor and also potentiates the vasoconstrictor response to ANG II [32,33], it is likely that complex alterations in CYP-derived metabolites of arachidonic acid in the nonclipped kidney contribute to the pathophysiology of Goldblatt hypertension during its sustained phase.
Conclusions
In summary, we showed that during the maintenance phase of hypertension small arteries of the nonclipped kidneys of 2K1C Goldblatt hypertensive rats exhibit vasodilator responses to EETs that are distinctly reduced compared to arteries from sham-operated normotensive rats. This fact may have pronounced physiological implications in hypertension that is characterized by an increased action of vasoactive hormones and enhanced microvascular reactivity to multiple vasoconstrictors. The present study shows that the increased vascular tone is not being compensated by the action of vasodilatory EETs. The attenuated EET–mediated vascular dilation could be partly responsible for reduced renal blood flow found in renovascular hypertension. Collectively, reduced vasodilatory action of EETs combined with decreased intrarenal EETs bioavailability and with intrarenal ANG II levels that are inappropriately high for hypertensive rats could importantly contribute to functional derangements of the nonclipped kidneys of 2K1C Goldblatt hypertensive rats. Such derangements might play an important role in the pathophysiology of sustained hypertension in this model of human renovascular hypertension.
Supplementary Material
Acknowledgments
Sources of support: This study was principally supported by grant No. NT/14011-3 awarded by the Internal Grant Agency of the Ministry of Health to L.K. Institute for Clinical and Experimental Medicine (IKEM) is recipient of the project of the Ministry of Health of the Czech Republic for the development of research organization 00023001 (institutional support). The Center for Experimental Medicine (IKEM) received financial support from the European Commission within the Operational Program Prague–Competitiveness; project “Rozvoj infrastruktury PEM” (#CZ.2.16/3.1.00/28025). J.D.I. was supported by NIH grant DK38226. J.R.F was supported by the Robert A. Welch Foundation (I-0011) and the USPHS NIH (DK38226, HL111392). A.S. was also supported by a Marie Curie Fellowship from the European Commission Program PEOPLE (IRG 247847).
Abbreviations
- 20-HETE
20-hydroxyeicosatetraenoic acid
- 2K1C
2-kidney, 1-clip Goldblatt hypertensive rat
- ANG II
angiotensin II
- BKCa
large conductance potassium channel
- BP
Blood pressure
- DHETEs
Dihydroxyeicosatrienoic acids
- EET
Epoxyeicosatrienoic acid
- ESRD
End-stage renal disease
- NO
nitric oxide
- PE
Phenylephrine
- RAS
Renin-angiotensin system
- VSMC
Vascular smooth muscle
- TRPC 3, TRCP 6
Transient receptor potential canonical channel 3 and 6
Footnotes
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Ploth DW. Angiotensin-dependent renal mechanism in two-kidney, one-clip renal vascular hypertension. Am J Physiol. 1983;245:F131–F141. doi: 10.1152/ajprenal.1983.245.2.F131. [DOI] [PubMed] [Google Scholar]
- 2.Navar LG, Zou L, Von Thun A, Wang CT, Imig JD, Mitchell KD. Unraveling the mystery of Goldblatt hypertension. News Physiol Sci. 1998;13:170–176. doi: 10.1152/physiologyonline.1998.13.4.170. [DOI] [PubMed] [Google Scholar]
- 3.Guan S, Fox K, Mitchell, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one-clip hypertensive rats. Hypertension. 1992;20:763–767. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- 4.El-Dahr SS, Dipp S, Guan S, Navar LG. Renin, angiotensinogen, and kallikrein gene expression in two-kidney Goldblatt hypertensive rats. Am J Hypertens. 1993;6:914–919. doi: 10.1093/ajh/6.11.914. [DOI] [PubMed] [Google Scholar]
- 5.Cervenka L, Wang CT, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension. 1999;33:102–107. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- 6.Červenka L, Vaněčková I, Husková Z, Vaňourková Z, Erbanová M, Thumová M, Škaroupková P, Opočenský M, Malý J, Čertíková Chábová V, Tesař V, Bürgelová M, Viklický O, Teplan V, Želízko M, Kramer HJ, Navar LG. Pivotal role of AT1A receptors in the development of two-kidney, one-clip hypertension: study in AT1A receptor knockout mice. J Hypertens. 2008;26:1379–1389. doi: 10.1097/HJH.0b013e3282fe6eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walkowska A, Škaroupková P, Husková Z, Vaňourková Z, Čertíková Chábová V, Tesař V, Kramer HJ, Falck JR, Imig JD, Kompanovska-Jezierska E, Sadowski J, Červenka L. Intrarenal cytochrome P-450 metabolites of arachidonic acid in the regulation of the nonclipped kidney function in two-kidney, one-clip Goldblatt hypertensive rats. J Hypertens. 2010;28:582–593. doi: 10.1097/HJH.0b013e328334dfd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell KD, Botros FT, Navar LG. Intrarenal renin-angiotensin system and counteracting protective mechanisms in angiotensin II-dependent hypertension. Acta Physiologica Hungarica. 2007;94:31–48. doi: 10.1556/APhysiol.94.2007.1-2.5. [DOI] [PubMed] [Google Scholar]
- 9.Rakušan D, Bürgelová M, Vaněčková I, Vaňourková Z, Husková Z, Škaroupková P, Mrázová I, Opočenský M, Kramer HJ, Netuka I, Malý J, Alenina N, Bader M, Santos RAS, Červenka L. Knockout of angiotensin 1-7 receptor mas worsens the course of two-kidney, one-clip Goldblatt hypertension: roles of nitric oxide deficiency and enhanced vascular responsiveness to angiotensin II. Kidney and Blood Press Res. 2010;33:476–488. doi: 10.1159/000320689. [DOI] [PubMed] [Google Scholar]
- 10.Sporkova A, Kopkan L, Varcabová A, Husková Z, Hwang SH, Hammock BD, Imig JD, Kramer HJ, Červenka L. Role of cytochrome P450 metabolites in the regulation of renal function and blood pressure in 2-kidney, 1-clip hypertensive rats. Am J Physiol. 2011;300:R1468–R1475. doi: 10.1152/ajpregu.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopkan L, Husková Z, Sporková A, Varcabová Š, Honetschlägerová Z, Hwang SH, Tsai HJ, Hammock BD, Imig JD, Kramer HJ, Bürgelová M, Vojtíšková A, Kujal P, Vernerová Z, Červenka L. Soluble epoxide hydrolase inhibition exhibits antihypertensive actions independently on nitric oxide in mice with renovascular hypertension. Kidney and Blood Press Res. 2012;35:595–607. doi: 10.1159/000339883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer SL, Gragasin FS, Xichen W, Wang S, McMurtry, Kim DH, Platonov M, Koshal A, Hashimoto Kyoko, BSc, William B, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human mammary artery is 11,12-Epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKca channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 13.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through guanine nucleotide binding protein. Circ Res. 1998;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 14.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman JR. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol. 1996;270:F822–832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 15.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau Ch, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 16.Madhun ZT, Goldthwait DA, McKay D, Hopfer U, Douglas JG. An epoxygenase metabolite of arachidonic acid mediates angiotensin II-induced rises in cytosolic calcium in rabbit proximal tubule epithelial cells. J Clin Invest. 1991;88:456–461. doi: 10.1172/JCI115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan F, Muoya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens. 2015;24:37–46. doi: 10.1097/MNH.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakairi Y, Jacobson HR, Noland DT, Capdevila JH, Falck JR, Breyer MD. 5,6-EET inhibits ion transport in collecting duct by stimulating endogenous prostaglandin synthesis. Am J Physiol. 1995;268:F931–F939. doi: 10.1152/ajprenal.1995.268.5.F931. [DOI] [PubMed] [Google Scholar]
- 19.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imig JD, Pham BT, LeBlanc EA, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension. 2000;35:307–312. doi: 10.1161/01.hyp.35.1.307. [DOI] [PubMed] [Google Scholar]
- 21.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br J Pharmacol. 1999;127:1399–1405. doi: 10.1038/sj.bjp.0702662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmarakby AA. Reno-protective mechanisms of epoxyeicosatrienoic acids in cardiovascular disease. Am J Physiol. 2012;302:R321–R330. doi: 10.1152/ajpregu.00606.2011. [DOI] [PubMed] [Google Scholar]
- 23.Imig JD, Deichmann PC. Afferent arteriolar responses to ANG II involve activation of PLA2 and modulation by lipoxygenase and P-450 pathways. Am J Physiol. 1997;273:F274–F282. doi: 10.1152/ajprenal.1997.273.2.F274. [DOI] [PubMed] [Google Scholar]
- 24.Imig JD, Zhao X, Falck JR, Wei S, Capdevila JH. Enhanced renal microvascular reactivity to angiotensin II in hypertension is ameliorated by the sulfonimide analog of 11,12-epoxyeicosatrienoic acid. J Hypertens. 2001;19:983–992. doi: 10.1097/00004872-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Kohagure K, Endo Y, Ito O, Arima S, Omata K, Ito S. Endogenous nitric oxide and epoxyeicosatrienoic acids modulate angiotensin II-induced constriction in the rabbit a | channel activation. Clin Exp Pharmacol Physiol. 2005;32:478–481. doi: 10.1046/j.1365-201X.2000.00638.x. [DOI] [PubMed] [Google Scholar]
- 26.Amberg GC, Santana LF. Downregulation of BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 27.Burnham MP, Johnson IT, Weston AH. Reduced Ca2+-dependent activation of large-conductance Ca2+-activated K channels from arteries of type 2 diabetic Zucker diabetic fatty rats. Am J Physiol. 2006;290:H1520–H1527. doi: 10.1152/ajpheart.00827.2005. [DOI] [PubMed] [Google Scholar]
- 28.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Phys Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 29.Fleming I. The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacol Rev. 2014;66:1106–1140. doi: 10.1124/pr.113.007781. [DOI] [PubMed] [Google Scholar]
- 30.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 31.Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56:336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 33.Joly E, Seqqat R, Flamion B, Caron N, Michal A, Imig JD, Kramp R. Increased renal vascular reactivity to angiotensin II after unilateral nephrectomy in the rat involves 20-HETE. Am J Physiol. 2006;291:R977–R986. doi: 10.1152/ajpregu.00401.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.