Abstract
IMPORTANCE
Abnormal cardiac metabolism contributes to the pathophysiology of advanced heart failure with reduced left ventricular ejection fraction (LVEF). Glucagon-like peptide 1 (GLP-1) agonists have shown cardioprotective effects in early clinical studies of patients with advanced heart failure, irrespective of type 2 diabetes status.
OBJECTIVE
To test whether therapy with a GLP-1 agonist improves clinical stability following hospitalization for acute heart failure.
DESIGN, SETTING, AND PARTICIPANTS
Phase 2, double-blind, placebo-controlled randomized clinical trial of patients with established heart failure and reduced LVEF who were recently hospitalized. Patients were enrolled between August 2013 and March 2015 at 24 US sites.
INTERVENTIONS
The GLP-1 agonist liraglutide (n = 154) or placebo (n = 146) via a daily subcutaneous injection; study drug was advanced to a dosage of 1.8 mg/d during the first 30 days as tolerated and continued for 180 days.
MAIN OUTCOMES AND MEASURES
The primary end point was a global rank score in which all patients, regardless of treatment assignment, were ranked across 3 hierarchical tiers: time to death, time to rehospitalization for heart failure, and time-averaged proportional change in N-terminal pro-B-type natriuretic peptide level from baseline to 180 days. Higher values indicate better health (stability). Exploratory secondary outcomes included primary end point components, cardiac structure and function, 6-minute walk distance, quality of life, and combined events.
RESULTS
Among the 300 patients who were randomized (median age, 61 years [interquartile range {IQR}, 52–68 years]; 64 [21%] women; 178 [59%] with type 2 diabetes; median LVEF of 25% [IQR, 19%–33%]; median N-terminal pro-B-type natriuretic peptide level of 2049 pg/mL [IQR, 1054–4235 pg/mL]), 271 completed the study. Compared with placebo, liraglutide had no significant effect on the primary end point (mean rank of 146 for the liraglutide group vs 156 for the placebo group, P = .31). There were no significant between-group differences in the number of deaths (19 [12%] in the liraglutide group vs 16 [11%] in the placebo group; hazard ratio, 1.10 [95% CI, 0.57–2.14]; P = .78) or rehospitalizations for heart failure (63 [41%] vs 50 [34%], respectively; hazard ratio, 1.30 [95% CI, 0.89–1.88]; P = .17) or for the exploratory secondary end points. Prespecified subgroup analyses in patients with diabetes did not reveal any significant between-group differences. The number of investigator-reported hyperglycemic events was 16 (10%) in the liraglutide group vs 27 (18%) in the placebo group and hypoglycemic events were infrequent (2 [1%] vs 4 [3%], respectively).
CONCLUSIONS AND RELEVANCE
Among patients recently hospitalized with heart failure and reduced LVEF, the use of liraglutide did not lead to greater posthospitalization clinical stability. These findings do not support the use of liraglutide in this clinical situation.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01800968
Heart failure is the leading cause of hospitalization in the United States with more than 4 million admissions per year from 2003–2009.1 Abnormal cardiac metabolism, including reduced fatty-acid oxidation and myocardial insulin resistance, contributes to the syndrome of heart failure.2 As heart failure progresses, these abnormalities become more pronounced and are observed in both patients with and without type 2 diabetes.3,4 No current heart failure therapy targets these metabolic derangements. In this context, agents that improve glucose metabolism could be repurposed as new therapies for patients with advanced heart failure.
Agents that increase glucagon-like peptide 1 (GLP-1) signaling have shown potential in preclinical and early clinical studies. Glucagon-like peptide 1 is an endogenous incretin hormone that improves insulin sensitivity with minimal risk of hypoglycemia. Recombinant GLP-1 increases myocardial insulin sensitivity5 and is cardioprotective during ischemia in model systems.6
In a pilot study,7 recombinant GLP-1 was associated with favorable effects on myocardial function and exercise tolerance in patients with advanced heart failure and reduced left ventricular ejection fraction (LVEF), and GLP-1 agonists reduced rates of hospitalization for patients with heart failure in a single-center, retrospective analysis.8 Together, these data suggest potential benefit of GLP-1 agonists for patients with advanced heart failure.
We performed the Functional Impact of GLP-1 for Heart Failure Treatment (FIGHT) study to test the hypothesis that sustained therapy with a GLP-1 agonist initiated during the postacute hospital discharge period is associated with greater clinical stability through 180 days in patients with advanced heart failure and reduced LVEF. Furthermore, we hypothesized that the treatment effects would be greater in patients with type 2 diabetes.
Methods
Study Design
The FIGHT trial was a multicenter, double-blind, placebo-controlled randomized clinical trial of patients with established heart failure and reduced LVEF. The trial was conducted by the NHLBI (National Heart, Lung, and Blood Institute) Heart Failure Clinical Research Network, which is funded by the NHLBI, with approval by the research network’s protocol review committee and monitored by an independent data and safety monitoring board. The ethics committee at each participating site approved the trial design (the full protocol appears in Supplement 2).
At 24 sites in the United States, patients with heart failure and reduced LVEF were identified based on hospital admission records. All participants provided written informed consent and were enrolled during either the last 24 hours of his or her hospitalization for heart failure or the 2-week interval after the hospitalization.
After baseline evaluations, including echocardiographic measures, the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire (KCCQ), and blood tests, patients were randomized in a 1:1 ratio to receive either the GLP-1 agonist liraglutide or placebo as a daily subcutaneous injection. At 30-, 90-, and 180-day study visits, follow-up testing was performed. Participants were called at a mean of 210 days (SD, 7 days) to determine their adverse event status. Details of the study design have been described.9
Study Population
Patients were required to have an established diagnosis of heart failure and a LVEF of 40% or lower during the preceding 3 months. To target a relatively high-risk patient population, the inclusion criteria were (1) a recent (within 14 days) hospitalization for an acute heart failure syndrome despite already receiving evidence-based therapies and (2) a preadmission oral diuretic dose of at least 40 mg of furosemide or an equivalent.
Key exclusion criteria were (1) recent acute coronary syndrome or coronary intervention, (2) known intolerance of GLP-1 agonist therapy, and (3) severe renal, hepatic, or pulmonary disease. Recognizing that myocardial insulin resistance has been observed in patients with advanced heart failure but without diabetes,3,4 this trial did not exclude patients who did not have type 2 diabetes.
Study Drug and Randomization
Active therapy in this trial consisted of a human GLP-1 analog with 97% homology to native GLP-1, liraglutide (Victoza), which has been approved for use by the US Food and Drug Administration. Patients were randomly assigned to liraglutide or placebo in a 1:1 ratio.
A permuted block randomization scheme stratified by clinical site and type 2 diabetes status was performed with an automated web-based system to ensure relatively equal distribution of patients to each group within each site. The protocol involved uptitration of study drug dosage as tolerated every 14 days from 0.6 mg/d to 1.2 mg/d to 1.8 mg/d during the first 30 days of the trial. Liraglutide and placebo were packaged identically to maintain blinding.
Concomitant Medications
The protocol allowed for adjustment of standard heart failure therapies, including attempted uptitration of neurohormonal antagonists, during and after the participants’ initial hospitalization. In patients with type 2 diabetes, plans for risk reduction of hypoglycemia included (1) adjustments to doses of insulin or insulin secretagogues (sulfonylureas or meglitinide), (2) at least daily monitoring of blood glucose, and (3) close follow-up with the treating physician managing the participant’s diabetes. All participants were counseled on the signs and symptoms of hypoglycemia and the appropriate treatment.
Study End Points
End point assessments were blinded to treatment assignment. The primary end point was a global rank score in which all participants, regardless of treatment assignment, were ranked across 3 hierarchical tiers: time to death, time to rehospitalization for heart failure, and time-averaged proportional change in N-terminal pro-B-type natriuretic peptide (NT-proBNP) level from baseline to 180 days. Higher values indicate better health (stability). Patients who died during the 180-day study period were ranked based on the time of their death with earliest death being ranked first and then later deaths. Next, patients who did not die were ranked based on the time to their first rehospitalization for heart failure, such that the individual with the earliest rehospitalization received the next lowest rank and then those with later rehospitalizations. Those patients who neither died nor were rehospitalized were ranked based on their time-averaged proportional changes in NT-proBNP levels from the least to most favorable change. The mean rank score was then compared between groups. In this scheme, a higher mean rank score indicates greater overall stability for patients with heart failure.10
All-cause mortality was considered an objective end point. Rehospitalization for heart failure was distinguished from rehospitalizations due to other causes by a blinded adjudication committee based on the presence of both clinical manifestations of worsening heart failure and additional or increased therapy specifically for the treatment of worsening heart failure. Levels of NT-proBNP were determined at a central core laboratory that was blinded to treatment assignment.
The key exploratory secondary end points included (1) the individual components of the primary end point, (2) time to other prespecified cardiac events (including emergency department visits), (3) changes in cardiac structure and function (by echocardiographic measures) from baseline to 180 days, (4) functional status based on 6-minute walk distances at 30, 90, and 180 days, and (5) changes in the KCCQ clinical summary score.
Comparison of treatment effects in patients by type 2 diabetes status was a prespecified subgroup analysis. Tertiary end points included changes in metabolic biomarkers (ie, hemoglobin A1c, weight, and fasting lipid levels) and changes in cystatin C. Safety end points were reported by site investigators using an online system and were not adjudicated.
Statistical Analysis
The full statistical analysis plan appears in Supplement 3. All analyses were conducted using the intention-to-treat principle and included all randomized patients (Figure 1). Analysis of the global rank end point was based on the Wilcoxon test statistic and calculated using the NPAR1WAY procedure within SAS software (SAS Institute Inc).
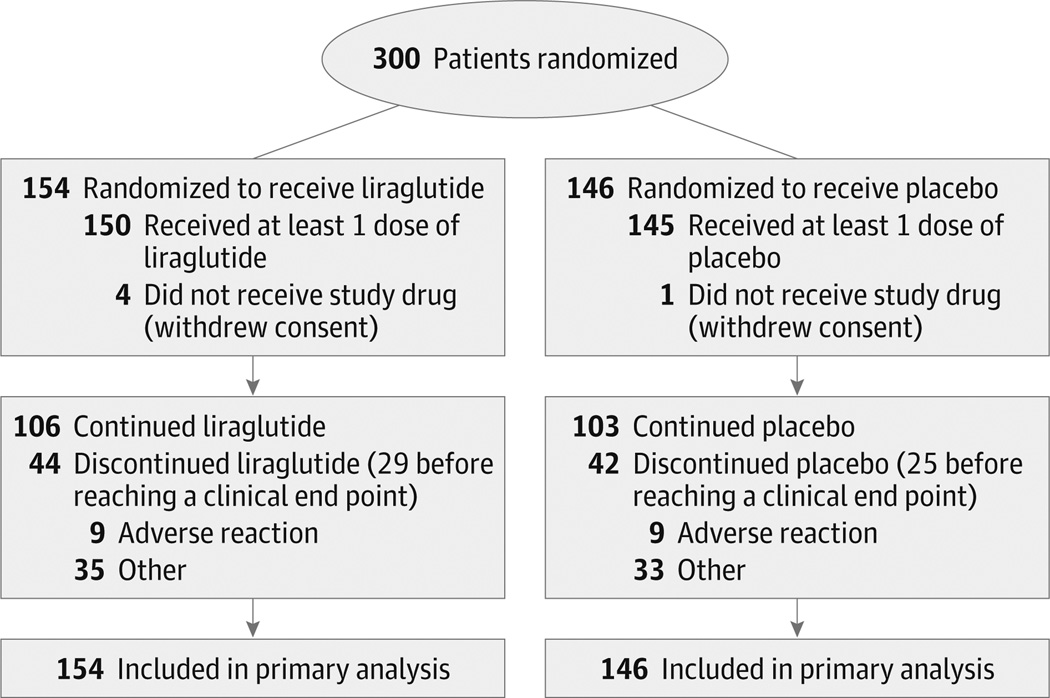
Figure 1. Patient Flow Diagram for the Functional Impact of GLP-1 for Heart Failure Treatment Study.
Data on patients screened for eligibility were not available. Secondary end points were analyzed with multiple imputation techniques when data were unavailable for the end point.
To evaluate for potential clustering of treatment responses by site, a mixed model was also computed using PROC MIXED with terms for treatment effect and random effects for sites. For binary outcomes, logistic regression analysis was used for the estimated odds ratio and associated 95% CI to compare liraglutide with placebo.
Unadjusted time-to-event comparisons were conducted using Kaplan-Meier survival estimates and log-rank tests. All P values are 2-sided with a level of significance of .05 and no adjustments for multiple comparisons.
Power Calculation
We regarded a reduction of 25% in deaths or rehospitalizations for heart failure as clinically significant based on the treatment effects of neurohormonal blockade,11 and a reduction of 0.5 SD in NT-proBNP level as clinically significant based on associations between serial natriuretic peptide levels and future events.12 Under these assumptions, the planned sample size of 300 patients (150 participants per treatment group) provided approximately 92% power.9 This planned sample size of 300 patients provided more than 90% power to detect differences of 0.4 SD for the continuous secondary end points; however, these secondary end points remain exploratory absent adjustment for multiple comparisons.
Missing data only affected the primary end point when patients did not die and were not rehospitalized for heart failure. Among patients who did not die or were not rehospitalized for heart failure, data were available for the time-averaged NT-proBNP levels in 136 cases and were missing in 35 cases. On these occasions, a LOESS smoother (adjusted for sex and treatment group) was used to impute missing values.
Multiple imputation was used to impute missing data for the secondary and tertiary end points. No adjustment for missing information was made for the safety end points. All statistical analyses were conducted using SAS software version 9.4.
Results
Patients and Treatment
Between August 2013 and March 2015, a total of 154 patients were randomized to receive liraglutide and 146 to receive placebo (Figure 1). Baseline characteristics were similar in the 2 groups (Table 1). Across the entire cohort, the median age was 61 years (interquartile range [IQR], 52–68 years), the median duration of heart failure was 6.2 years (IQR, 3.3–11.2 years), and more than 85% of participants in both groups had been hospitalized for heart failure at least once during the year prior to the hospitalization for heart failure that was required for study eligibility.
Table 1.
Baseline Characteristics
| Liraglutide (n = 154) |
Placebo (n = 146) |
|
|---|---|---|
| Age, median (IQR), y | 62 (52–68) | 61 (51–67) |
| Female sex, No. (%) | 31 (20) | 33 (23) |
| White race, No. (%)a | 82 (53) | 90 (62) |
| Hispanic, No. (%)a | 4 (3) | 11 (8) |
| Body mass index, median (IQR)b | 31 (26–36) | 33 (25–38) |
| Functional Measures | ||
| New York Heart Association Classification, No. (%) | ||
| II | 49 (32) | 36 (25) |
| III | 93 (60) | 96 (66) |
| IV | 8 (5) | 6 (4) |
| Kansas City Cardiomyopathy Questionnaire summary scores, median (IQR)c |
||
| Clinicald | 46 (32–65) | 44 (29–65) |
| Overalle | 43 (30–61) | 41 (28–61) |
| 6-min walk distance, median (IQR), m | 234 (143–313) | 212 (141–311) |
| Physical Examination | ||
| Weight, median (IQR), kg | 93 (79–113) | 96 (76–117) |
| Systolic blood pressure, median (IQR), mm Hg | 108 (99–120) | 108 (99–118) |
| Heart rate, median (IQR), beats/min | 75 (68–85) | 76 (68–88) |
| Elevated jugular venous pressure, No. (%) | 72 (47) | 66 (45) |
| Edema, No. (%) | 82 (53) | 88 (60) |
| Duration since diagnosis of heart failure, median (IQR), y |
6.6 (3.3–12.5) | 6.1 (3.2–10.8) |
| Medical History, No. (%) | ||
| Prior hospitalization for heart failure within past year | 137 (89) | 125 (86) |
| Ischemic heart disease | 133 (86) | 113 (77) |
| Hypertension | 121 (79) | 114 (78) |
| Atrial fibrillation | 74 (48) | 70 (48) |
| Type 2 diabetes mellitus | 91 (59) | 87 (60) |
| Stage ≥3 chronic kidney diseasef | 65 (42) | 53 (36) |
| Heart Failure Medications at Enrollment, No. (%) | ||
| β-Blocker | 143 (93) | 139 (95) |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker |
112 (73) | 104 (71) |
| Hydralazine | 51 (33) | 47 (32) |
| Long-acting nitrates | 60 (39) | 53 (36) |
| Aldosterone antagonist | 88 (57) | 89 (61) |
| Loop diuretic | 151 (98) | 146 (100) |
| Digoxin | 51 (33) | 51 (35) |
| Calcium-channel blocker | 12 (8) | 5 (3) |
| Lipid-lowering agent | 110 (71) | 110 (75) |
| Antiplatelet agent | 114 (74) | 102 (70) |
| Anticoagulant agent | 79 (51) | 80 (55) |
| Laboratory or Echocardiographic Measures, median (IQR) | ||
| Creatinine, mg/dL | 1.5 (1.1–1.8) | 1.5 (1.2–1.9) |
| Hemoglobin A1c, % | 6.6 (6.0–7.6) | 6.7 (5.9–7.9) |
| Total cholesterol, mg/dL | 132 (110–161) | 131 (108–164) |
| High-density lipoprotein cholesterol, mg/dL | 36 (28–47) | 35 (29–47) |
| Low-density lipoprotein cholesterol, mg/dL | 72 (54–96) | 68 (57–92) |
| Triglycerides, mg/dL | 97 (72–138) | 97 (73–145) |
| N-terminal pro-B-type natriuretic peptide, pg/mLg | 1936 (1075–4231) | 2083 (1020–4333) |
| Cystatin C, mg/Lg | 1.3 (1.0–1.7) | 1.5 (1.1–1.9) |
| Left ventricular ejection fraction, % | 25 (20–33) | 25 (19–32) |
| Left ventricular end-diastolic volume index, mL/m2 | 140 (112–173) | 137 (115–174) |
| Left ventricular end-systolic volume index, mL/m2 | 104 (78–130) | 100 (80–133) |
| Ratio of early mitral inflow velocity to early diastolic medial mitral annular velocity |
22 (17–28) | 23 (18–30) |
Abbreviation: IQR, interquartile range.
SI conversion factors: To calculate creatinine to µmol/L, multiply by 88.4; low-density lipoprotein, high-density lipoprotein, and total cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
Race and ethnicity were self-reported.
Calculated as weight in kilograms divided by height in meters squared.
Range from 1 to 100; higher scores indicate better function.
A composite score for functional status, quality of life, and social limitation.
Derived from the physical function, symptom (frequency and severity), social function, and quality-of-life domains.
Determined by enrollment site.
Determined at a central core laboratory that was blinded to treatment assignment.
Ischemic heart disease was the primary origin of heart failure in 82% of participants, and most patients had 1 or more comorbid conditions. Fifty-nine percent of randomized participants (178 patients) had type 2 diabetes. The median body mass index (calculated as weight in kilograms divided by height in meters squared) was 32 (IQR, 26–37).
Twenty-nine percent of participants had New York Heart Association class II heart failure, 63% had class III, and 5% had class IV. Despite high rates of disease-modifying therapies for heart failure, the median NT-proBNP level at the time of enrollment was 2049 pg/mL (IQR, 1054–4235 pg/mL) and the median LVEF was 25% (IQR, 19%–33%).
At least 1 dose of study drug was received by 150 of the 154 randomized to receive liraglutide and 145 of the 146 patients randomized to receive placebo. In the liraglutide group, 60% of participants achieved the target maximal dose of 1.8 mg/d, 21% received a maximum of 1.2 mg/d, and 16% received a maximum of 0.6 mg/d, whereas the corresponding proportions were 71%, 19%, and 10% for the placebo group.
In both groups, 29% of patients permanently discontinued use of the study drug prior to study termination. Of those who discontinued the study drug prior to rehospitalization for heart failure or death, this occurred in 19% of patients in the liraglutide group and in 17% of patients in the placebo group. The median duration that participants received the study drug was 25.0 weeks (IQR, 8.6–25.9 weeks) in the liraglutide group and 25.0 weeks (IQR, 11.4–26.0 weeks) in the placebo group (eFigure 1 in Supplement 1).
Primary End Point
In the primary intention-to-treat analysis, there was no significant between-group difference in the global rank scores (mean rank of 146 in the liraglutide group vs 156 in the placebo group; Wilcoxon rank sum P = .31), with higher rank indicating better stability (Table 2). To account for possible clustering of responses by enrollment site, we conducted a sensitivity analysis with site as a covariate; the results were nearly identical to the primary analysis (eTable 1 in Supplement 1).
Table 2.
Study End Points
| Liraglutide (n = 154) |
Placebo (n = 146) |
Treatment Effect (95% CI)a |
P Value |
|
|---|---|---|---|---|
| Primary End Point | ||||
| Mean global rank scoreb | 146 | 156 | .31c | |
| Secondary End Points | ||||
| Change from baseline to 180 d, mean (95% CI) | ||||
| Left ventricular end-diastolic volume index, mL/m2 | 3.4 (−3.7 to 10.4) | −2.9 (−9.7 to 3.9) | 6.7 (−2.6 to 16.0) | .16 |
| Left ventricular end-systolic volume index, mL/m2 | 1.2 (−4.6 to 6.9) | −3.5 (−9.0 to 2.1) | 5.0 (−2.6 to 12.7) | .19 |
| Left ventricular ejection fraction, % | 1.1 (−0.7 to 2.8) | 1.4 (−0.4 to 3.2) | −0.1 (−2.3 to 2.1) | .95 |
| 6-min walk distance, m | 56 (30 to 81) | 55 (29 to 81) | 5 (−29 to 39) | .79 |
| Kansas City Cardiomyopathy Questionnaire summary scoresd | ||||
| Clinicale | 14 (10 to 18) | 13 (9 to 17) | 1.3 (−4.0 to 6.5) | .64 |
| Overallf | 13 (10 to 17) | 13 (9 to 17) | 0.6 (−4.5 to 5.8) | .81 |
| Events from baseline to 180 d, No. (%)g | ||||
| Death | 19 (12) | 16 (11) | 1.10 (0.57 to 2.14)h | .78 |
| Rehospitalization for heart failure | 63 (41) | 50 (34) | 1.30 (0.89 to 1.88)h | .17 |
| Death or rehospitalization for heart failure | 72 (47) | 57 (39) | 1.30 (0.92 to 1.83)h | .14 |
| N-terminal pro-B-type natriuretic peptide level from baseline to 180 d, mean (95% CI) |
||||
| Total change, pg/mL | 1055 (201 to 1909) | 1216 (336 to 2096) | −155 (−1368 to 1058) | .80 |
| Time-averaged proportional change expressed as ratio vs baseline value |
1.9 (1.4 to 2.3) | 1.8 (1.4 to 2.1) | 0.1 (−0.4 to 0.7) | .65 |
| Tertiary End Points | ||||
| Change from baseline to 180 d, mean (95% CI) | ||||
| Cystatin C, mg/L | 0.08 (−0.01 to 0.17) | −0.09 (−0.18 to 0) | 0.16 (0.04 to 0.28) | .009 |
| Hemoglobin A1c, % | −0.21 (−0.45 to 0.03) | 0.10 (−0.14 to 0.34) | −0.33 (−0.67 to 0) | .05 |
| Total cholesterol, mg/dL | 8 (0 to 15) | 17 (9 to 24) | −9.9 (−19.9 to 0.1) | .05 |
| High-density lipoprotein cholesterol, mg/dL | 4 (2 to 7) | 3 (1 to 5) | 2 (−2 to 5) | .37 |
| Low-density lipoprotein cholesterol, mg/dL | 0.3 (−6 to 6) | 7 (0 to 13) | −7 (−15 to 1) | .08 |
| Triglycerides, mg/dL | 15 (−1 to 32) | 39 (20 to 57) | −22 (−47 to 3) | .08 |
| Weight, kg | −1.5 (−3.0 to 0) | 0.3 (−1.2 to 1.8) | −1.8 (−3.9 to 0.3) | .09 |
| Other Clinical End Points | ||||
| Events from baseline to 180 d | ||||
| Rehospitalization for cardiovascular reasons, No. (%) | 78 (51) | 62 (42) | 1.33 (0.95 to 1.85)h | .09 |
| ED visit, No. (%) | 39 (25) | 28 (19) | 1.41 (0.87 to 2.30)h | .16 |
| Death, rehospitalization for cardiovascular reasons, or ED visit, No. (%) |
97 (63) | 81 (55) | 1.34 (1.00 to 1.80)h | .05 |
| Death, rehospitalization for heart failure, or ED visit, No. (%) | 89 (58) | 72 (49) | 1.36 (0.99 to 1.85)h | .05 |
| Change in heart rate (95% CI), beats/min | 1.0 (−1.72 to 3.63) | 1.2 (−1.5 to 3.8) | −1.6 (−4.8 to 1.6) | .33 |
Abbreviation: ED, emergency department.
SI conversion factors: To calculate low-density lipoprotein, high-density lipoprotein, and total cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
Defined as between-group difference, adjusted for baseline value.
Ranked across 3 hierarchical tiers: time to death, time to rehospitalization for heart failure, and time-averaged proportional change in N-terminal pro-B-type natriuretic peptide level from baseline to 180 days. Higher values indicate better health (range, 1 [an early death] to 300 [a patient who survived free from rehospitalization and had an improvement in N-terminal pro-B-type natriuretic peptide level]). This nonparametric analysis does not provide an informative estimate of variability.
Determined using a Wilcoxon test statistic for the primary end point.
Range from 1 to 100; higher scores indicate better function.
A composite score for functional status, quality of life, and social limitation.
Derived from the physical function, symptom (frequency and severity), social function, and quality-of-life domains.
Patients with a left ventricular assist device or heart transplantation were not included.
Data are expressed as hazard ratio (95% CI).
Components of the Primary End Point
For the components of the primary end point, there was no significant between-group difference in the number of deaths (19 [12%] in the liraglutide group vs 16 [11%]) in the placebo group; hazard ratio [HR], 1.10 [95% CI, 0.57–2.14]; P = .78) (Figure 2A), rehospitalizations for heart failure (63 [41%] in the liraglutide group vs 50 (34%) in the placebo group; HR, 1.30 [95% CI, 0.89–1.88]; P = .17), or the composite of death or rehospitalization for heart failure (72 [47%] in the liraglutide group vs 57 [39%] in the placebo group; HR, 1.30 [95% CI, 0.92–1.83]; P = .14) (Figure 2B). Among participants who were alive and not rehospitalized for heart failure, the time-averaged proportional change in NT-proBNP level was 1.52 (SD, 1.71) times the baseline levels in the liraglutide group and 1.44 (SD, 1.22) times the baseline levels in the placebo group (P = .94; Figure 2C).
Figure 2. Components of the Primary End Point.
HR indicates hazard ratio. A and B, y-axis scale in blue indicates range from 0% to 16%. The median duration of follow-up was 179 days (IQR, 157–182 days) in the liraglutide group and 178 days (IQR, 150–183 days) in the placebo group. In part C, the box plots were formed by the 25th and 75th percentiles and the line within the box is the median; the error bars indicate the 95% CIs and the data markers indicate the means.
aWithout missing data.
Exploratory Secondary End Points
Compared with placebo, there was no significant effect of liraglutide on any of the prespecified secondary end points including changes in cardiac structure and function from baseline to 180 days, 6-minute walk test distances, the KCCQ clinical summary score, emergency department visits, and the composites of death and rehospitalization for heart failure with or without emergency department visits (Table 2).
Exploratory Tertiary End Points
Compared with placebo, liraglutide treatment was associated with greater weight decreases at 30 days (intergroup difference, −1.7 kg [95% CI, −2.9 to −0.5 kg]; P = .004) and 90 days (intergroup difference, −1.9 kg [95% CI, −3.4 to −0.4 kg]; P = .01), with no significant difference at 180 days (intergroup difference, −1.8 kg [95% CI, −3.9 to 0.3 kg], P = .09). Increases in cystatin C (a marker of renal dysfunction) were significantly greater at 180 days in patients in the liraglutide group compared with those in the placebo group (between-group difference, 0.16 mg/L [95% CI, 0.04 to 0.28] mg/L; P = .009). An exploratory analysis limited to patients who continued taking the study drug was not significant (eFigure 2 in Supplement 1).
Diabetes Subgroup Analysis
Among the 178 patients with diabetes (baseline characteristics appear in eTable 2 in Supplement 1), there was no statistically significant between-group difference in global rank score (mean rank of 85 for the liraglutide group vs 94 for the placebo group; P = .27) (eTable 3 in Supplement 1). The P value for interaction was 0.60 for treatment based on type 2 diabetes status.
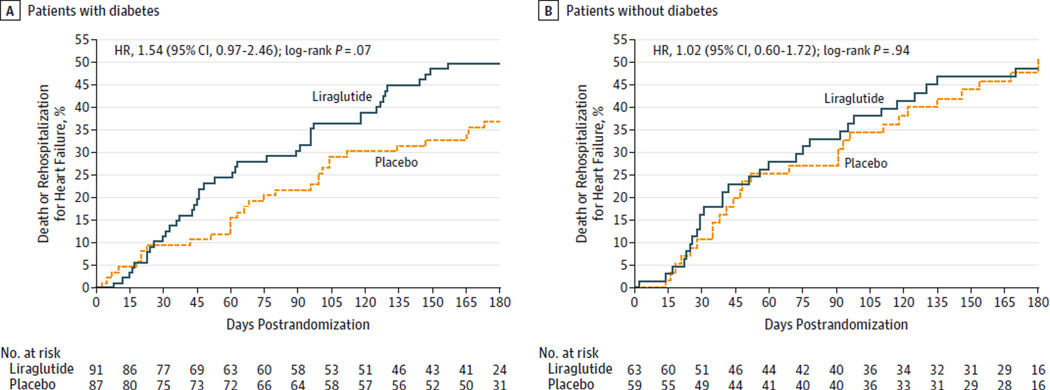
Similar to the overall study population, the findings for the secondary clinical outcomes were not significantly different between the liraglutide and placebo groups. However, the point estimates consistently suggested higher risk of heart failure–related events with liraglutide and were higher in magnitude in patients with diabetes than in the overall study population (Table 2, Figure 3, and eTable 3 in Supplement 1).
Figure 3. Prespecified Subgroup Analysis of Patients Who Died or Experienced Rehospitalization for Heart Failure by Type 2 Diabetes Status.
HR indicates hazard ratio. The median duration of follow-up was 179 days (interquartile range, 157–182 days) in the liraglutide group and 178 days (interquartile range, 150–183 days) in the placebo group.
Investigator-Reported Safety Events
Severe adverse events observed with liraglutide and placebo are reported in eTable 4 in Supplement 1. The number of investigator-reported hyperglycemic events was 16 (10%) in the liraglutide group vs 27 (18%) in the placebo group and hypoglycemic events were infrequent (2 [1%] in the liraglutide group vs 4 [3%] in the placebo group). The study was not adequately powered for statistical comparisons of the rates of adverse events between groups.
Discussion
The GLP-1 agonist liraglutide did not improve posthospitalization clinical stability in patients with advanced heart failure and reduced LVEF despite prior studies indicating that GLP-1 therapy might ameliorate mechanisms of myocardial insulin resistance reported in patients with severe cardiomyopathies.3,4 The absence of favorable effects of liraglutide on secondary end points based on echocardiographic measures, 6-minute walk distance, or quality of life based on the KCCQ clinical summary score also supports the conclusion that liraglutide did not improve heart failure status.
Although the global rank end point based on a hierarchy of death, rehospitalization, and biomarker analysis does not allow definitive conclusions about the effect of liraglutide on clinical outcomes, this study effectively excludes any large favorable effects with liraglutide on the composite of time to death or rehospitalization for heart failure (Figure 2B).
To our knowledge, this is the first multicenter randomized clinical trial specifically designed to determine whether a GLP-1 agonist benefits a high-risk subset of patients with established heart failure. The high-risk features of this trial’s population were evident in (1) their recent hospitalization for heart failure at entry, (2) the substantial majority with New York Heart Association class III or IV symptoms, (3) their low median LVEF (25%) and 6-minute walk distance (<240 m), and (4) the elevated baseline levels of serum creatinine (1.5 mg/dL) and NT-proBNP (2049 pg/mL) despite treatment with evidence-based medical therapies. High risk was ultimately confirmed by high rates of death (11.7%) and rehospitalization for heart failure (37.7%) during the 6-month follow-up period in the entire cohort.
There are several potential explanations for failure of liraglutide to improve heart failure status in this trial. Because GLP-1 agonists promote glucose-dependent insulin secretion, this trial raises concerns about whether enhancing endogenous insulin secretion is advantageous in the setting of heart failure. To the extent that GLP-1 agonists mitigate insulin resistance, this trial also raises questions about whether previously demonstrated2 myocardial insulin resistance in heart failure models is a maladaptive mechanism in patients with advanced heart failure and receiving standard medical therapy. These concerns are supported by the fact that other agents that augment insulin secretion and insulin sensitivity, specifically dipeptidyl peptidase 4 inhibitors and thiazolidinediones, have generated adverse heart failure signals.13,14
Alternatively, differences between the results in this trial and the encouraging earlier study using recombinant GLP-17 could be due to the cardioprotective roles of GLP-1 metabolites, such as GLP-1(9–36)amide, which act independently of the GLP-1 receptor and are not generated by GLP-1 agonists like liraglutide.15 It is also possible that patients with advanced heart failure, like those in this trial, are refractory to the otherwise beneficial effects of GLP-1 agonists or are prone to detrimental extracardiac actions of GLP-1, such as impairment of renal function, that are not apparent in other populations.16 Even though most baseline parameters were equivalent in the 2 treatment groups, a slightly higher proportion of ischemic heart disease (86%) among liraglutide-treated patients (vs 77% in the placebo group) could have increased their risk of adverse outcomes.
This trial provides complementary information to an increasing number of large randomized trials that assess cardiovascular outcomes, including heart failure, associated with treatments for type 2 diabetes. These include cardiovascular safety studies of sulfonylureas, thiazolidinediones, dipeptidyl-dipeptidase inhibitors,13,17,18 GLP-1 agonists,19 and sodium-glucose co-transporter 2 inhibitors.20 In the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI-53) trial,13 there was an increase in hospitalizations for heart failure in the patients with diabetes randomized to the dipeptidyl peptidase 4 inhibitor saxagliptin who had no prior diagnosis of heart failure (HR, 1.30 [95% CI, 1.03–1.65]; P = .03). Among the 234 patients with New York Heart Association class III or IV symptoms of heart failure in the SAVOR-TIMI-53 trial,13 there was a nonstatistically significant increase in hospitalizations for heart failure in patients treated with saxagliptin (HR, 1.75 [95% CI, 0.94–3.36]). The increase in risk in SAVOR-TIMI-53 was highest among patients with elevated levels of natriuretic peptides, symptomatic heart failure, or chronic kidney disease. Similar increases in risk of heart failure were noted in patients with renal insufficiency or elevation in natriuretic peptides, which are cardinal features of advanced heart failure. To our knowledge, in trials of patients with diabetes taking GLP-1 agonists, there are no prior reports focusing on patients who already have advanced heart failure.
In the absence of a beneficial effect on heart failure status, it is important to define the efficacy and safety of liraglutide for diabetes management in patients already at high risk. In the patients with advanced heart failure and type 2 diabetes mellitus in this trial, the efficacy of liraglutide for diabetes management was supported by a reduction in hemoglobin A1c and weight loss compared with the placebo-treated participants. However, nonsignificant increases in the numbers of patients with diabetes experiencing adverse heart failure outcomes, including the composite end point of time to death, rehospitalization for heart failure or emergency department visit, and signals of worsening renal function raise safety concerns about the use of liraglutide in this patient population.
Together, these nonsignificant signals of harm suggest the need for caution and close monitoring among clinicians considering initiation of liraglutide and other GLP-1 agonists for weight loss or diabetes management in patients with heart failure and reduced LVEF. Our findings are not relevant to patients already treated with GLP-1 agonists because such patients were specifically excluded from this trial. Larger safety trials21 may provide complementary insight into the safety of liraglutide and other GLP-1 agonists in patients with less severe and earlier stages of heart failure.
This study has some important limitations. Although supported by prior work10 and appropriate for a phase 2 trial, the global rank score end point in this trial has not been validated in patients with heart failure. In addition, this trial was not powered to detect differences in clinical events or safety end points, and it was not powered for subgroup analyses. The patients enrolled in this trial had advanced heart disease, and as expected there were missing data, especially for functional metrics such as the 6-minute walk test. It is also possible that patients seen at referral centers within the NHLBI Heart Failure Network are not representative of larger populations of patients with advanced heart failure treated outside academic medical centers.
Conclusions
Among patients recently hospitalized with heart failure and reduced LVEF, the use of liraglutide did not lead to greater posthospitalization clinical stability. These findings do not support the use of liraglutide in this clinical situation.
Supplementary Material
Key Points.
Question
Does therapy with the glucagon-like peptide 1 agonist liraglutide improve clinical stability in patients with advanced heart failure?
Findings
In this randomized clinical trial of 300 adults with advanced heart failure, liraglutide had no significant effect on posthospitalization clinical stability based on a global rank score of time to death, time to rehospitalization for heart failure, and time-averaged proportional change in N-terminal pro-B-type natriuretic peptide level. There was no benefit observed among the 178 patients with type 2 diabetes.
Meaning
These findings do not support the use of liraglutide for improving clinical stability in patients with advanced heart failure and reduced left ventricular ejection fraction.
Acknowledgments
Dr Margulies reported receiving grants from Juventis Therapeutics, Celladon Corporation, Thoratec Corporation, Innolign Biomedical LLC, and Merck Sharp and Dohme; and serving as a consultant for Janssen Pharmaceuticals, Merck Sharp and Dohme, Pfizer, and Ridgetop Research. Dr Hernandez reported receiving grants from GlaxoSmithKline; grants and personal fees from AstraZeneca, Amgen, Novartis, Merck Sharp and Dohme, and Bristol-Myers Squibb; and personal fees from Janssen.
Dr Redfield reported receiving honoraria from the Heart Failure Society of America; and receiving royalties from Anexion. Dr Givertz reported serving as a consultant for Merck Sharp and Dohme and Novartis. Dr Oliveira reported receiving grants from the Frankino-Dodero Foundation; serving on the speakers bureau for Amgen and Novartis; and serving as a consultant for Abiomed. Dr Whellan reported receiving grants from ResMed and Poszen. Dr Felker reported receiving grants and personal fees from Novartis and Amgen; grants from Roche Diagnostics, Otsuka, and Singulex; personal fees from Bristol-Myers Squibb, Trevena, Myokardia, Merck Sharp and Dohme, and Celladon; and serving as a consultant for Novartis and Amgen. Dr Anstrom reported receiving grants from AstraZeneca; and serving as a consultant for Pfizer, Abbot Vascular, and AstraZeneca. Dr Braunwald reported receiving grants from AstraZeneca, Novartis, Merck, Daiichi Sankyo, GlaxoSmithKline, Bristol-Myers Squibb, Johnson & Johnson, and Sanofi-Aventis; and personal fees from The Medicines Company, Sanofi-Aventis, Theravance, Daiichi Sankyo, Menarini International, Medscape, and Bayer. Dr Cappola reported receiving personal fees from Novartis and Teva Pharmaceuticals.
Funding/Support: This research was supported by grants U10 HL084904 (awarded to the coordinating center) and U01 HL084861, U10 HL110312, U10 HL110337, U10 HL110342, U10 HL110262, U10 HL110297, U10 HL110302, U10 HL110309, U10 HL110336, and U10 HL110338 (awarded to the regional clinical centers) from the National Heart, Lung, and Blood Institute (NHLBI). The study drug (liraglutide) and matching placebo injections were supplied by NovoNordisk Inc.
Role of the Funder/Sponsor: The NHLBI contributed to the design and oversight of the study; interpretation of the data; and preparation, review and approval of the manuscript. NovoNordisk only provided the study drug and had no input in the design or conduct of the trial, interpretation of the data, preparation, review, or approval of the manuscript. Neither the NHLBI nor NovoNordisk were able to prevent manuscript submission.
Footnotes
Author Contributions: Drs Margulies and Cappola had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Margulies, Hernandez, Redfield, Givertz, Felker, Anstrom, Shah, Braunwald, Cappola.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Margulies, Cappola.
Critical revision of the manuscript for important intellectual content: Margulies, Hernandez, Redfield, Givertz, Oliveira, Cole, Mann, Whellan, Kiernan, Felker, McNulty, Anstrom, Shah, Braunwald.
Statistical analysis: McNulty, Anstrom.
Obtained funding: Margulies, Hernandez, Redfield, Givertz, Mann, Whellan, Felker, Braunwald, Cappola.
Administrative, technical, or material support: Hernandez, Oliveira, Cole, Shah.
Study supervision: Margulies, Hernandez, Givertz, Whellan, Felker, Shah, Braunwald, Cappola.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
No other disclosures were reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure–associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61(12):1259–1267. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(2):219–226. doi: 10.2174/1568008054064869. [DOI] [PubMed] [Google Scholar]
- 3.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61(2):297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Swan JW, Anker SD, Walton C, et al. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30(2):527–532. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 5.Grigoropoulou P, Eleftheriadou I, Zoupas C, Diamanti-Kandarakis E, Tentolouris N. Incretin-based therapies for type 2 diabetes mellitus: effects on insulin resistance. Curr Diabetes Rev. 2013;9(5):412–417. doi: 10.2174/15733998113099990070. [DOI] [PubMed] [Google Scholar]
- 6.Clarke SJ, McCormick LM, Dutka DP. Optimising cardioprotection during myocardial ischaemia: targeting potential intracellular pathways with glucagon-like peptide-1. Cardiovasc Diabetol. 2014;13:12. doi: 10.1186/1475-2840-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12(9):694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 8.Velez M, Peterson EL, Wells K, et al. Association of antidiabetic medications targeting the glucagon-like peptide 1 pathway and heart failure events in patients with diabetes. J Card Fail. 2015;21(1):2–8. doi: 10.1016/j.cardfail.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margulies KB, Anstrom KJ, Hernandez AF, et al. Heart Failure Clinical Research Network. GLP-1 agonist therapy for advanced heart failure with reduced ejection fraction: design and rationale for the Functional Impact of GLP-1 for Heart Failure Treatment study. Circ Heart Fail. 2014;7(4):673–679. doi: 10.1161/CIRCHEARTFAILURE.114.000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lachin JM. Worst-rank score analysis with informatively missing observations in clinical trials. Control Clin Trials. 1999;20(5):408–422. doi: 10.1016/s0197-2456(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 11.Packer M, Coats AJ, Fowler MB, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 12.Anand IS, Fisher LD, Chiang YT, et al. Val-HeFT Investigators. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107(9):1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM, Braunwald E, Raz I, et al. SAVOR-TIMI-53 Steering Committee and Investigators. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [published correction appears in Circulation. 2015;132(15):e198] [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Hopper I, Skiba M, Krum H. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: meta-analysis of randomized clinical trials with 55,141 participants. Cardiovasc Ther. 2014;32(4):147–158. doi: 10.1111/1755-5922.12075. [DOI] [PubMed] [Google Scholar]
- 15.Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114(11):1788–1803. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 16.Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of Liraglutide Versus Placebo as Add-on to Glucose-Lowering Therapy in Patients With Type 2 Diabetes and Moderate Renal Impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care. 2016;39(2):222–230. doi: 10.2337/dc14-2883. [DOI] [PubMed] [Google Scholar]
- 17.Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 18.Green JB, Bethel MA, Armstrong PW, et al. TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer MA, Claggett B, Diaz R, et al. ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 20.Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 21.Marso SP, Poulter NR, Nissen SE, et al. Design of the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial. Am Heart J. 2013;166(5):823.e5–830.e5. e825. doi: 10.1016/j.ahj.2013.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.