Abstract
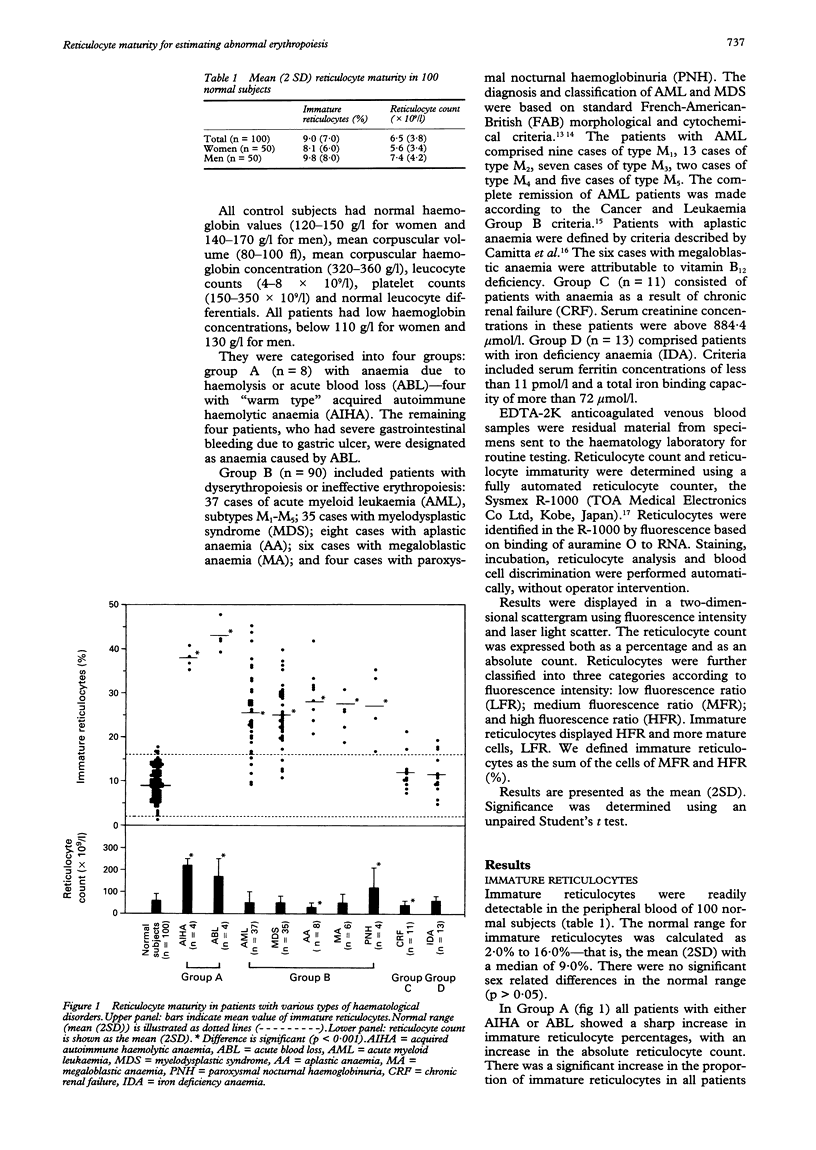
AIMS--To determine the maturity of reticulocytes in patients with anaemia as a result of various haematological disorders including those with qualitative abnormalities such as ineffective erythropoiesis or dyserythropoiesis. METHODS--The number of mature reticulocytes was measured with flow cytometry in venous blood samples from 122 patients with haematological disorders and 100 healthy controls. Reticulocytes were classified into three categories by the fluorescence intensity of auramin O staining: low fluorescence ratio (LFR), medium fluorescence ratio (MFR), and high fluorescence ratio (HFR). Immature reticulocytes were determined as the aggregate of MFR and HFR (%). RESULTS--The mean (2SD) number of immature reticulocytes in 100 normal subjects was 9.0 (7.0)%. Significantly high mean values of immature reticulocytes with a normal or reduced reticulocyte count were shown in 90 patients with dyserythropoietic or ineffective erythropoietic conditions, such as acute myeloid leukaemia (AML) (n = 37), myelodysplastic syndrome (MDS) (n = 35), aplastic anaemia (AA) (n = 8), or megaloblastic anaemia (MA), (n = 6). Reticulocyte ratios returned to normal after successful treatment of patients with AML (n = 10) and MA (n = 3). However, high percentages of immature reticulocytes with increased reticulocyte counts were consistently observed in patients with enhanced erythropoiesis such as those with acquired autoimmune haemolytic anaemias (AIHA) (n = 4) or acute blood loss (ABL) (n = 4). Reticulocyte maturity was within the normal range in patients with reduced erythropoiesis such as occurs in chronic renal failure (CRF) (n = 11), or in iron deficiency anaemia (IDA) (n = 13). CONCLUSIONS--The evaluation of reticulocyte maturity with total reticulocyte count seems to be clinically useful for estimating the qualitative impairment of erythropoiesis, and so could help differentiate haematological disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abkowitz J. L., Fialkow P. J., Niebrugge D. J., Raskind W. H., Adamson J. W. Pancytopenia as a clonal disorder of a multipotent hematopoietic stem cell. J Clin Invest. 1984 Jan;73(1):258–261. doi: 10.1172/JCI111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum F. R., Fefer A. The pathogenesis of aplastic anemia. Semin Hematol. 1981 Oct;18(4):241–257. [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982 Jun;51(2):189–199. [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985 Oct;103(4):620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- Camitta B. M., Thomas E. D., Nathan D. G., Gale R. P., Kopecky K. J., Rappeport J. M., Santos G., Gordon-Smith E. C., Storb R. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979 Mar;53(3):504–514. [PubMed] [Google Scholar]
- Crouch J. Y., Kaplow L. S. Relationship of reticulocyte age to polychromasia, shift cells, and shift reticulocytes. Arch Pathol Lab Med. 1985 Apr;109(4):325–329. [PubMed] [Google Scholar]
- Davis B. H., Bigelow N. C. Clinical flow cytometric reticulocyte analysis. Pathobiology. 1990;58(2):99–106. doi: 10.1159/000163571. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Bigelow N. C. Flow cytometric reticulocyte quantification using thiazole orange provides clinically useful reticulocyte maturity index. Arch Pathol Lab Med. 1989 Jun;113(6):684–689. [PubMed] [Google Scholar]
- Davis B. H., Bigelow N., Ball E. D., Mills L., Cornwell G. G., 3rd Utility of flow cytometric reticulocyte quantification as a predictor of engraftment in autologous bone marrow transplantation. Am J Hematol. 1989 Oct;32(2):81–87. doi: 10.1002/ajh.2830320202. [DOI] [PubMed] [Google Scholar]
- Ellison R. R., Holland J. F., Weil M., Jacquillat C., Boiron M., Bernard J., Sawitsky A., Rosner F., Gussoff B., Silver R. T. Arabinosyl cytosine: a useful agent in the treatment of acute leukemia in adults. Blood. 1968 Oct;32(4):507–523. [PubMed] [Google Scholar]
- Gavosto F., Gabutti V., Masera P., Pileri A. The problem of anaemia in the acute leukaemias. Kinetic study. Eur J Cancer. 1970 Feb;6(1):33–38. doi: 10.1016/0014-2964(70)90051-4. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Löwenberg B. Clonogenic cells in acute myeloblastic leukemia. Blood. 1986 Dec;68(6):1185–1195. [PubMed] [Google Scholar]
- Hillman R. S. Characteristics of marrow production and reticulocyte maturation in normal man in response to anemia. J Clin Invest. 1969 Mar;48(3):443–453. doi: 10.1172/JCI106001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepke J. F., Koepke J. A. Reticulocytes. Clin Lab Haematol. 1986;8(3):169–179. doi: 10.1111/j.1365-2257.1986.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Kojima K., Niri M., Setoguchi K., Tsuda I., Tatsumi N. An automated optoelectronic reticulocyte counter. Am J Clin Pathol. 1989 Jul;92(1):57–61. doi: 10.1093/ajcp/92.1.57. [DOI] [PubMed] [Google Scholar]
- MYHRE E. STUDIES ON THE ERYTHROKINETICS IN PERNICIOUS ANEMIA. Scand J Clin Lab Invest. 1964;16:391–402. doi: 10.3109/00365516409060531. [DOI] [PubMed] [Google Scholar]
- Oni S. B., Osunkoya B. O., Luzzatto L. Paroxysmal nocturnal hemoglobinuria: evidence for monoclonal origin of abnormal red cells. Blood. 1970 Aug;36(2):145–152. [PubMed] [Google Scholar]
- Perrotta A. L., Finch C. A. The polychromatophilic erythrocyte. Am J Clin Pathol. 1972 Apr;57(4):471–477. doi: 10.1093/ajcp/57.4.471. [DOI] [PubMed] [Google Scholar]
- Raskind W. H., Tirumali N., Jacobson R., Singer J., Fialkow P. J. Evidence for a multistep pathogenesis of a myelodysplastic syndrome. Blood. 1984 Jun;63(6):1318–1323. [PubMed] [Google Scholar]
- Tanke H. J., Rothbarth P. H., Vossen J. M., Koper G. J., Ploem J. S. Flow cytometry of reticulocytes applied to clinical hematology. Blood. 1983 Jun;61(6):1091–1097. [PubMed] [Google Scholar]
- Tanke H. J., van Vianen P. H., Emiliani F. M., Neuteboom I., de Vogel N., Tates A. D., de Bruijn E. A., van Oosterom A. T. Changes in erythropoiesis due to radiation or chemotherapy as studied by flow cytometric determination of peripheral blood reticulocytes. Histochemistry. 1986;84(4-6):544–548. doi: 10.1007/BF00482989. [DOI] [PubMed] [Google Scholar]
- Thaer A., Becker H. Microscope fluorometric investigations on the reticulocytic maturation distribution as diagnostic criterion of disordered erythropoiesis. Blut. 1975 May;30(5):339–348. doi: 10.1007/BF01633653. [DOI] [PubMed] [Google Scholar]
- Tichelli A., Gratwohl A., Driessen A., Mathys S., Pfefferkorn E., Regenass A., Schumacher P., Stebler C., Wernli M., Nissen C. Evaluation of the Sysmex R-1000. An automated reticulocyte analyzer. Am J Clin Pathol. 1990 Jan;93(1):70–78. doi: 10.1093/ajcp/93.1.70. [DOI] [PubMed] [Google Scholar]
- Wells D. A., Daigneault-Creech C. A., Simrell C. R. Effect of iron status on reticulocyte mean channel fluorescence. Am J Clin Pathol. 1992 Jan;97(1):130–134. doi: 10.1093/ajcp/97.1.130. [DOI] [PubMed] [Google Scholar]
- Young N. S. The problem of clonality in aplastic anemia: Dr Dameshek's riddle, restated. Blood. 1992 Mar 15;79(6):1385–1392. [PubMed] [Google Scholar]
- van Kamp H., Landegent J. E., Jansen R. P., Willemze R., Fibbe W. E. Clonal hematopoiesis in patients with acquired aplastic anemia. Blood. 1991 Dec 15;78(12):3209–3214. [PubMed] [Google Scholar]