Abstract
Background
Variability of blood pressure (BP) across outpatient visits is frequently dismissed as random fluctuation around a patient’s underlying BP. Objective: Examine the association between visit-to-visit variability (VVV) of systolic and diastolic BP (SBP and DBP) on cardiovascular disease and mortality outcomes.
Design
Prospective cohort study
Setting
Post-hoc analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Participants
25,814 ALLHAT participants.
Measurements
VVV of SBP was defined as the standard deviation (SD) across BP measurements obtained at 7 visits conducted from 6 to 28 months following ALLHAT enrollment. Participants free of cardiovascular disease events during the first 28 months of follow-up were followed from the month 28 study visit through the end of active ALLHAT follow-up. Outcomes included fatal coronary heart disease or non-fatal myocardial infarction, all-cause mortality, stroke and heart failure.
Results
There were 1194 cases of fatal CHD or non-fatal MI, 1948 deaths, 606 cases of stroke and 921 cases of heart failure during follow-up. After multivariable adjustment including mean SBP, the hazard ratio comparing participants in the highest versus lowest quintile of SD of SBP (≥14.4 mmHg versus <6.5 mmHg) was 1.30 (1.06–1.59) for fatal coronary heart disease or non-fatal myocardial infarction, 1.58 (1.32–1.90) for all-cause mortality, 1.46 (1.06–2.01) for stroke, and 1.25 (0.97–1.61) for heart failure. Higher VVV of DBP was also associated with cardiovascular disease events and mortality.
Limitations
Long-term outcomes were not available.
Conclusions
Higher VVV of SBP is associated with increased cardiovascular disease and mortality risk. Future studies should examine whether reducing VVV of BP lowers this risk.
Primary funding source
National Institutes of Health
The prognostic value of blood pressure (BP) is mainly based on measurements obtained in a clinic setting, typically from a few visits.1 Until recently, variability of BP across outpatient visits was dismissed as random fluctuation around a patient’s true underlying BP.2,3 While some studies have reported associations between higher visit-to-visit variability (VVV) of systolic blood pressure (SBP) and increased stroke and coronary heart disease (CHD) risk, other analyses have failed to demonstrate such associations. 4–10
The methodology applied in estimating VVV of BP varied widely across previous studies. In addition, studies have used as few as three visits and as many as 56 visits to estimate VVV of BP and the duration of time between visits has ranged from 2 days to as long as 4 years.5,11,12 These factors not only influence VVV of BP but could also impact the strength of the association between VVV of BP and CVD outcomes.4,13
To address the inconsistent findings from previous studies, we conducted a secondary data analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) to examine whether VVV of BP is associated with CVD and mortality events. ALLHAT provides the opportunity to evaluate the association of VVV of BP and major clinical outcomes in a large diverse population of individuals with hypertension where visits were conducted at set time intervals, BP was measured following a standardized protocol, and outcomes were evaluated over several years of follow-up.
METHODS
Study design
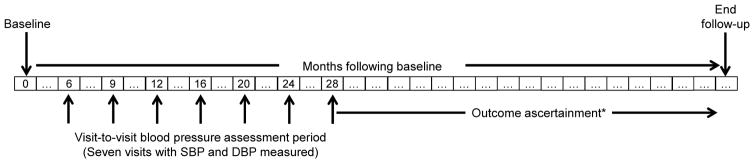
We conducted a cohort study as a secondary analysis using data from ALLHAT. Figure 1 shows the timeline for assessment of VVV of BP and outcome events within ALLHAT. The primary exposure of interest, VVV of SBP, was ascertained at the 7 study visits conducted between 6 and 28 months following randomization. We studied four outcomes: fatal coronary heart disease (CHD) or nonfatal myocardial infarction, all-cause mortality, stroke and heart failure. Participants were followed from their month 28 study visit until the occurrence of an outcome event or the end of active ALLHAT follow-up (October 2001 to March 2002). Participants who had an event before their month 28 study visit were excluded from all analyses.
Figure 1.
Design of the study evaluating the association of visit-to-visit variability of blood pressure and cardiovascular outcomes and all-cause mortality in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
*Participants were followed for a mean of 2.7 to 2.9 years depending on the outcome (maximum: 5.7 years) following assessment of visit-to-visit variability of blood pressure.
ALLHAT was a multicenter randomized, double-blind, clinical trial sponsored by the National Heart, Lung, and Blood Institute of the U.S. National Institutes of Health. A complete description of the rationale and design of ALLHAT has been previously published.14 In brief, ALLHAT was designed to determine whether treatment initiated with a calcium channel blocker (amlodipine), an angiotensin converting enzyme inhibitor (lisinopril), or an alpha-blocker (doxazosin), each compared to treatment initiated with a diuretic (chlorthalidone), would lower major cardiovascular outcomes. The primary endpoint was incidence of fatal coronary heart disease (CHD) or nonfatal myocardial infarction. A total of 42,418 hypertensive adults aged 55 years or older with one or more additional risk factor for CVD were enrolled at 623 clinical sites across the United States, Canada, Puerto Rico and the US Virgin Islands between February 1994 and January 1998. The doxazosin treatment arm was discontinued in 2000 due to little chance of finding a benefit on CHD outcomes and an increased risk of CVD compared with the chlorthalidone arm.15 The main results comparing participants randomized to chlorthalidone, amlodipine, and lisinopril, were published in December 2002.16 ALLHAT was approved by local Institutional Review Boards and all participants provided written informed consent. The current analysis was approved by the Institutional Review Board at the University of Alabama at Birmingham.
Study visits, blood pressure measurements and calculation of VVV of BP
To calculate intra-individual VVV of SBP and DBP, we used data from 7 follow-up visits that occurred 6, 9, 12, 16, 20, 24 and 28 months following randomization. We chose to begin the VVV of BP assessment period at the 6-month follow-up visit to avoid confounding by the initial reduction in BP that occurred between randomization and the 6-month follow-up visit (6 to 10 mmHg for SBP and ~5 mmHg for DBP) due to early medication titration. To increase the precision of VVV of BP estimates, we calculated VVV of BP for participants with BP measurements at 5, 6 or 7 visits between month 6 and 28 of follow-up. As described below, VVV of BP was imputed for participants who attended fewer than 5 visits between month 6 and 28. To maximize follow-up time available, assessment of VVV of BP did not extend past the month 28 follow-up visit. At each follow-up visit, BP was measured two times by a trained observer following a standardized technique.17 Using the average BP at each visit, VVV of BP was defined by the intra-individual standard deviation (SD) across visits. Average real variability (ARV) and standard deviation independent of the mean (SDIM) were calculated as alternative VVV of BP metrics (Appendix Figure 1).18 VVV of DBP was also calculated in secondary analyses.
Outcome ascertainment
In this report, we studied four outcomes including fatal CHD or nonfatal MI, all-cause mortality, stroke, and heart failure. Details of the event ascertainment process are provided elsewhere.14,16 Participants were followed from the end of the VVV of BP assessment period to the date of each outcome, their date of death, or end of active ALLHAT follow-up (October 1, 2001 through March, 31, 2002).
Covariate information
Covariates used for adjustment were selected a priori based on their potential role as confounders. Baseline (pre-randomization) variables included age, gender, race/ethnicity, education, current cigarette smoking, body mass index, use of aspirin, high-density lipoprotein cholesterol <35 mg/dL (at ≥2 occasions in the 5 years prior to ALLHAT enrollment), total cholesterol, estimated glomerular filtration rate (eGFR), diabetes, history of myocardial infarction or stroke, documentation of other atherosclerotic CVD, history of revascularization, atrial fibrillation on electrocardiogram, left ventricular hypertrophy, the presence of ST depression and T-wave inversion, and use of antihypertensive medication prior to ALLHAT randomization. eGFR was calculated using the Chronic Kidney Disease Epidemiology Study (CKD-EPI) equation.19 Data collected at visits conducted 6 to 28 months following randomization were used to calculate the following covariates: mean SBP and DBP, pulse pressure, the use of antihypertensive medications beyond the randomization drug, changes in antihypertensive medication regimen (adding, stopping or changing antihypertensive medications), use of statins, and low adherence, defined as participant report of taking <80% of the randomization drug at any visits between months 6 and 28. Participants who reported taking ≥80% of their randomization drug at every visit between months 6 and 28 were considered to have high adherence.
Statistical Analysis
Due to limited follow-up available following the VVV of BP assessment period for participants randomized to the doxazosin treatment arm, we restricted our analyses to the 33,357 ALLHAT participants randomized to the chlorthalidone, amlodipine, or lisinopril arms. We excluded 7,543 participants who had CVD events or died prior to the 28 month visit. We imputed VVV of BP for participants with fewer than 5 visits with BP measurements between months 6 and 28 of follow-up. Additionally, missing data for covariates were imputed (Appendix Table 1). Imputation was performed with 10 data sets using chained equations.
Characteristics of participants included in the current analyses were calculated by quintile of SD of SBP. The Kaplan-Meier method was used to calculate the cumulative incidence of each outcome by quintile of SD of SBP. Cox proportional hazards regression was used to calculate multivariable adjusted hazard ratios for each outcome associated with quintile of SD of SBP with the lowest quintile serving as the reference. Four nested models were constructed: Model 1 included adjustment for age, race/ethnicity, gender, region of residence, and antihypertensive randomization assignment. Model 2 included additional adjustment for education, smoking status, body mass index, use of aspirin, low high density lipoprotein cholesterol, total cholesterol, eGFR, diabetes, history of MI or stroke, history of other atherosclerotic cardiovascular disease, history of coronary revascularization, atrial fibrillation on electrocardiogram, major ST depression or T wave inversion, left ventricular hypertrophy, use of antihypertensive medications prior to ALLHAT randomization, and statin use during the VVV of SBP assessment period (i.e., month 6 through 28 of follow-up). Model 3 additionally included mean pulse pressure, medication adherence, use of antihypertensive medications beyond the randomized drug, and changes in antihypertensive medication regimen, all during the VVV of BP assessment period (i.e., month 6 through month 28 of follow-up). Model 4 also included mean SBP across the visits used to calculate VVV of SBP. Trends across quintiles were calculated by modeling quintile of SD of SBP as an ordinal variable. We repeated the above analyses for ARV and SDIM of SBP and for VVV of DBP using SD, ARV and SDIM and in a complete case analyses (i.e., analyses without multiple imputation).
Next, we calculated fully-adjusted (Model 4) hazard ratios for the highest versus the lowest quintile of SD of SBP for each outcome stratified by age (<65 and 65+ years), gender, race (black and non-black), diabetes status, antihypertensive medication adherence, BP control (mean SBP/DBP across visits 6 to 28 months following randomization <140/90 mmHg and ≥140/90 mmHg), left ventricular hypertrophy, history of CVD, and antihypertensive randomization group, and tested for multiplicative interactions between these groups. Finally, the multivariable-adjusted hazard ratios for each outcome associated with SD of SBP, modeled as a continuous variable, were calculated using restricted quadratic splines. All analyses were conducted in Stata 13.0 (Stata Corporation, College Station, TX).
Role of the funding source: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under contracts NO1-HC-35130 and HHSN268201100036C and under Award Number R01 HL110993. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
RESULTS
Characteristics of study participants included in the current analysis by quintile of SD of SBP are presented in Table 1. Older age and being non-Hispanic black were associated with higher SD of SBP while men were more likely to be in the lower quintiles of SD of SBP. Participants with higher SD of SBP had less education, were less likely to be taking aspirin and have low high density lipoprotein cholesterol, had higher total cholesterol and lower eGFR, and were more likely to have major ST depression or T wave inversion or LVH by electrocardiogram. Use of antihypertensive medication prior to baseline was more common at higher SD of SBP. Mean SBP and pulse pressure were higher at higher levels of SD of SBP. Higher mean SBP, DBP and pulse pressure were associated with higher SD of SBP. Additionally, those with higher SD of SBP were more likely to have low adherence and change medication classes. Participants randomized to chlorthalidone and amlodipine were less likely, while those randomized to lisinopril were more likely, to be in the highest quintiles of SD of SBP.
Table 1.
Characteristics of ALLHAT participants by standard deviation of systolic blood pressure.
| Quintile of standard deviation of SBP, mmHg (range) | ||||||
|---|---|---|---|---|---|---|
| Q1 (<6.5) | Q2 (6.5 to <8.7) | Q3 (8.7 to <11.0) | Q4 (11.0 to <14.4) | Q5 (≥14.4) | p-trend | |
| N, mean | 5205 | 5179 | 5206 | 5163 | 5061 | - |
| Variables assessed at baseline | ||||||
| Age, years, mean (SE) | 65.7 (0.11) | 65.9 (0.10) | 66.3 (0.11) | 66.6 (0.11) | 67.4 (0.12) | <0.001 |
| Race, % | ||||||
| Non-Hispanic White | 47.5% | 47.5% | 47.5% | 46.4% | 41.6% | <0.001 |
| Non-Hispanic Black | 25.5% | 29.3% | 31.3% | 33.7% | 39.9% | |
| Hispanic White | 18.0% | 15.0% | 12.7% | 10.8% | 8.8% | |
| Hispanic Black | 4.0% | 3.7% | 3.4% | 2.8% | 2.4% | |
| Men, % | 55.3% | 53.0% | 52.1% | 50.8% | 47.1% | <0.001 |
| Years of education, mean (SE) | 11.3 (0.07) | 11.2 (0.06) | 11.1 (0.06) | 10.9 (0.6) | 10.4 (0.06) | <0.001 |
| Current smoking, % | 20.1% | 21.5% | 20.5% | 21.7% | 22.8% | 0.006 |
| BMI, kg/m2, mean (SE) | 29.9 (0.09) | 29.9 (0.10) | 30.1 (0.10) | 29.8 (0.09) | 29.5 (0.10) | 0.003 |
| Aspirin use, % | 36.2% | 35.7% | 34.4% | 33.0% | 32.1% | <0.001 |
| Low HDL-cholesterol (<35mg/dl), % | 14.3% | 13.0% | 12.5% | 10.8% | 9.1% | <0.001 |
| Total cholesterol, mg/dL, mean (SE) | 213.6 (0.62) | 215.1 (0.68) | 216.2 (0.67) | 216.3 (0.68) | 217.8 (0.67) | <0.001 |
| eGFR, ml/min/1.73 m2, mean (SE) | 76.6 (0.24) | 76.4 (0.25) | 75.7 (0.26) | 74.7 (0.27) | 73.0 (0.30) | <0.001 |
| Diabetes (type 2), % | 34.8% | 34.3% | 36.0% | 34.3% | 35.7% | 0.49 |
| History of MI or stroke, % | 20.5% | 21.6% | 21.3% | 20.4% | 19.4% | 0.097 |
| History of other ASCVD, % | 22.4% | 21.2% | 21.6% | 22.6% | 23.1% | 0.185 |
| History of revascularization, % | 11.3% | 11.0% | 11.2% | 11.4% | 10.0% | 0.136 |
| Atrial fibrillation by electrocardiogram, % | 0.9% | 0.9% | 0.9% | 0.7% | 0.9% | 0.99 |
| Major ST depression, % | 9.1% | 10.2% | 10.1% | 10.9% | 11.1% | 0.004 |
| LVH by electrocardiogram, % | 14.8% | 14.8% | 16.1% | 16.7% | 19.9% | <0.001 |
| Use of antihypertensive medication prior to baseline, % | 88.8% | 89.4% | 90.2% | 89.9% | 91.2% | <0.001 |
| Variables assessed during the visit-to- visit variability of BP assessment period | ||||||
| Statin use, % | 31.0% | 30.1% | 30.5% | 29.6% | 29.5% | 0.143 |
| Mean SBP, mm Hg, mean (SE) | 132.7 (0.13) | 134.0 (0.16) | 136.0 (0.16) | 138.4 (0.17) | 144.2 (0.20) | <0.001 |
| Mean DBP, mm Hg, mean (SE) | 78.3 (0.09) | 78.4 (0.10) | 78.6 (0.11) | 79.3 (0.11) | 80.2 (0.12) | <0.001 |
| Mean pulse pressure, mm Hg, mean (SE) | 54.4 (0.13) | 55.6 (0.16) | 57.4 (0.15) | 59.1 (0.16) | 64.0 (0.20) | <0.001 |
| Max. number of antihypertensive meds at any visit (6–28m) | 1.4 (0.01) | 1.5 (0.01) | 1.6 (0.01) | 1.7 (0.01) | 2.0 (0.02) | <0.001 |
| Low adherence at any visit, % | 10.3% | 11.6% | 12.6% | 15.0% | 18.1% | <0.001 |
| Changes in medication classes, % | 27.3% | 35.0% | 41.3% | 50.1% | 64.1% | <0.001 |
| Randomization group | ||||||
| Chlorthalidone, % | 49.6% | 48.1% | 45.6% | 45.6% | 41.2% | <0.001 |
| Amlodipine, % | 29.5% | 29.0% | 28.6% | 26.9% | 22.8% | |
| Lisinopril, % | 20.9% | 22.9% | 25.8% | 27.5% | 30.0% | |
Standard deviation of systolic blood pressure was assessed using data from visits conducted 6 to 28 months following randomization in ALLHAT.
Numbers in table are mean (standard error) or column percent (%).
BMI – body mass index; eGFR – estimated glomerular filtration rate; MI – myocardial infarction; ASCVD – atherosclerotic cardiovascular disease; LVH – left ventricular hypertrophy; SBP – systolic blood pressure; DBP – diastolic blood pressure
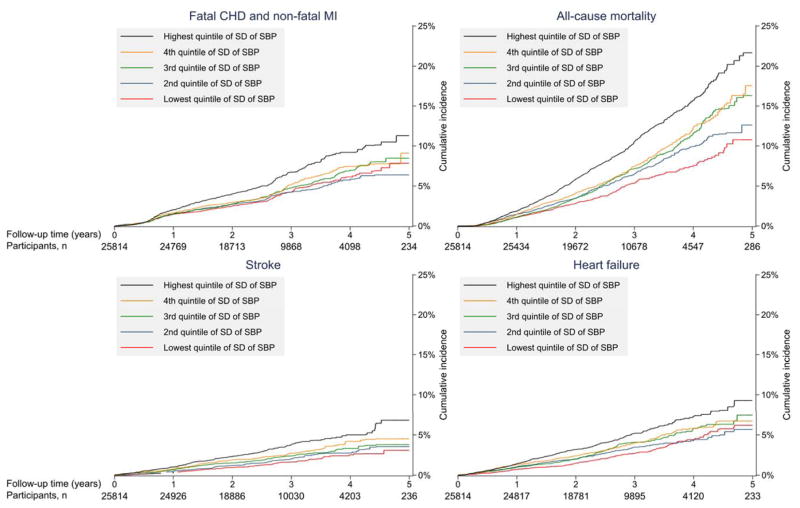
Following the VVV of SBP assessment period, there were 1,194 cases of fatal CHD or non-fatal MI, 1,948 deaths, 606 cases of stroke and 921 cases of heart failure over a mean follow-up of 2.7 to 2.9 (maximum 5.7) years. The incidence of fatal CHD and non-fatal MI, mortality, stroke, and heart failure were each progressively higher at higher levels of SD of SBP (Figure 2 and Table 2). After full multivariable adjustment, participants in the highest versus lowest quintile of SD of SBP had a statistically significantly higher risk for fatal CHD and non-fatal MI, mortality, and stroke. After full multivariable adjustment, the hazard ratio for heart failure comparing the highest to lowester quintile of SD of SBP was 1.25 (0.97–1.61). Results were consistent when modeling VVV of SBP using ARV and SDIM (Appendix Tables 2 – 3), in analyses without multiple imputation (Appendix Table 4) and for VVV of DBP (Appendix Tables 5–8).
Figure 2.
Cumulative incidence of fatal coronary heart disease and non-fatal myocardial infarction, all-cause mortality, stroke, and heart failure by quintile of intra-individual standard deviation of systolic blood pressure.
Table 2.
Incidence rates and hazard ratios for fatal coronary heart disease and non-fatal myocardial infarction, all-cause mortality, stroke, and heart failure associated with standard deviation of systolic blood pressure.
| Quintile of standard deviation of SBP, mmHg | ||||||
|---|---|---|---|---|---|---|
| Q1 (<6.5) | Q2 (6.5 to <8.7) | Q3 (8.7 to <11.0) | Q4 (11.0 to <14.4) | Q5 (≥14.4) | p-trend | |
| Fatal CHD and non-fatal MI (1194 events total) | ||||||
| Number of cases | 203 | 199 | 234 | 244 | 314 | - |
| Incidence rate* (95% CI) | 14.5 (12.4–16.5) | 14.2 (12.1–16.3) | 16.3 (14.0–18.5) | 17.1 (14.9–19.3) | 22.7 (20.0–25.4) | <0.001 |
| Hazard ratio | ||||||
| Model 1 | 1 (ref) | 0.98 (0.80–1.21) | 1.10 (0.90–1.35) | 1.15 (0.95–1.40) | 1.53 (1.28–1.84) | <0.001 |
| Model 2 | 1 (ref) | 0.97 (0.79–1.19) | 1.07 (0.87–1.31) | 1.12 (0.92–1.36) | 1.46 (1.21–1.76) | <0.001 |
| Model 3 | 1 (ref) | 0.95 (0.77–1.16) | 1.03 (0.84–1.26) | 1.05 (0.86–1.28) | 1.30 (1.07–1.59) | 0.005 |
| Model 4 | 1 (ref) | 0.95 (0.77–1.16) | 1.03 (0.84–1.26) | 1.05 (0.86–1.28) | 1.30 (1.06–1.59) | 0.006 |
| All-cause mortality (1948 events total) | ||||||
| Number of cases | 259 | 331 | 389 | 412 | 557 | - |
| Incidence rate* (95% CI) | 17.9 (15.5–20.3) | 22.9 (20.2–25.5) | 26.0 (23.1–28.9) | 27.6 (24.8–30.4) | 38.2 (34.9–41.5) | <0.001 |
| Hazard ratio | ||||||
| Model 1 | 1 (ref) | 1.25 (1.05–1.49) | 1.35 (1.12–1.62) | 1.41 (1.19–1.67) | 1.85 (1.57–2.19) | <0.001 |
| Model 2 | 1 (ref) | 1.24 (1.04–1.47) | 1.31 (1.09–1.58) | 1.36 (1.15–1.61) | 1.75 (1.47–2.07) | <0.001 |
| Model 3 | 1 (ref) | 1.21 (1.02–1.45) | 1.26 (1.05–1.52) | 1.27 (1.07–1.51) | 1.55 (1.29–1.86) | <0.001 |
| Model 4 | 1 (ref) | 1.21 (1.02–1.45) | 1.27 (1.05–1.53) | 1.28 (1.08–1.52) | 1.58 (1.32–1.90) | <0.001 |
| Stroke (606 events total) | ||||||
| Number of cases | 79 | 96 | 117 | 137 | 177 | - |
| Incidence rate* (95% CI) | 5.6 (4.3–6.8) | 6.8 (5.4–8.3) | 8.1 (6.6–9.7) | 9.5 (7.8–11.3) | 12.6 (10.7–14.6) | <0.001 |
| Hazard ratio | ||||||
| Model 1 | 1 (ref) | 1.18 (0.87–1.62) | 1.34 (1.00–1.81) | 1.52 (1.12–2.07) | 1.87 (1.40–2.52) | <0.001 |
| Model 2 | 1 (ref) | 1.17 (0.86–1.60) | 1.29 (0.96–1.74) | 1.50 (1.11–2.04) | 1.80 (1.34–2.42) | <0.001 |
| Model 3 | 1 (ref) | 1.15 (0.85–1.57) | 1.22 (0.91–1.65) | 1.39 (1.01–1.90) | 1.52 (1.12–2.08) | 0.004 |
| Model 4 | 1 (ref) | 1.15 (0.84–1.57) | 1.22 (0.90–1.64) | 1.36 (0.99–1.87) | 1.46 (1.06–2.01) | 0.013 |
| Heart failure (921 events total) | ||||||
| Number of cases | 136 | 156 | 188 | 196 | 245 | - |
| Incidence rate* (95% CI) | 9.7 (7.9–11.5) | 11.1 (9.3–13.0) | 13.1 (11.1–15.0) | 13.7 (11.7–15.7) | 17.6 (15.4–19.9) | <0.001 |
| Hazard ratio | ||||||
| Model 1 | 1 (ref) | 1.12 (0.87–1.44) | 1.26 (0.99–1.59) | 1.30 (1.01–1.66) | 1.62 (1.29–2.04) | <0.001 |
| Model 2 | 1 (ref) | 1.10 (0.86–1.42) | 1.21 (0.95–1.54) | 1.27 (0.99–1.62) | 1.56 (1.24–1.97) | <0.001 |
| Model 3 | 1 (ref) | 1.07 (0.83–1.37) | 1.12 (0.88–1.43) | 1.11 (0.86–1.43) | 1.22 (0.95–1.57) | 0.125 |
| Model 4 | 1 (ref) | 1.07 (0.83–1.38) | 1.13 (0.89–1.44) | 1.12 (0.87–1.45) | 1.25 (0.97–1.61) | 0.084 |
Per 1,000 person-years
CI – confidence interval; SBP – systolic blood pressure; p-y – person years.
Model 1 includes adjustment for age, gender, race/ethnicity, region of residence and randomization assignment.
Model 2 includes variables in model 1 and smoking status, body mass index, estimated glomerular filtration rate, diabetes, total cholesterol, history of myocardial infarction or stroke, history of coronary revascularization, atrial fibrillation on electrocardiogram, history of other atherosclerotic cardiovascular disease, major ST depression or T wave inversion, left ventricular hypertrophy, low high density lipoprotein cholesterol, aspirin use, statin use, use of blood pressure medications prior to study randomization and years of education.
Model 3 includes variables in model 2 and pulse pressure, medication adherence, antihypertensive medication classes being taken, and changes in antihypertensive medication classes being taken.
Model 4 includes variables in model 3 and mean systolic blood pressure.
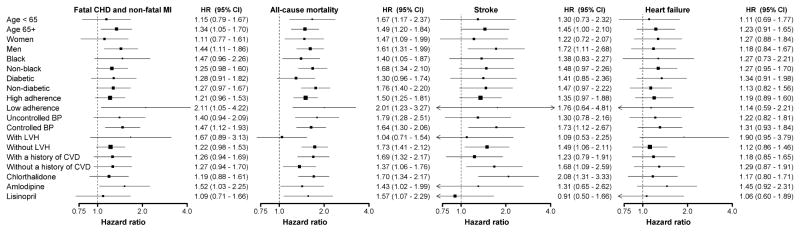
The association of SD of SBP and outcomes were consistent across sub-groups defined by age group, gender, race, diabetes status, antihypertensive medication adherence, BP control, left ventricular hypertrophy, history of CVD and randomization assignment with two exceptions (Figure 3). The highest versus lowest quintile of SD of SBP was associated with all-cause mortality for participants without but not with left ventricular hypertrophy (p-interaction=0.016). Also, no association was present between the highest versus lowest quintile of SD of SBP and stroke risk for participants randomized to lisinopril or amlodipine but the highest quintile of SD of SBP was associated with increased stroke risk for participants randomized to chlorthalidone (p-interaction = 0.083 comparing the lisinopril and chlorthalidone sub-groups).
Figure 3.
Hazard ratios for fatal coronary heart disease and non-fatal myocardial infarction, all-cause mortality, stroke, and heart failure associated with the highest versus lowest quintile of intra-individual standard deviation across ALLHAT follow-up visits conducted 6 to 28 months following baseline in selected sub-groups.
Includes adjustment for age, gender, race/ethnicity, region of residence, randomization assignment, smoking status, body mass index, estimated glomerular filtration rate, diabetes, total cholesterol, history of myocardial infarction or stroke, history of coronary revascularization, atrial fibrillation on electrocardiogram, history of other atherosclerotic cardiovascular disease, major ST depression or T wave inversion, left ventricular hypertrophy, low high density lipoprotein cholesterol, aspirin use, use of blood pressure medications prior to study randomization, statin use, pulse pressure, medication adherence, antihypertensive medication classes being taken, changes in antihypertensive medication classes being taken, and mean systolic blood pressure.
Blood pressure control was defined as systolic/diastolic blood pressure < 140/90 mm Hg.
All p-values for interaction > 0.10 for each sub-group and outcome except comparing LVH on all-cause mortality (p=0.016) and Lisinopril and Chlorthalidone on stroke (p=0.083).
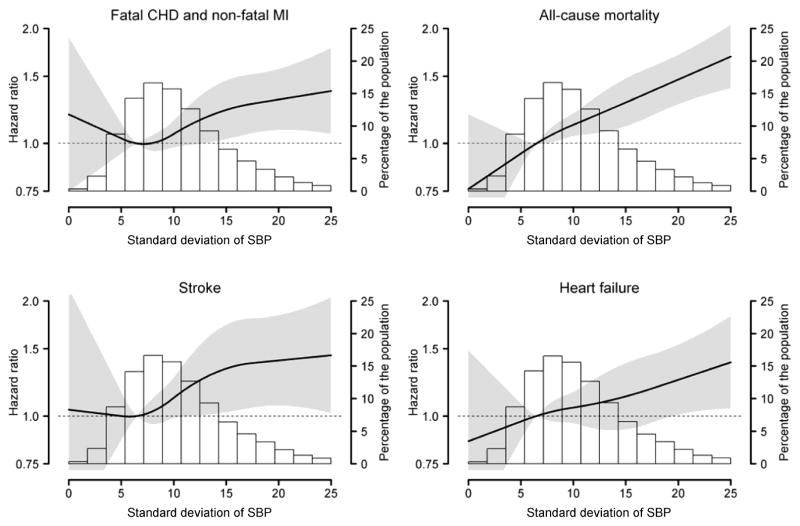
The association between SD of SBP, modeled as a continuous variable, and fatal CHD or non-fatal MI was j-shaped with the risk being lowest at an SD of SBP of ~6 mm Hg (Figure 4). A graded association was present between higher SD of SBP and increased risk for all-cause mortality, stroke and heart failure.
Figure 4.
Hazard ratios for fatal coronary heart disease and non-fatal myocardial infarction, all-cause mortality, stroke, and heart failure associated with intra-individual standard deviation across ALLHAT follow-up visits conducted 6 to 28 months following baseline using restricted quadratic splines.
The solid black line in each figure represents the hazard ratio and the grey shaded area represents the 95% confidence interval. The histogram represents the distribution of standard deviation of systolic blood pressure in the ALLHAT population.
Includes adjustment for the following age, gender, race/ethnicity, region of residence, randomization assignment, smoking status, body mass index, estimated glomerular filtration rate, diabetes, total cholesterol, history of myocardial infarction or stroke, history of coronary revascularization, atrial fibrillation on electrocardiogram, history of other atherosclerotic cardiovascular disease, major ST depression or T wave inversion, left ventricular hypertrophy, low high density lipoprotein cholesterol, aspirin use, use of blood pressure medications prior to study randomization, statin use, pulse pressure, medication adherence, antihypertensive medication classes being taken, changes in antihypertensive medication classes being taken, and mean systolic blood pressure.
DISCUSSION
In this secondary analysis of ALLHAT, we showed strong associations between VVV of SBP and fatal CHD or non-fatal MI, all-cause mortality, stroke and heart failure. These associations were present after multivariable adjustment and was consistent across several sub-groups and when modeling VVV of SBP as a continuous variable. A statistically significant association was also present for VVV of DBP. These data add to the growing body of evidence on the prognostic value of VVV of BP on CVD events and all-cause mortality risks.
In 2010, Rothwell and colleagues published a comprehensive series of analyses from four studies showing strong associations between VVV of BP and stroke and CHD risk.4 For example, comparing the highest to lowest decile of the SD of SBP, the multivariable adjusted hazard ratio for stroke was 4.84 (95% CI: 3.03 – 7.74) in the United Kingdom Transient Ischaemic Attack (TIA) Aspirin Trial; 4.29 (95% CI: 1.78 – 10.4) and 4.39 (95% CI: 1.68 – 11.5) in the Anglo-Scandinavian Cardiac Outcomes Trial Blood Pressure Lowering Arm (ASCOT-BPLA) atenolol group and amlodipine group, respectively; 1.78 (95% CI: 1.21 – 2.62) in the European Stroke Prevention Study; and 3.35 (95% CI: 1.63 – 6.87) in the Dutch TIA trial. Additionally, higher VVV of SBP was associated with an increased risk of CHD. VVV of DBP was also associated with an increased stroke risk in these studies.
Over the past 4 years, a number of studies have evaluated the associations between VVV of BP and outcomes with conflicting results.5,6,20–23 The heterogeneity of study designs used in these analyses may have contributed to the conflicting results. For example, prior studies have varied widely with respect to number of visits used to calculate VVV of BP, duration of time between visits, and study populations enrolled. Some prior studies relied on only three visits to define VVV of BP and the duration of time between visits ranged from days to years across studies.5,24–26 Using data from the Trial of Preventing Hypertension (TROPHY), Levitan and colleagues found that the number of visits and duration of time between visits affect VVV of SBP.13 Given the large sample size, frequent monitoring of BP at set time intervals and long duration of follow-up in ALLHAT, we were able to overcome many of the limitations present in prior analyses.
The association between VVV of BP and fatal CHD and non-fatal MI, all-cause mortality, and heart failure was consistent for ALLHAT participants randomized to chlorthalidone, amlodipine and lisinopril. However, an association between higher VVV of BP and increased stroke risk was present for participants randomized to chlorthalidone but not lisinopril or amlodipine. Given the number of comparisons being made in the subgroup analyses, this finding may be due to chance. Additional studies are needed to confirm this finding and further investigate whether certain drug classes attenuate the effect of VVV of BP on cardiovascular outcomes.
There is potential utility of clarifying the prognostic value of VVV of BP among individuals with hypertension. While antihypertensive treatment reduces the risk of mortality and CVD outcomes, hypertensive individuals with controlled BP continue to have measureable excess risk.27,28 Novel therapies or drug combinations addressing VVV of BP might further reduce this excess risk. Studies of the association of VVV of BP and CVD outcomes may help understand mechanistic links between hypertension and CVD and, thus, lead to more efficacious therapy.
Low medication adherence has been proposed as a mechanism underlying the increased CVD risk at higher levels of VVV of BP.29 In a prior study, Muntner and colleagues found only a small percentage of VVV of BP could be explained by low medication adherence.30 Also, higher VVV of SBP was associated with increased stroke risk among individuals with high adherence in the ASCOT-BPLA.4 In the current study, associations of higher CVD and mortality risk at higher VVV of BP were present after adjustment for medication adherence and in analyses restricted to participants with high adherence at every study visit. Taken together, these data suggest that medication adherence does not explain the association of VVV of SBP and outcomes.
Arterial stiffness, endothelial dysfunction and subclinical inflammation have been proposed as mechanisms underlying the association between VVV of BP and outcomes.28–33 Shimbo and colleagues found that higher levels of VVV of SBP were associated with both lower aortic distensibility by magnetic resonance imaging and lower large and small artery elasticity, assessed by pulse contour analysis.31 Also, Nagai and colleagues reported an association between common carotid artery stiffness and VVV of BP.32 Diaz and colleagues found decreased endothelial function to be associated with higher VVV of BP.33 We are not aware of formal studies of the association of VVV of BP with subclinical inflammation. Studies aimed at better understanding the reasons underlying high VVV of BP may provide useful information for reducing CVD risk.
There are many strengths associated with using ALLHAT data to study VVV of BP and outcomes including a large sample size of whites, blacks and Hispanics. BP was measured in ALLHAT following a standardized protocol during visits at set time points. For outcomes reported here, events were ascertained and diagnoses assigned following a standardized protocol. Additional strengths of ALLHAT include its collection of a large number of potential confounders. The association between higher VVV of BP and outcomes after multivariable adjustment for these variables is noteworthy. There are also known and potential limitations associated with the current analysis. The analysis we present was a post-hoc analysis of ALLHAT using an observational study design. Therefore, causality cannot be determined. ALLHAT is a community-clinical-practice based randomized trial, which may limit the generalizability of the current analysis. Although outcomes were collected and ascertained following a standardized protocol, given the size of ALLHAT, adjudication by a blinded committee did not occur for all events. Approximately 30% of ALLHAT participants did not have ≥5 BP measurements during the VVV of BP assessment period. For these participants, we imputed their VVV of BP. Additionally, many patients changed antihypertensive medication regimens during the VVV of BP assessment period which could affect the associations we report. While we conducted statistical adjustment for regimen changes, residual confounding may remain present. Low adherence was defined based on self-report and more robust information was not available.
In this secondary analysis of ALLHAT, higher VVV of SBP and VVV of DBP were associated with an increased risk for CVD and all-cause mortality. The current study adds to the growing body of evidence on the prognostic importance of VVV of BP as a CVD risk factor. Future studies are needed to identify the mechanisms underlying high VVV of BP and its relationship with CVD and mortality, and to determine whether lowering VVV of BP reduces this risk.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under contracts NO1-HC-35130 and HHSN268201100036C and under Award Number R01 HL110993. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial investigators acknowledge study medications contributed by Pfizer, Inc., (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURE
Dr. Muntner has received research support and an honorarium from Amgen Inc.
Dr. Levitan has received research support from Amgen Inc.
Dr. Cushman has received research support from Merck Inc. and Eli Lilly Inc.
Dr. Oparil has received honoraria from Daiichi Sankyo Inc. and Novartis Inc.
No other authors have disclosures to report.
This study was presented as a poster at the 2014 American Society of Hypertension meeting on May 19, 2014 in New York, NY.
Study protocol
Available from Paul Muntner (pmuntner@uab.edu)
Statistical code: not available
Data set: available from: https://biolincc.nhlbi.nih.gov/home/
References
- 1.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 2.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999 Aug 15;150(4):341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 3.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease: Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990 Mar 31;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010 Mar 13;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011 Feb;57(2):160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 6.Shimbo D, Newman JD, Aragaki AK, et al. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women's Health Initiative. Hypertension. 2012 Sep;60(3):625–630. doi: 10.1161/HYPERTENSIONAHA.112.193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hata J, Arima H, Rothwell PM, et al. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation. 2013 Sep 17;128(12):1325–1334. doi: 10.1161/CIRCULATIONAHA.113.002717. [DOI] [PubMed] [Google Scholar]
- 8.McMullan CJ, Bakris GL, Phillips RA, Forman JP. Association of BP variability with mortality among African Americans with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2013 May;8(5):731–738. doi: 10.2215/CJN.10131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation. 2012 Jul 31;126(5):569–578. doi: 10.1161/CIRCULATIONAHA.112.107565. [DOI] [PubMed] [Google Scholar]
- 10.Carr MJ, Bao Y, Pan J, Cruickshank K, McNamee R. The predictive ability of blood pressure in elderly trial patients. Journal of Hypertension. 2012 Sep;30(9):1725–1733. doi: 10.1097/HJH.0b013e3283568a73. [DOI] [PubMed] [Google Scholar]
- 11.Brickman AM, Reitz C, Luchsinger JA, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67(5):564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pringle E, Phillips C, Thijs L, et al. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21(12):2251–2257. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Blood Pressure Measurement Device, Number and Timing of Visits, and Intra-Individual Visit-to-Visit Variability of Blood Pressure. Journal of clinical hypertension. 2012 Nov;14(11):744–750. doi: 10.1111/jch.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis BR, Cutler JA, Gordon DJ, et al. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. Am J Hypertens. 1996;9(4 Pt 1):342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 15.Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) ALLHAT Collaborative Research Group. JAMA. 2000 Apr 19;283(15):1967–1975. [PubMed] [Google Scholar]
- 16.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002 Dec 18;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 17.Cushman WC, Ford CE, Einhorn PT, et al. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Journal of clinical hypertension. 2008 Oct;10(10):751–760. doi: 10.1111/j.1751-7176.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Relationships between metrics of visit-to-visit variability of blood pressure. Journal of human hypertension. 2013 doi: 10.1038/jhh.2013.19. In press. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability in the European Lacidipine Study on Atherosclerosis: methodological aspects and effects of antihypertensive treatment. J Hypertens. 2012 Jun;30(6):1241–1251. doi: 10.1097/HJH.0b013e32835339ac. [DOI] [PubMed] [Google Scholar]
- 21.Poortvliet RKE, Ford I, Lloyd SM, et al. Blood Pressure Variability and Cardiovascular Risk in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) PLoS ONE [Electronic Resource] 2012;7(12):e52438. doi: 10.1371/journal.pone.0052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hastie CE, Jeemon P, Coleman H, et al. Long-term and ultra long-term blood pressure variability during follow-up and mortality in 14,522 patients with hypertension. Hypertension. 2013 Oct;62(4):698–705. doi: 10.1161/HYPERTENSIONAHA.113.01343. [DOI] [PubMed] [Google Scholar]
- 23.Eguchi K, Hoshide S, Schwartz JE, Shimada K, Kario K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. American Journal of Hypertension. 2012 Sep;25(9):962–968. doi: 10.1038/ajh.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yinon L, Chen Y, Parvez F, et al. A prospective study of variability in systolic blood pressure and mortality in a rural Bangladeshi population cohort. Preventive medicine. 2013 Dec;57(6):807–812. doi: 10.1016/j.ypmed.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tozawa M, Iseki K, Yoshi S, Fukiyama K. Blood pressure variability as an adverse prognostic risk factor in end-stage renal disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1999 Aug;14(8):1976–1981. doi: 10.1093/ndt/14.8.1976. [DOI] [PubMed] [Google Scholar]
- 26.Di Iorio B, Pota A, Sirico ML, et al. Blood pressure variability and outcomes in chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012 Sep 7;27(12):4404–4410. doi: 10.1093/ndt/gfs328. [DOI] [PubMed] [Google Scholar]
- 27.Lawlor DA, Kim L, Morris R, Amuzu A, Whincup P, Ebrahim S. Survival with treated and well-controlled blood pressure: findings from a prospective cohort study. PloS one. 2011;6(4):e17792. doi: 10.1371/journal.pone.0017792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson OK, Almgren T, Persson B, Samuelsson O, Hedner T, Wilhelmsen L. Survival in treated hypertension: follow up study after two decades. BMJ. 1998 Jul 18;317(7152):167–171. doi: 10.1136/bmj.317.7152.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krakoff LR. Fluctuation: does blood pressure variability matter? Circulation. 2012 Jul 31;126(5):525–527. doi: 10.1161/CIRCULATIONAHA.112.124750. [DOI] [PubMed] [Google Scholar]
- 30.Muntner P, Levitan EB, Joyce C, et al. Association between antihypertensive medication adherence and visit-to-visit variability of blood pressure. Journal of clinical hypertension. 2013 Feb;15(2):112–117. doi: 10.1111/jch.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimbo D, Shea S, McClelland RL, et al. Associations of aortic distensibility and arterial elasticity with long-term visit-to-visit blood pressure variability: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertens. 2013 Jul;26(7):896–902. doi: 10.1093/ajh/hpt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. Journal of the American Society of Hypertension : JASH. 2011 May-Jun;5(3):184–192. doi: 10.1016/j.jash.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Diaz KM, Veerabhadrappa P, Kashem MA, et al. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertension research : official journal of the Japanese Society of Hypertension. 2012 Jan;35(1):55–61. doi: 10.1038/hr.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.