Abstract
Introduction
National Comprehensive Cancer Network (NCCN) guidelines recommend wide excision without axillary staging to treat phyllodes tumors of the breast. Without prospective trials to guide management, NCCN also recommends consideration of radiation therapy (XRT). We describe current patterns of care for the multidisciplinary management of phyllodes tumors.
Methods
Using Surveillance, Epidemiology and End Results Program (SEER) data, we identified women diagnosed with phyllodes tumors between 2000 and 2012 who underwent surgical therapy. Trends in breast conserving surgery (BCS), nodal sampling and XRT were assessed using the Cochrane-Armitage test. Multivariable logistic regression was used to identify factors associated with treatment.
Results
Of 1,238 patients, 56.9% underwent BCS and 23.6% underwent nodal sampling (10.5% after BCS vs 40.9% after mastectomy). After surgery, 15.4% received adjuvant XRT (BCS, 12.9% and mastectomy, 18.8%). XRT utilization increased significantly over the study period (BCS, p=<0.0001; mastectomy, p=0.0003) while nodal sampling did not change significantly. Women were more likely to receive mastectomy if they were older or had larger tumors. Nodal sampling was also associated with older age, larger tumor size and receipt of mastectomy. Receipt of XRT was associated with later year of diagnosis, larger tumors and nodal assessment.
Conclusion
Over time, an increasing number of women received XRT after surgical management of phyllodes tumor, and one in four women underwent nodal sampling. While some of this practice can be attributed to concern about more advanced disease in the absence of strong data, there may be an educational gap regarding current guidelines and appropriate management.
INTRODUCTION
Phyllodes tumors of the breast are rare fibroepithelial lesions that comprise less than 1% of all breast malignancies.1 An analysis of the Surveillance, Epidemiology, and End Results Program (SEER) data registry from 2000 to 2004 reported that 500 women are diagnosed with malignant phyllodes tumors in the United States annually.2 Phyllodes tumors usually present as mobile painless breast masses, however, approximately 20% of tumors are identified on screening mammography and are non-palpable.3 They are characterized by hypercellular stroma growth into epithelial lined cyst spaces and the epithelial element is responsible for the distinction from stromal sarcomas.4,5 Although phyllodes tumors are similar to fibroadenomas, suspicion for a phyllodes tumor is based on large size, a rapid growth rate, and findings of stromal hyperplasia and atypia on microscopic examination.3 Based on the degree of stromal hyperplasia and atypia, the World Health Organization (WHO) categorizes phyllodes tumors as benign, borderline and malignant with malignant tumors accounting for 25% of resected tumors.1,5,6
National Comprehensive Cancer Network (NCCN) guidelines for the management of phyllodes tumors recommend wide excision with margins ≥1cm and recommend against axillary staging.7 Without randomized studies supporting the use of post-operative radiation (XRT), routine use of XRT is not recommended, except in specific circumstances where a local recurrence would result in significant morbidity. In this setting, guidelines recommend XRT, following a sarcoma treatment paradigm.7 The objective of our study is to examine current patterns of care for the treatment of phyllodes tumors relative to current guideline recommendations and to assess temporal trends in management.
METHODS
Data Source and Study Subjects
The SEER program is comprised of 18 population-based cancer registries around the United States. Records from SEER registries are publicly available and managed by the National Cancer Institute.8 It currently represents approximately 30% of the US population and is comparable to the general population with regards to poverty and education.8 We utilized the SEER-18 submission spanning the following geographic regions: Metropolitan Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Los Angeles, San Jose-Monterey, Utah, rural and greater Georgia, Alaska, greater California, Kentucky, Louisiana, and New Jersey. SEER routinely collects data on demographics (age at diagnosis, gender, race) tumor-specific information (primary site, stage, size, grade) and first course local-regional therapy defined as treatment plan at diagnosis, conducted prior to disease progression or recurrence.
We identified patients with a histologic diagnosis of malignant phyllodes tumors of the breast (International Classification of Diseases for Oncology, 3rd edition [ICD-O-3] histology codes 9020/3) diagnosed between 2000 and 2012. We limited our cohort to patients over the age of 18 who underwent breast surgery. We excluded patients with a prior history of cancer (n=266), who were diagnosed only at autopsy or on a death-certificate (n=89) and women with unknown XRT status (n=23). Patient factors examined include age, race, year of diagnosis, and tumor size. Treatment factors include type of primary surgery (BCS vs mastectomy), nodal examination, and receipt of XRT.
Statistical Analysis
Patient and treatment characteristics were described stratified by type of breast surgery using summary statistics and p-values for comparing categorical data were computed using chi-square. We assessed changes in annual rates of BCS, lymph node evaluation and adjuvant XRT over time using the Cochran-Armitage test for trend. We utilized separate multivariable logistic regression models to identify factors independently associated with the receipt of BCS, nodal sampling and XRT. P-values were 2 sided and p<0.05 was used as threshold for statistical significance. Analyses were conducted using SEER*Stat 8.1.5 statistical software (National Cancer Institute, Bethesda, MD, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patient Characteristics
We identified 1,238 patients who were diagnosed with phyllodes tumor between 2000 and 2012 and underwent surgical therapy (Table 1). The mean age at diagnosis was 50.2 years and the median tumor size was 4.8cm (mean tumor size 6cm). In our cohort, 56.9% (n=705) underwent BCS while 43.1% (n=533) underwent mastectomy. Tumor size was greater in women undergoing mastectomy versus BCS (66.6% vs 28.7% with size ≥5cm). Nodal sampling was performed in 23.6% (n=292) of patients and was more common for patients receiving mastectomy compared with BCS (40.9%, n=218 vs 10.5%, n=74). Of the patients who had their nodes examined, 4.0% (n=12) had positive nodes. XRT was administered to 15.4% (n=191) of the cohort overall and was more common after mastectomy (18.8%, n=100) compared to BCS (12.9%, n=91) (Table 1).
Table 1.
Characteristics of women diagnosed with phyllodes tumors of the breast from 2000 to 2012 who underwent surgical therapy
| Characteristic | Total (n = 1,238) | BCS (n = 705) | Mastectomy (n = 533) | P value |
|---|---|---|---|---|
| Age (years) | 0.012 | |||
| <40 | 254 (20.5) | 166 (23.6) | 88 (16.5) | |
| 40–49 | 339 (27.4) | 193 (27.4) | 146 (27.4) | |
| 50–59 | 376 (30.4) | 207 (29.4) | 169 (31.7) | |
| 60 and older | 269 (21.7) | 139 (19.6) | 130 (24.4) | |
| Race | 0.746 | |||
| White | 907 (73.3) | 519 (73.6) | 388 (72.8) | |
| Other/unknown | 331 (26.7) | 186(26.4) | 145 (27.2) | |
| Year of diagnosis | 0.581 | |||
| 2000–2004 | 476 (38.5) | 270 (38.3) | 206 (38.7) | |
| 2005–2008 | 366 (29.6) | 216 (30.6) | 150 (28.1) | |
| 2009–2012 | 396 (31.9) | 219 (31.1) | 177 (33.2) | |
| Tumor size (cm) | <.0001 | |||
| <5 | 593 (47.9) | 443 (62.8) | 150 (28.1) | |
| ≥5 | 557 (45.0) | 202 (28.7) | 355 (66.6) | |
| Unknown | 88 (7.1) | 60 (8.5) | 28 (5.3) | |
| Nodal Status | ||||
| Negative | 280 (22.6) | 73 (10.4) | 207 (38.8) | <.0001 |
| Positive | 12 (1.0) | 1 (0.1) | 11 (2.1) | |
| Not examined | 946 (76.4) | 631 (89.5) | 315 (59.1) | |
| Radiotherapy | ||||
| No | 1047 (84.6) | 614 (87.1) | 433 (81.2) | <.0001 |
| Yes | 191 (15.4) | 91 (12.9) | 100 (18.8) | |
Data are expressed as n(%)
Abbreviations: BCS Breast conserving surgery
Temporal Trends
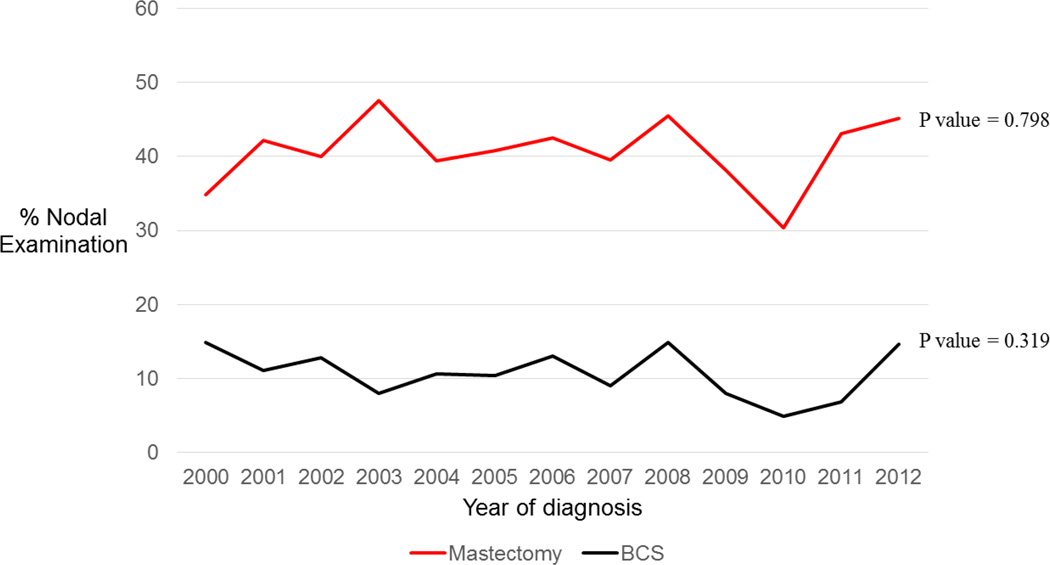
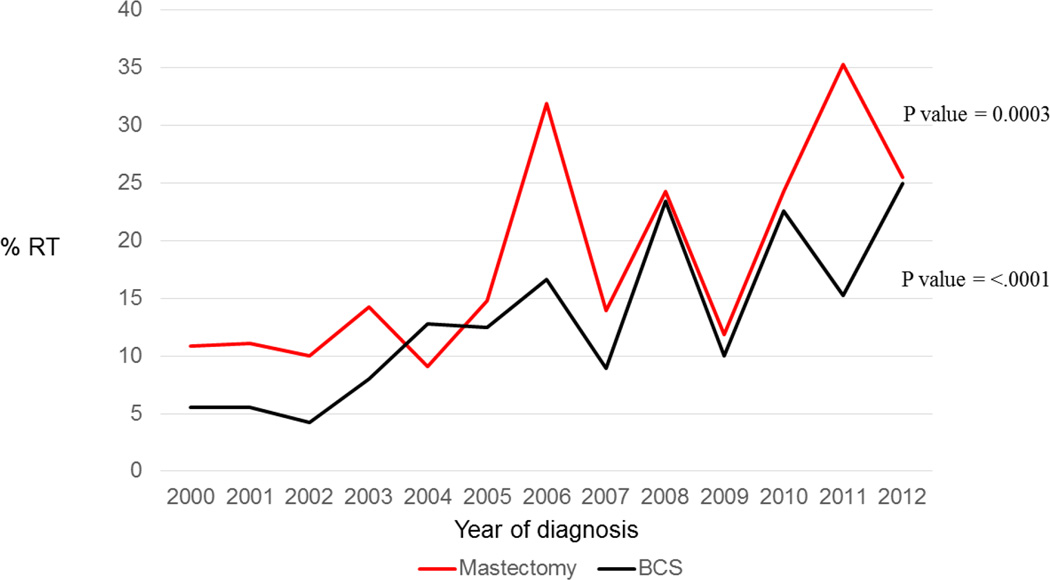
There has been no change in the proportion of women who underwent BCS versus mastectomy (54.0% underwent BCS in 2000 and 48.0% in 2012, p= 0.58). Rates of nodal examination likewise did not change significantly during this time period, regardless of the surgery received (BCS, p=0.32; Mastectomy, p=0.80) (Figure 1). XRT utilization increased significantly after both BCS and mastectomy from 2000 to 2012, with a rate increase from 5.6% to 25.0% after BCS (p<0.0001) and 10.9% to 25.5% after mastectomy (p=0.0003) (Figure 1).
Figure 1.
Rate of nodal examination stratified by surgery type in patients diagnosed with phyllodes tumors from 2000 to 2012
Abbreviations: BCS Breast conserving surgery
Multivariate Logistic Regression
In adjusted analysis, women were more likely to undergo mastectomy if they were older or had larger tumors (Table 2). Patients were more likely to receive nodal evaluation if they were older, had a larger tumor size, or underwent mastectomy. Receipt of mastectomy was the strongest predictor of nodal evaluation (OR 5.12; 95% CI 3.73–7.02). Receipt of post-BCS XRT was significantly associated with later years of diagnosis, larger tumor size, and nodal examination (Table 3). Similar associations were seen for receipt of post-mastectomy XRT.
Table 2.
Adjusted odds ratio for receipt of breast conserving surgery in women diagnosed with phyllodes tumor who underwent surgical therapy, 2000 – 2012 (n = 1,238)
| Characteristic | Adjusted OR (95% CI) |
|---|---|
| Age (years) | |
| <40 | 1 |
| 40–49 | 0.66 (0.46 – 0.94)‡ |
| 50–59 | 0.59 (0.41 – 0.84)‡ |
| 60 and older | 0.50 (0.34 – 0.73)‡ |
| Race | |
| White | 1 |
| Other/unknown | 1.08 (0.82 – 1.43) |
| Year of diagnosis | |
| 2000–2004 | 1 |
| 2005–2008 | 1.24 (0.92 – 1.67) |
| 2009–2012 | 1.11 (0.83 – 1.49) |
| Tumor size (cm) | |
| <5 | 1 |
| ≥5 | 0.18 (0.14 – 0.24)‡ |
| Unknown | 0.69 (0.42 – 1.13) |
Abbreviations: BCS Breast conserving surgery, OR odds ratio, CI confidence interval
Statistically significant (P<0.05) after adjusting for all other variables in the table
Table 3.
Adjusted odds ratio for receipt of radiotherapy (RT) in women diagnosed with phyllodes tumor who underwent surgical therapy, 2000 – 2012 (n = 1,238)
| Characteristic | Post-BCS XRT (n=91) n (%) |
P value |
Adjusted OR (95% CI) |
Post- mastectomy XRT (n=100) n (%) |
P value |
Adjusted OR (95% CI) |
|---|---|---|---|---|---|---|
| Age (years) | 0.189 | 0.264 | ||||
| <40 | 19 (20.9) | 1 | 18 (18) | 1 | ||
| 40–49 | 18 (19.8) | 0.75 (0.37 – 1.52) | 29 (29) | 1.05 (0.53 – 2.09) | ||
| 50–59 | 32 (35.2) | 1.40 (0.75 – 2.63) | 36 (36) | 1.08 (0.55 – 2.12) | ||
| 60 and older | 22 (24.2) | 1.45 (0.73 – 2.87) | 17 (17) | 0.53 (0.25 – 1.14) | ||
| Race | 0.93 | 0.149 | ||||
| White | 67 (73.6) | 1 | 67 (67) | 1 | ||
| Other/unknown | 24 (26.4) | 1.05 (0.62 – 1.76) | 33 (33) | 1.18 (0.72 – 1.92) | ||
| Year of diagnosis | 0.001 | 0.001 | ||||
| 2000–2004 | 19 (20.9) | 1 | 23 (23) | 1 | ||
| 2005–2008 | 32 (35.2) | 2.28 (1.24 – 4.19)‡ | 33 (33) | 2.12 (1.16 – 3.87)‡ | ||
| 2009–2012 | 40 (43.9) | 2.99 (1.66 – 5.42)‡ | 44 (44) | 2.43 (1.37 – 4.30)‡ | ||
| Tumor size (cm) | 0.014 | <.0001 | ||||
| <5 | 54 (59.3) | 1 | 12 (12) | 1 | ||
| ≥5 | 35 (38.5) | 1.46 (0.90 – 2.37)‡ | 85 (85) | 3.34 (1.74 – 6.39)‡ | ||
| Unknown | 2 (2.2) | 0.26 (0.26 – 1.11) | 3 (3) | 1.56 (0.40 – 6.06) | ||
| Nodal Status | 0.001 | 0.04 | ||||
| Not examined | 72 (79.1) | 1 | 50 (50) | 1 | ||
| Examined | 19 (20.8) | 2.92 (1.59–5.36)‡ | 50 (50) | 1.66 (1.05 – 2.62)‡ | ||
Abbreviations: BCS Breast conserving surgery, XRT radiation therapy, OR odds ratio, CI confidence interval
Statistically significant (P<0.05) after adjusting for all other variables in the table
DISCUSSION
Using a population-based cohort from the SEER database, we examined patterns of care in the local-regional management of patients diagnosed with phyllodes tumors of the breast. Our results demonstrate that approximately 50% of patients are treated with breast conservation. Our findings also demonstrate an increasing use of XRT over the study period after both BCS and mastectomy, despite the absence of Level 1 data supporting this practice. Finally, we determined that approximately one in four women receive axillary nodal staging despite guidelines recommending against this additional surgery.
BCS is appropriate treatment for a phyllodes tumor if a good cosmetic and oncologic outcome are feasible.7,9 In an analysis of the SEER database from 1988–2002, Mcdonald et al suggested that BCS is equivalent to mastectomy and larger tumors can be effectively removed with wide excision without increasing the risk of cancer-specific death.9 They also noted an increase in BCS rates in their analysis from 41% in 1988 to 56% in 2002. Our results suggest rates of BCS have remained relatively unchanged since that time. This stability in BCS rates may be related to the large average presenting tumor size for phyllodes tumors as well as the wider excision margins required, which can limit the surgeon’s ability to achieve a good cosmetic outcome and negative margins with BCS alone. This is supported by our data, where the average tumor size was 6cm, and 45% of patients had a tumor ≥5cm in size, and is also consistent with prior literature.1,10–13 We found that the type of surgical procedure varied with tumor size and 36% of patients with tumors ≥5cm received BCS compared to 75% of patients with tumors <5cm.
Over the study period, utilization of XRT more than doubled following both mastectomy and BCS. This is similar to trends previously reported by Gnerlich et al using the National Cancer Data Base (NCDB).14 Currently, there are no randomized controlled trials that have examined the efficacy of XRT after margin-negative surgery for phyllodes tumors; however, the relatively high rate of local recurrence (LR) has generated interest in the potential role of XRT. There have been several retrospective studies examining the impact of XRT on local recurrence. The results of these single institution experiences are mixed however.1,2,9,10,12,14 A retrospective study by Belkacemi et al evaluated 446 patients treated for phyllodes tumors with a 45% negative margin rate between 1971 and 2003. Adjuvant XRT was administered to 38 (8.5%) patients and they reported a significant decrease in 10 year LR rates from 86% to 56%.12 In a study of 101 largely margin negative patients with phyllodes tumors by Chaney et al, local failure occurred in 4 (4%) patients. The authors here suggest that BCS with appropriate margins is sufficient primary therapy.10 The only prospective study in the literature is from Barth et al who conducted a multi-institutional study of 46 patients where XRT was administered after margin-negative surgery for borderline and malignant tumors. No local recurrence occurred in this cohort after 56 months of follow up.2 In the absence of better data, the NCCN recommends consideration of XRT if surgical management of a local recurrence would be especially morbid.7 The increasing rate that we observe may reflect a propensity to utilize XRT for larger tumors where margins may be difficult to obtain. As a result, this may be in accordance with guidelines as larger tumors would most likely result in a morbid local recurrence.
In this cohort, one in four women underwent axillary nodal sampling despite guidelines recommending against it. Phyllodes tumors rarely metastasize to the lymph nodes, confirmed in this data by the low rate of positive nodes (0.1% and 2.1% after BCS and mastectomy, respectively). Because of the presumed hematogenous spread of these tumors if metastatic, it is uncertain what the goal of axillary surgery was in these cases. We identify four possible explanations for the unexpectedly high utilization of axillary sampling in this study. First, we identified an association between nodal sampling and mastectomy. Given limitations in the extent of axillary surgery variables, we used the number of nodes examined to determine whether axillary sampling was performed. It is possible that some of these cases represent removal of intramammary or inadvertent axillary lymph nodes during the mastectomy. A second explanation is that surgeons may be concerned about finding an occult primary invasive cancer in the mastectomy specimen and are therefore performing a prophylactic sentinel lymph node biopsy as is the standard recommendation for patients undergoing mastectomy for ductal carcinoma in situ (DCIS).15–17 Another explanation is that patients are taken to the operating room with a presumed diagnosis of adenocarcinoma and undergo lymph node sampling. It is only on final pathology that the diagnosis of phyllodes is made. This etiology is supported by our observation that the utilization of lymph node sampling (24%) is greater than radiation (15%). However, if this were the case, we would have anticipated a temporal decrease in the utilization of lymph node sampling over time with the increasing utilization of percutaneous core biopsy and a higher rate with BCS as mastectomy is unlikely without a tissue diagnosis neither of which is observed. Finally, given the rarity of phyllodes tumors, it is possible some patients are being treated according to adenocarcinoma paradigms. This etiology is further supported by the strong association between nodal examination and XRT utilization in adjusted analysis for both BCS and mastectomy.
While SEER is a comprehensive and geographically representative registry, there are several limitations to this study. First, this is a retrospective study subject to bias as the allocation of patients to receive a particular intervention is not random. We were unable to evaluate diagnostic uncertainty prior to surgery, as histology in SEER is coded based on the final diagnosis obtained from a pathology report. Nodal examination is based on nodes removed during surgery rather than surgery type as discussed above. Several important prognostic data elements specifically relevant to phyllodes tumors, including grade, number of mitosis, cellular atypia, stromal overgrowth, and margin status, are either absent or incomplete in SEER. We therefore cannot assess how the presence of these factors may have influenced choice of XRT use in this cohort.12,13,18 In addition, we lack data on comorbidities which can be a surrogate for overall health status and may significantly affect which patients are selected for certain treatments. SEER also lacks information on local or distant recurrence, preventing the assessment of the impact of XRT use on this outcome.
CONCLUSION
We determined that there is evidence of frequent nodal examination and increasing utilization of adjuvant XRT in patients diagnosed with phyllodes tumors of the breast. These findings have important implications for practice and policy. Continued efforts to increase pre-operative tissue diagnosis via core biopsy should be emphasized and may help to decrease the rate of lymph node surgery for phyllodes tumors. Additionally, the majority of breast cancer surgery in the United States is performed at institutions treating less than 10 cases per year; thus, most centers will rarely encounter a phyllodes tumor due to its low incidence. As such, our findings may represent an educational gap in the surgical care of patients with phyllodes tumors. Improving the use of current guidelines in routine practice, such as those from the NCCN, for both common and rare conditions may help to ensure appropriate treatment, especially at institutions not routinely treating rare diseases such as phyllodes tumor.
Figure 2.
Rate of radiotherapy utilization stratified by surgery type in patients diagnosed with phyllodes tumors from 2000 to 2012
Abbreviations: BCS Breast conserving surgery, RT radiotherapy
Synopsis.
Using Surveillance, Epidemiology and End Results Program (SEER) data (2000–2012), we demonstrate a significant increase in the number of women received adjuvant radiation therapy after surgical management of phyllodes tumor. Additionally, one in four women underwent nodal sampling despite guidelines recommending against this procedure. These findings have implications for practice and policy highlighting the need for stronger evidence and education about current guidelines to inform practice and ensure appropriate care.
Acknowledgments
Financial support: Taiwo Adesoye receives support from the University of Wisconsin Surgical Oncology Research Training Program (T32 CA090217). Heather B. Neuman is supported through the Building Interdisciplinary Research Careers in Women’s Health Scholar Program (NIH K12 HD055894).
Footnotes
Disclosures: None
REFERENCES
- 1.Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer. 1996;77:910–916. doi: 10.1002/(sici)1097-0142(19960301)77:5<910::aid-cncr16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Barth RJ, Wells WA, Mitchell SE, Cole BF. A Prospective, Multi-Institutional Study of Adjuvant Radiotherapy After Resection of Malignant Phyllodes Tumors. Ann Surg Oncol. 2009;16(8):2288–2294. doi: 10.1245/s10434-009-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AHS. Recent developments in the histological diagnosis of spindle cell carcinoma, fibromatosis and phyllodes tumour of the breast: Spindle cell and fibroepithelial lesions. Histopathology. 2007;52(1):45–57. doi: 10.1111/j.1365-2559.2007.02893.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein L, Deapen D, Ross RK. The descriptive epidemiology of malignant cystosarcoma phyllodes tumors of the breast. Cancer. 1993;71:3020–3024. doi: 10.1002/1097-0142(19930515)71:10<3020::aid-cncr2820711022>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Parker SJ, Harries SA. Phyllodes tumours. Postgrad Med J. 2001;77(909):428–435. doi: 10.1136/pmj.77.909.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvadori B, Cusumano F, Del Bo R, et al. Surgical treatment of phyllodes tumors of the breast. Cancer. 1989;63:2532–2536. doi: 10.1002/1097-0142(19890615)63:12<2532::aid-cncr2820631229>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Phyllodes Tumor (Version 1.2016) [Accessed January 25, 2016]; http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 8.National Cancer Institute. Surveillance Epidemiology and End Results (SEER) Program. [Accessed January 1, 2016]; http://seer.cancer.gov/about/overview.
- 9.Macdonald OK, Lee CM, Tward JD, Chappel CD, Gaffney DK. Malignant phyllodes tumor of the female breast: Association of primary therapy with cause-specific survival from the Surveillance, Epidemiology, and End Results (SEER) program. Cancer. 2006;107(9):2127–2133. doi: 10.1002/cncr.22228. [DOI] [PubMed] [Google Scholar]
- 10.Chaney AW, Pollack A, Mcneese MD, et al. Primary treatment of cystosarcoma phyllodes of the breast. Cancer. 2000;89(7):1502–1511. doi: 10.1002/1097-0142(20001001)89:7<1502::aid-cncr13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero MA, Ballard BR, Grau AM. Malignant phyllodes tumor of the breast: Review of the literature and case report of stromal overgrowth. Surg Oncol. 2003;12:27–37. doi: 10.1016/s0960-7404(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 12.Belkacémi Y, Bousquet G, Marsiglia H, et al. Phyllodes Tumor of the Breast. Int J Radiat Oncol. 2008;70(2):492–500. doi: 10.1016/j.ijrobp.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 13.Barrio AV, Clark BD, Goldberg JI, et al. Clinicopathologic Features and Long-Term Outcomes of 293 Phyllodes Tumors of the Breast. Ann Surg Oncol. 2007;14(10):2961–2970. doi: 10.1245/s10434-007-9439-z. [DOI] [PubMed] [Google Scholar]
- 14.Gnerlich JL, Williams RT, Yao K, Jaskowiak N, Kulkarni SA. Utilization of Radiotherapy for Malignant Phyllodes Tumors: Analysis of the National Cancer Data Base, 1998–2009. Ann Surg Oncol. 2014;21(4):1222–1230. doi: 10.1245/s10434-013-3395-6. [DOI] [PubMed] [Google Scholar]
- 15.Pilewskie M, Karsten M, Radosa J, Eaton A, King TA. Is Sentinel Lymph Node Biopsy Indicated at Completion Mastectomy for Ductal Carcinoma In Situ? Ann Surg Oncol. 2016 Mar; doi: 10.1245/s10434-016-5145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. Invasive Breast Cancer (Version 1.2016) [Accessed January 25, 2016]; http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 17.Lyman GH, Temin S, Edge SB, et al. Sentinel Lymph Node Biopsy for Patients With Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2014;32(13):1365–1383. doi: 10.1200/JCO.2013.54.1177. [DOI] [PubMed] [Google Scholar]
- 18.Kapiris I, Nasiri N, A'Hern R, Healy V, Gui GP. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur J Surg Oncol EJSO. 2001;27(8):723–730. doi: 10.1053/ejso.2001.1207. [DOI] [PubMed] [Google Scholar]