Abstract
Response rates of unselected non–small cell lung cancer (NSCLC) patients to the epidermal growth factor receptor inhibitor erlotinib are low and range from 10% to 20%. Early response assessments are needed to avoid costs and side effects of inefficient treatments. Here we determined whether early changes in tumor uptake of 18F-FDG can predict progression-free and overall survival in NSCLC patients who are treated with erlotinib.
Methods
Twenty-two patients (6 men, 16 women; mean age ± SD, 64 ± 13 y) with stage III or stage IV NSCLC who received erlotinib treatment were enrolled prospectively. 18F-FDG PET/CT was performed before the initiation of treatment (n = 22), after 2 wk (n = 22), and after 78 ± 21 d (n = 11). Tumor maximum standardized uptake values were measured for a maximum of 5 lesions for each patient. Tumor responses were classified using modified PET Response Criteria in Solid Tumors (use of maximum standardized uptake values). Median overall survival by Kaplan–Meier analysis was compared between groups using a log-rank test.
Results
The overall median time to progression was 52 d (95% confidence interval, 47–57 d). The overall median survival time was 131 d (95% confidence interval, 0–351 d). Patients with progressive metabolic disease on early follow-up PET showed a significantly shorter time to progression (47 vs. 119 d; P < 0.001) and overall survival (87 vs. 828 d; P = 0.01) than patients classified as having stable metabolic disease or partial or complete metabolic response.
Conclusion
These data suggest that 18F-FDG PET/CT performed early after the start of erlotinib treatment can help to identify patients who benefit from this targeted therapy.
Keywords: 18F-FDG, PET/CT, NSCLC, erlotinib, treatment monitoring
Kinase inhibitors targeting the epidermal growth factor receptor (EGFR) can improve progression-free (PFS) and overall survival (OS) in some non–small cell lung cancer (NSCLC) patients (1–4). For instance, the OS was 6.7 and 4.7 mo in NSCLC patients treated with erlotinib (Tarceva; Astellas Pharma Inc.) versus placebo after the failure of first-line or second-line chemotherapy (P < 0.001) (5). Response rates in patients with specific EGFR mutations were higher than those without these mutations (1). However, even for the latter group, significantly improved PFS and OS were reported (6). Nevertheless, overall response rates to erlotinib are modest and survival benefits are limited. Given the less than perfect predictability of erlotinib responses by EGFR genotyping and the considerable costs of this treatment, different approaches to assess treatment efficacy early during the course of therapy are needed.
18F-FDG PET and 18F-FDG PET/CT improve the staging of NSCLC (7–10). Moreover, early glucose metabolic PET during cytotoxic therapy predicts long-term patient survival (11,12).
Two recently published studies have investigated the usefulness of 18F-FDG PET/CT for predicting responses to first-line treatment with erlotinib in NSCLC patients (13,14). In one study, erlotinib was given as neoadjuvant treatment (13), and the second study was performed in unselected patients with advanced disease (14).
However, in clinical practice, erlotinib is frequently administered as a second- or third-line treatment in patients for whom multiple other therapies have failed. Thus, the reported ability of 18F-FDG PET to predict treatment response to erlotinib as first-line therapy might not apply to these patients. Only 1 group has reported that early changes in tumor 18F-FDG uptake in response to second- or third-line EGFR inhibition are predictive of OS and PFS (15). The current study aimed to determine whether early 18F-FDG PET/CT is able to predict response and outcome in unselected patients with advanced NSCLC using the recently proposed criteria for assessment of tumor response by 18F-FDG PET (PET response criteria in solid tumors [PERCIST]) (16).
MATERIALS AND METHODS
Twenty-two patients (age, >18 y) with stage IIIB or IV NSCLC who were scheduled to undergo erlotinib treatment were enrolled in this study.
A baseline 18F-FDG PET/CT scan was obtained 7 ± 9 d (median, 3 d; range, 0–32 d) before the start of erlotinib treatment, followed by an early follow-up 18F-FDG PET/CT study 14 ± 1 d (median, 14 d; range, 13–19 d) after the initiation of erlotinib therapy.
Eleven patients (50%) underwent a third 18F-FDG PET/CT study 78 ± 21 d (median, 89 d; range, 49–104 d) after the start of erlotinib treatment. In the remaining 11 patients, therapy was discontinued before the third scan could be obtained.
The study endpoints were PFS and OS of metabolic responders and nonresponders. All patients gave written informed consent to participate. This study was approved by the UCLA Institutional Review Board and the UCLA Medical Radiation Safety Committee.
PET/CT Image Acquisition
To standardize imaging conditions, patients were instructed to fast for at least 6 h before 18F-FDG PET/CT. Blood glucose levels were measured before the injection of 18F-FDG. Only patients with serum glucose levels less than 150 mg/dL were included (17).
18F-FDG PET/CT studies were performed in 12 patients on a dual-slice PET/CT scanner and in 10 patients on a 64-slice PET/CT scanner. The CT image acquisition parameters were 130 kVp, 120 mAs, 1-s rotation, 4-mm slice collimation, and 8-mm/s bed speed.
Patients were injected intravenously with 18F-FDG (7.77 MBq [0.21 mCi]/kg) at a median of 75 min before image acquisition. PET emission scan duration per bed position ranged between 1 and 5 min, depending on patient body weight, as previously described (18,19).
To minimize misregistration between the CT and PET images, patients were instructed to use shallow breathing during the image acquisition (20). The CT images were reconstructed using conventional filtered backprojection, at 3.4-mm axial intervals to match the slice separation of the PET data.
PET images were reconstructed using iterative algorithms (ordered-subset expectation maximization, 2 iterations, 8 subsets). To correct for photon attenuation, a previously published CT-based algorithm was applied (21).
Image Analysis
PET/CT scans were analyzed using the Osirix software. The maximum standardized uptake value (SUVmax) was used to measure tumor 18F-FDG uptake in up to 5 tumor lesions per patient on baseline and follow-up scans, and changes in tumor SUVmax were recorded.
To quantify tumor 18F-FDG uptake, loosely fitting regions of interest covering the whole tumor were placed manually over every axial image plane in which tumor tissue was visualized by tumor 18F-FDG uptake (22). Then the SUVmax in this set of regions of interest was determined.
Side-by-side image review and analysis were performed to ascertain that the SUVmax was derived from the same lesions on baseline and follow-up scans.
On the basis of the previously published PERCIST criteria, patients were classified as complete metabolic responders (CMR; complete resolution of tumor 18F-FDG uptake), partial metabolic responders (PMR; reduction of a minimum of 30% in target measurable lesion), stable metabolic disease (SMD; not CMR, PMR, or progressive metabolic disease (PMD)), or progressive metabolic disease (PMD; increase of a minimum of 30% in target measurable lesion or presentation of a new lesion) (16). In contrast to the PERCIST suggestions, tumor SUVmax rather than peak SUV was measured.
Follow-up of patients was performed by chart review, clinical assessments by the treating physician, and follow-up anatomic imaging (radiography, CT, or MRI) and laboratory testing. Disease progression was determined by response evaluation criteria in solid tumors (version 1.0) on follow-up imaging (23). Time to progression was calculated from initiation of erlotinib to first evidence of progression.
Statistical Analysis
Quantitative data are expressed as median, mean ± SD, and range. Median OS was estimated by Kaplan–Meier analysis. Survival in patients with and without metabolic response was compared by log-rank test. Time to progression and death served as endpoints.
Data were analyzed using PASW Statistics 18 (IBM SPSS, IBM Corp.) for Windows (Microsoft). P values less than 0.05 were considered statistically significant.
RESULTS
Patient and Tumor Characteristics
Sixteen women (73%) and 6 men (27%) (mean age ± SD, 64 ± 13 y; median, 64 y; age range, 42–86 y) were included in the study. Ten of the 22 patients (45%) had a history of smoking. The study population included 14 Caucasians (64%), 6 Asians (27%; Asians have a higher response rate to EGFR inhibitors, compared with other races), and 2 others (9%). The histopathologic diagnoses were adenocarcinoma (n = 17; 77%), squamous cell carcinoma (n = 3; 14%), large cell carcinoma (n = 1; 4%), and lung carcinoma not otherwise specified (n = 1; 4%). Thus, the population was enriched for patients who were more likely to respond to erlotinib treatment (24–26).
At the time of enrollment, 3 patients (14%) had stage IIIB and 19 (86%) had stage IV disease. Seven patients (32%) presented with nonresectable primary disease and 15 patients (68%) with recurrent or residual disease.
Inclusion was not based on the mutational status of the EGFR, which was known in only 5 of 22 patients (positive for EGFR mutation, n = 4; negative for EGFR mutation, n = 1).
Seventeen patients (77%) received erlotinib as a single drug. Erlotinib was combined with the estrogen receptor antagonist fulvestrant in 3 patients (14%) and with the nonsteroidal antiinflammatory drug celecoxib in 2 patients (9%).
Seven of 22 patients received erlotinib as first-line treatment, and 15 of 22 patients had other prior therapies (chemotherapy in 7, radiotherapy in 2, combined chemo- and radiotherapy in 4, and resection in combination with chemo- and radiotherapy in 2 patients).
The patient characteristics are depicted in Table 1.
TABLE 1.
Clinical, Pathologic, and Treatment Characteristics (n = 22)
| Characteristic | n |
|---|---|
| Sex | |
| Male | 6 (27) |
| Female | 16 (73) |
| Presentation status | |
| Primary | 7 (32) |
| Recurrent or residual | 15 (68) |
| Histology | |
| Adenocarcinoma | 17 (77) |
| Squamous cell carcinoma | 3 (14) |
| Not otherwise specified | 1 (4.5) |
| Large cell carcinoma | 1 (4.5) |
| Stage | |
| IIIB | 3 (14) |
| IV | 19 (86) |
| Tumor size (cm) | |
| Median | 2.4 |
| Range | 1.0–9.0 |
| Smoker | |
| Yes | 10 (45) |
| No | 12 (55) |
| Treatment | |
| Erlotinib | 17 (77) |
| Erlotinib plus fulvestrant | 3 (14) |
| Erlotinib plus celecoxib | 2 (9) |
Data in parentheses are percentages. Median age was 64 y, and age range was 42–86 y.
Seventy-five lesions were analyzed on baseline scans and on the corresponding early follow-up scans. One to 5 lesions per patient were analyzed (median, 3.0 lesions per patient). In the subgroup of patients with three 18F-FDG PET/CT scans, 41 lesions were evaluated at baseline, early, and late follow-up scans (median, 4.0 lesions; range, 2–5 lesions).
Tumor 18F-FDG Uptake
The tumor SUVmax of the most 18F-FDG–avid lesion averaged 11.0 ± 4.9 g/mL (median, 10.9 g/mL; range, 1.8–19.8 g/mL) at baseline and decreased to 9.6 ± 7.4 g/mL (median, 8.0 g/mL; range, 0.7–24.4 g/mL) at early follow-up (P = 0.25).
The tumor SUVmax of all analyzed tumor lesions averaged 8.0 ± 4.2 g/mL (median, 8.0 g/mL; range, 1.4–19.8 g/mL) at baseline and 6.3 ± 5.2 g/mL (median, 4.7 g/mL; range, 0.6–24.4 g/mL) at early follow-up (P = 0.001).
Metabolic Response Classification According to PERCIST
Early 18F-FDG PET classified 6 patients (27%) as CMR–PMR, 7 patients (32%) as SMD, and 9 patients as PMD (41%).
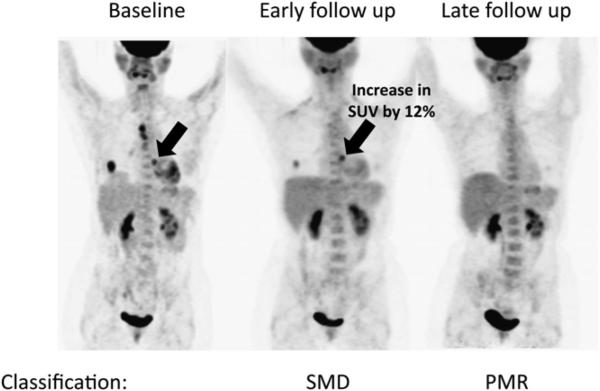
In 9 of 11 patients (82%), the late follow-up PET scan revealed response information concordant with the early follow-up PET studies. Discordant response information was evident in 2 patients who were classified as SMD on the early follow-up PET but as PMR (Fig. 1) and PMD on the late follow-up PET scans.
FIGURE 1.
Baseline, early, and late follow-up PET of 62-y-old woman with stage IV adenocarcinoma of lung. Patient was classified as SMD on early follow-up PET but as PMR on late follow-up PET.
Early Changes in 18F-FDG Uptake Versus Time to Progression
The overall median time to progression was 52 d (95% confidence interval [CI], 47–57 d).
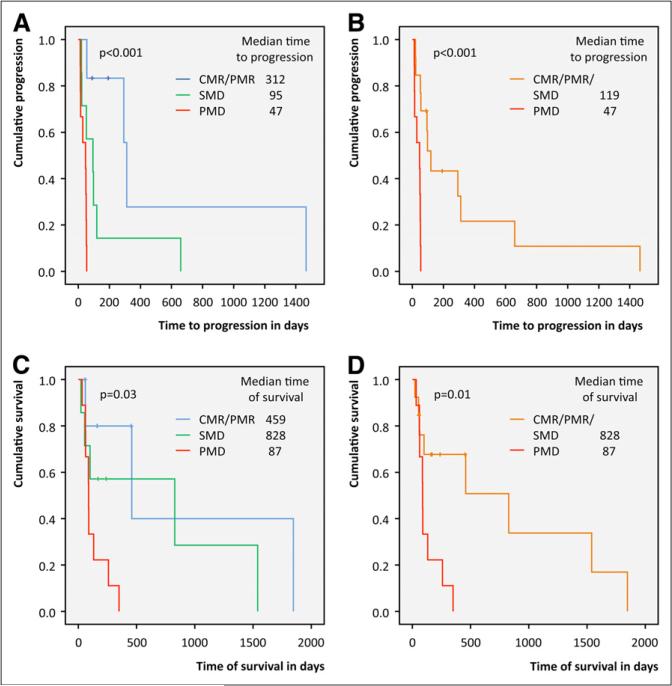
Patients classified as PMD on early follow-up PET had a median time to progression of 47 d (95% CI, 0–103 d), whereas it was 95 d (95% CI, 0–205 d) and 312 d (95% CI, 208–343 d) in patients with SMD, or PMR or CMR (P < 0.001), respectively (Fig. 2A).
FIGURE 2.
Time to progression (A and B) and OS (C and D) in patients stratified by modified PERCIST.
When grouped together, patients with SMD and those with PMR or CMR had a median time to progression of 119 d (95% CI, 80–158 d), as compared with 47 d (95% CI, 0–103 d) for patients with PMD (P < 0.001) (Fig. 2B).
When SMD patients were combined with those with PMD, the time to progression was significantly shorter than that of patients with PMR and CMR (P = 0.005) (median time to progression, 49 d [95% CI, 41–57 d] vs. 312 d [95% CI, 281–343 d]).
Early Changes in 18F-FDG Uptake Versus OS
The median OS duration was 131 d (95% CI, 0–351 d).
Patients classified as PMD on early follow-up PET showed a significantly shorter OS than patients classified as SMD, or PMR or CMR (P = 0.03) (median OS, 87 d [95% CI, 84–90 d], 828 d [95% CI, 0–1,938 d], and 459 d [95% CI, 0–1,039 d], respectively) (Fig. 2C).
The overlap in survival between the groups of early CMR or PMR and SMD (Fig. 2C) provided the justification to pool metabolic responders and patients with SMD into 1 group. Stratifying patients into SMD plus PMR or CMR versus PMD yielded comparable results (P = 0.01) (median OS, 87 d [95% CI, 84–90 d] and 828 d [95% CI, 62–1,594 d], respectively) (Fig. 2D).
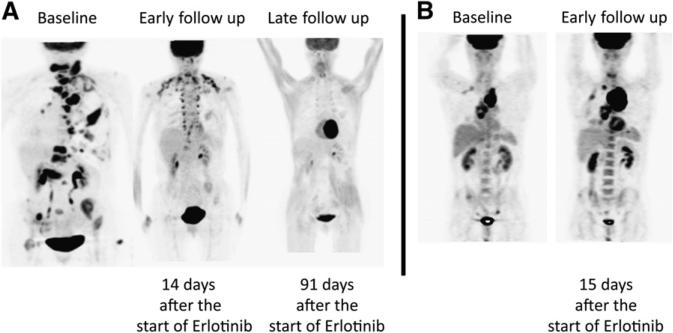
In contrast, the median OS of SMD plus PMD patients did not differ from that of patients with PMR plus CMR (P = 0.07) (89 d [95% CI, 62–116 d] vs. 459 d [95% CI, 0–1,039 d], respectively). An imaging example of a patient classified as PMR and PMD is shown in Figure 3.
FIGURE 3.
18F-FDG PET at baseline, early follow-up, and late follow-up in patient classified as PMR and PMD.
DISCUSSION
The current study demonstrates that changes in tumor 18F-FDG uptake at approximately 2 wk after the start of erlotinib treatment can identify erlotinib responders and nonresponders. These findings are relevant because early identification of treatment response versus nonresponse could potentially reduce unnecessary annual costs, ranging from $14,800 to $26,400/patient (27), and side effects, including rash and diarrhea, that occur in up to 60% of patients (6).
18F-FDG uptake decreased within 2 h in H3255 cell lines that harbor sensitizing EGFR mutations after incubation with 0.2 μM (28) of gefitinib. This effect was due to translocation of glucose transporters from the cell membrane to the cytoplasm. A similar effect on 18F-FDG tumor uptake was observed in animal experiments after only 2 doses of gefitinib (28).
These studies provided the motivation to determine the ability of 18F-FDG PET for early treatment response assessments to EGFR inhibition in humans. NSCLC patients with EGFR mutations derive the greatest benefit from erlotinib treatment. The response rate in a previous study in patients with metastatic NSCLC and EGFR mutations who had not previously received chemotherapy was 73.7% (2). However, EGFR mutations are present in only 10%–20% of all NSCLC patients. Therefore, the response rates in unselected populations range from only 10% to 19% (29,30). However, EGFR inhibition also resulted in improved survival in some patients without specific mutations (6). The presence of specific mutations is therefore not the only determinant of responses to erlotinib. Secondary EGFR mutations (31,32) or the activation of other oncogenes, such as KRAS, can also cause resistance to EGFR kinase inhibitors (33). Moreover, the EGFR status of primary lung carcinomas differs from that of metastatic lesions in more than 30% of patients (34).
Predicting tumor responses to EGFR inhibition by genotyping is therefore not entirely reliable. Consequently, effective approaches to determine the net effects of therapeutic interventions on tumor growth and viability early during the course of treatment are needed. In concordance with other studies, we found that such response assessments can be performed successfully in human lung cancer patients (13–15).
However, there are several differences between the current report and other reports. In 2 studies, erlotinib was administered in previously untreated patients (13,14). In one of these studies, changes in 18F-FDG tumor uptake were correlated with histopathologic responses, but no survival analysis was performed (13). EGFR kinase inhibitors are increasingly used as second- or third-line treatment. To serve as a clinically useful intermediate endpoint biomarker, 18F-FDG PET needs to provide accurate response assessments in these patients. The current unselected population appropriately represents these patients.
Consistent with our findings, Mileshkin et al. (15) reported that early glucose metabolic responses by PET were associated with improved PFS and OS in patients who failed conventional chemotherapy. In their study, metabolic response was assessed according to the criteria of the European Organization for Research and Treatment of Cancer, whereas we relied on the more recently published PERCIST criteria (16). However, both response assessment systems provided identical survival predictions in the current study.
The outcome of patients with early SMD is thus far unknown, as illustrated in Figure 1. The 18F-FDG uptake of 3 thoracic tumor lesions decreased significantly as early as 14 d after the start of erlotinib therapy. However, 1 lesion exhibited a minor increase in 18F-FDG uptake on early follow-up PET. According to PERCIST, this patient was classified as SMD. Near-complete disappearance of metabolic tumor activity 91 d after the initiation of erlotinib reclassified this patient as PMR.
Such discordant findings between early and late 18F-FDG PET were observed in 2 of 11 patients who were initially classified as having stable disease, suggesting that a late scan might be important for correctly classifying responses to EGFR inhibition, especially in patients with SMD on early 18F-FDG PET.
The current study has some limitations. First, 16 of 22 patients were women; this higher number of women is likely explained by the fact that lung cancer in never-smokers affects women disproportionately more than men and that never-smokers have higher response rates to EGFR inhibition (35). Second, the EGFR mutational status was unknown in all but 5 patients. Four of these had sensitizing mutations. Three of these 4 patients were metabolic responders on early and late follow-up PET, whereas 1 had SMD. The mutational status was unknown in 17 patients, likely reflecting the clinical practice during the enrollment period when EGFR sequencing was not routinely done. Third, 5 of 22 patients received the estrogen receptor fulvestrant (n = 3) or the nonsteroidal antiinflammatory drug celecoxib (n = 2), which, although unlikely, might have affected tumor glucose metabolic activity.
Fourth, only 11 of 22 patients underwent a late follow-up PET scan. It is therefore unknown whether additional patients might have exhibited discordant responses between early and late follow-up. Future studies will be needed to determine whether discordant responses are indeed limited to patients initially classified as having SMD.
CONCLUSION
Our results may suggest that early changes in tumor 18F-FDG uptake can predict PFS and OS in unselected patients undergoing treatment with an EGFR inhibitor. Future studies will need to confirm these findings in larger study populations and will have to address the potential need for a third PET scan to elucidate the outcome of patients with SMD on early follow-up PET.
ACKNOWLEDGMENT
No potential conflict of interest relevant to this article was reported.
Footnotes
DISCLOSURE STATEMENT
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 6.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosini V, Nicolini S, Caroli P, et al. PET/CT imaging in different types of lung cancer: an overview. Eur J Radiol. 2011;78:30–40. doi: 10.1016/j.ejrad.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 9.Maziak DE, Darling GE, Inculet RI, et al. Positron emission tomography in staging early lung cancer: a randomized trial. Ann Intern Med. 2009;151:221–228. W–248. doi: 10.7326/0003-4819-151-4-200908180-00132. [DOI] [PubMed] [Google Scholar]
- 10.Schreyogg J, Weller J, Stargardt T, et al. Cost-effectiveness of hybrid PET/CT for staging of non-small cell lung cancer. J Nucl Med. 2010;51:1668–1675. doi: 10.2967/jnumed.109.072090. [DOI] [PubMed] [Google Scholar]
- 11.Hicks RJ. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J Nucl Med. 2009;50(suppl 1):31S–42S. doi: 10.2967/jnumed.108.057216. [DOI] [PubMed] [Google Scholar]
- 12.Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–2657. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Aukema TS, Kappers I, Olmos RA, et al. Is 18F-FDG PET/CT useful for the early prediction of histopathologic response to neoadjuvant erlotinib in patients with non-small cell lung cancer? J Nucl Med. 2010;51:1344–1348. doi: 10.2967/jnumed.110.076224. [DOI] [PubMed] [Google Scholar]
- 14.Zander T, Scheffler M, Nogova L, et al. Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using [18F]fluorodeoxyglucose and [18F]fluorothymidine positron emission tomography. J Clin Oncol. 2011;29:1701–1708. doi: 10.1200/JCO.2010.32.4939. [DOI] [PubMed] [Google Scholar]
- 15.Mileshkin L, Hicks RJ, Hughes BG, et al. Changes in 18F-fluorodeoxyglucose and 18F-fluorodeoxythymidine positron emission tomography imaging in patients with non-small cell lung cancer treated with erlotinib. Clin Cancer Res. 2011;17:3304–3315. doi: 10.1158/1078-0432.CCR-10-2763. [DOI] [PubMed] [Google Scholar]
- 16.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 18.Halpern BS, Dahlbom M, Auerbach MA, et al. Optimizing imaging protocols for overweight and obese patients: a lutetium orthosilicate PET/CT study. J Nucl Med. 2005;46:603–607. [PubMed] [Google Scholar]
- 19.Halpern BS, Dahlbom M, Quon A, et al. Impact of patient weight and emission scan duration on PET/CT image quality and lesion detectability. J Nucl Med. 2004;45:797–801. [PubMed] [Google Scholar]
- 20.Beyer T, Antoch G, Muller S, et al. Acquisition protocol considerations for combined PET/CT imaging. J Nucl Med. 2004;45(suppl 1):25S–35S. [PubMed] [Google Scholar]
- 21.Kinahan PE, Townsend DW, Beyer T, Sashin D. Attenuation correction for a combined 3D PET/CT scanner. Med Phys. 1998;25:2046–2053. doi: 10.1118/1.598392. [DOI] [PubMed] [Google Scholar]
- 22.Benz MR, Evilevitch V, Allen-Auerbach MS, et al. Treatment monitoring by 18F-FDG PET/CT in patients with sarcomas: interobserver variability of quantitative parameters in treatment-induced changes in histopathologically responding and nonresponding tumors. J Nucl Med. 2008;49:1038–1046. doi: 10.2967/jnumed.107.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial). J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 25.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 26.Miller VA, Kris MG, Shah N, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:1103–1109. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 27. [September 19, 2011];Erlotinib Pricing & Ordering Comparisons. Available at: www.pharmacychecker. com/Pricing.asp?DrugName5Erlotinib&DrugId565757&DrugStrengthId5124815.
- 28.Su H, Bodenstein C, Dumont RA, et al. Monitoring tumor glucose utilization by positron emission tomography for the prediction of treatment response to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2006;12:5659–5667. doi: 10.1158/1078-0432.CCR-06-0368. [DOI] [PubMed] [Google Scholar]
- 29.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 30.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 32.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monaco SE, Nikiforova MN, Cieply K, Teot LA, Khalbuss WE, Dacic S. A comparison of EGFR and KRAS status in primary lung carcinoma and matched metastases. Hum Pathol. 2010;41:94–102. doi: 10.1016/j.humpath.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]