Abstract
Smoking continues to be the leading cause of preventable death in the USA, despite the vast and widely publicized knowledge about the negative health effects of tobacco smoking. Data show that smoking cessation is often accompanied by weight gain and an improvement in insulin sensitivity over time. However, paradoxically, post-cessation-related obesity might contribute to insulin resistance. Furthermore, post-cessation weight gain is reportedly the number one reason why smokers, especially women, fail to initiate smoking cessation or relapse after initiating smoking cessation. In this Review, we discuss the metabolic effects of stopping smoking and highlight future considerations for smoking cessation programs and therapies to be designed with an emphasis on reducing post-cessation weight gain.
Globally, reducing tobacco use and/or smoking cessation needs to be a health priority. The WHO estimates that each year ~6 million individuals die prematurely from smoking-related diseases worldwide, with the majority of deaths occurring in middle-income and low-income countries1. In 2008, the WHO introduced a practical, cost-effective way to help reduce worldwide tobacco use called MPOWER1. The six MPOWER measures include: monitoring tobacco use and prevention policies; protecting people from tobacco use; offering help to quit tobacco use; warning about the dangers of tobacco; enforcing bans on tobacco advertising, promotion and sponsorship; and raising taxes on tobacco1.
Although >5 million of the 6 million deaths from smoking-related diseases are the result of direct tobacco use, >600,000 deaths are the result of nonsmokers being exposed to second-hand smoke1. Smoking is associated with an increased risk of multiple conditions including several types of cancer, type 2 diabetes mellitus (T2DM), heart disease, chronic obstructive pulmonary disease, congenital defects, adverse reproductive effects in men (such as erectile dysfunction), osteoporosis and hip and/or vertebral fractures, and overall diminished health2–5. Adverse reproductive effects in women (such as decreased fertility, preterm delivery and ectopic pregnancies) are more common in smokers than in nonsmokers5,6. Smoking decreases estrogen levels in the body7. Furthermore, in 2015, smoking and nicotine were shown to be associated with an increased relative risk of accidents and suicide; the association with suicide possibly being mediated by modulation of serotonin levels by nicotine8. In a review published in 2014, some of the carcinogenic effects of nicotine were also highlighted9. In addition, passive smoking (that is, second-hand smoking) has been linked to severe negative health consequences, such as a low birth rate in offspring of mothers exposed to second-hand smoke, sudden infant death syndrome and T2DM10. In 2012, we reported that second-hand smoking is associated with both T2DM and obesity, whereas primary smoke is only associated with T2DM11. Moreover, smoking leads to substantial financial costs to society. Between 2009 and 2012, smoking cost the USA $289–332.5 billion, with 46–53% of this amount spent on adult medical care and the rest due to loss of workplace productivity5. The negative effects of smoking, thus, lead to reduced quality of life and loss of life, and can lead to personal and national financial burdens. Smoking cessation is, therefore, a personal (for smokers), national and global imperative. One of the major advances in understanding the link between cigarette smoke and cardiovascular diseases came in 1992 from a study that used insulin-mediated glucose uptake techniques to show that smokers are more insulin resistant (and had compensatory hyperinsulinaemia) than nonsmokers12. Smokers also have higher plasma levels of triglycerides and lower levels of HDL cholesterol12. These findings have since been reproduced in numerous studies13–19 and support the notion that insulin resistance leads to dyslipidaemia20 and endothelial dysfunction21 in smokers. Smoking is also a major risk factor for nonalcoholic fatty liver disease22. Although normal in some individuals and animals, excess fat deposition in the liver can lead to liver inflammation and scarring and, in severe cases, liver failure23–25.
Nicotine-mediated weight loss
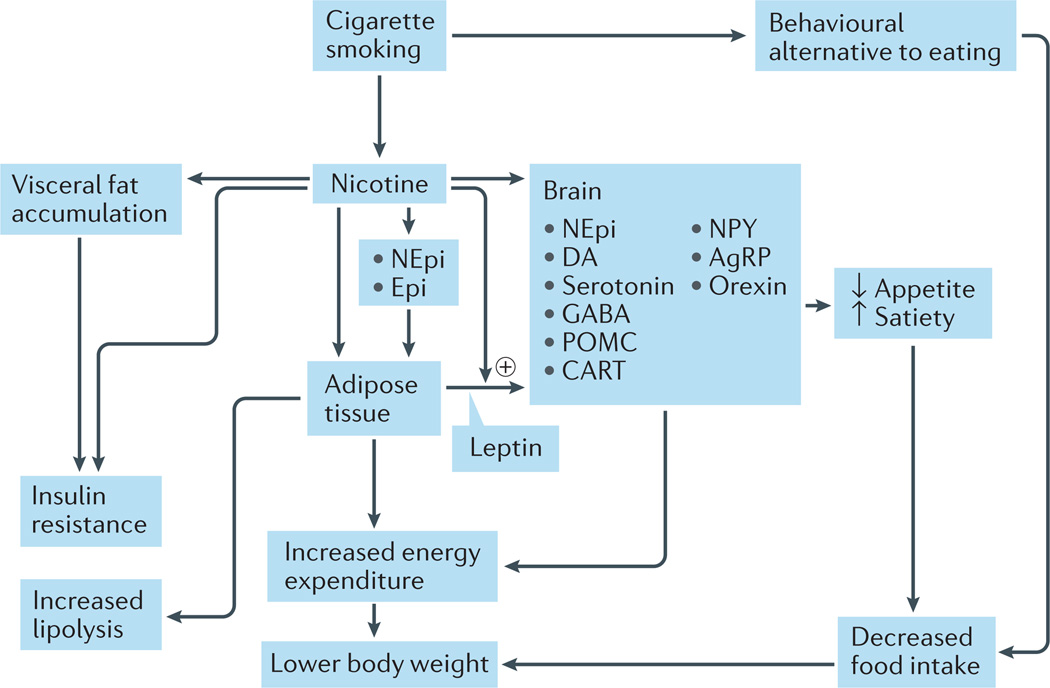
In order to understand the effects of smoking cessation on metabolism, we must first understand the effects of smoking and nicotine on body weight and metabolic parameters. Smoking and nicotine directly affect glucose metabolism and body weight as a result of changes in metabolism, activation of lipoprotein lipase that breaks down triglycerides to form free fatty acids, sympathetic nervous system activation, and other changes that lead to increased energy consumption and weight loss26. Nicotine use results in anorexia and increased metabolism, which leads to weight loss in rodents and humans27. Nicotine use can, therefore, result in reduced body weight (FIG. 1) and other metabolic and endocrine effects. Some of the mechanisms by which nicotine causes weight loss include: direct stimulation of melanocortin receptor 4 (MC4-R), which results in reduced food consumption28 and serum levels of leptin29,30; increased stimulation of the sympathetic nervous system, which results in increased levels of adrenaline and noradrenaline31 (FIG. 1); lipolysis32 (FIG. 2) and other processes30–32.
Figure 1. Mechanisms by which cigarette smoking reduces body weight.
Smoking reduces body weight by increasing energy expenditure and inhibiting the expected compensatory increase in caloric intake. Nicotine increases energy expenditure both by direct effects on peripheral tissues (largely mediated by catecholamines) and by effects on neuroendocrine circuits in the central nervous system. The effects of nicotine on the brain also lead to suppression of appetite; smoking per se can serve as a behavioural alternative to eating. AgRP, agouti-related protein; CART, cocaine- and amphetamine-regulated transcript protein; DA, dopamine; Epi, adrenaline; GABA, γ-aminobutyric acid; NEpi, noradrenaline; NPY, neuropeptide Y; POMC, proopiomelanocortin. Modified with permission from Wiley © Audrain-McGovern, J. & Benowitz, N. L. Clin. Pharmacol. Ther. 90, 164–168 (2011).
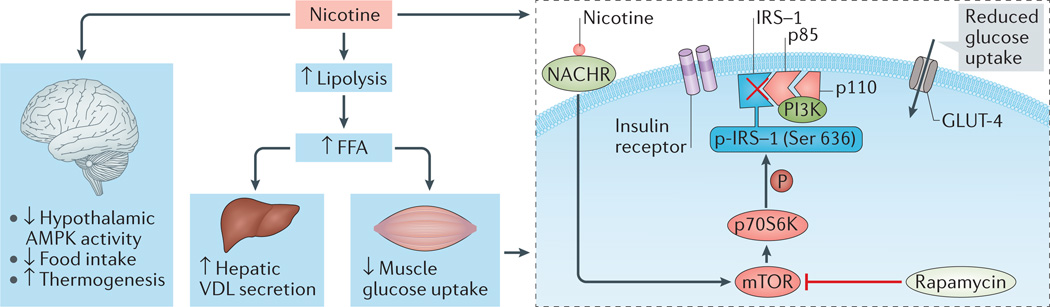
Figure 2. Mechanisms by which nicotine leads to insulin resistance.
Nicotine inhibits hypothalamic 5´-AMP-activated protein kinase (AMPK) activity, decreases food intake and increases thermogenesis. Nicotine also enhances lipolysis and increases the delivery of free fatty acids (FFA) to the liver and skeletal muscle. These effects of nicotine are associated with increased hepatic secretion of VLDL cholesterol and intramyocellular lipid saturation, as well as peripheral insulin resistance. Nicotine increases mammalian target of rapamycin (mTOR) and/or p70S6 kinase (p70S6K) activity in cultured L6 myotubes in association with increased phosphorylation (P) of insulin receptor substrate 1 (IRS-1) at Ser636 and reduced insulin-stimulated glucose uptake; the mTOR inhibitor rapamycin blocks these effects of nicotine. GLUT-4, glucose transporter type 4, insulin-responsive; NACHR, nicotinic acetylcholine receptor; p85, PI3K regulatory subunit-α; p110, PI3K catalytic subunit polypeptide; PI3K, phosphoinositide 3-kinase. American Diabetes Association, Bajaj, M. et al. Nicotine and insulin resistance: when the smoke clears. Diabetes 61, 3078–3080 (2012). Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association.
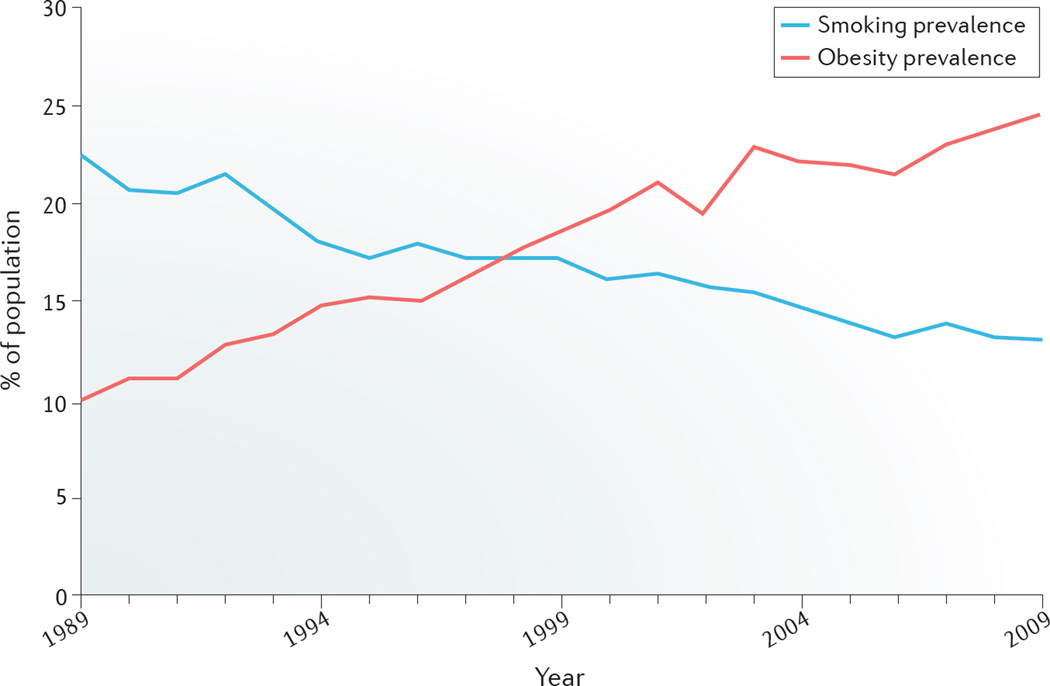
Although causality has not been demonstrated, the decline in the rate of smoking parallels the increased prevalence of obesity (FIG. 3). An inverse dose-response relationship exists between smoking and BMI, with some smokers having a BMI lower than the healthy reference range33. By contrast, a positive correlation exists between the number of cigarettes smoked and central fat accumulation34; many individuals appreciate the weight-suppressive effects of nicotine35. In general, both active and passive smoking increase the risk of T2DM10. Although use of electronic cigarettes results in weight loss in mice36, no studies examining the consequences of electronic cigarettes on body weight or glycaemic parameters in humans have been reported.
Figure 3. Prevalence of smoking and obesity.
Data shows the prevalence of smoking and obesity in individuals aged ≥18 years in California, USA, for the period 1989–2009. Modified with permission from the Legislative Analyst’s Office. 201 Cal Facts. California’s economy and budget in perspective [online], http://www.lao.ca.gov/reports/2011/calfacts/calfacts_010511.aspx (2011)109.
Nicotine-induced weight loss is a result of reduced appetite signalling in the hypothalamus and increased energy expenditure owing to increased locomotor activity, increased thermogenesis of brown adipose tissue, increased expression of UCP1 and UCP3 in brown adipose tissue and alterations in the utilization of fuel substrates29,37. At the biochemical level, nicotine-induced weight loss has been shown to result from inactivation of the cellular energy sensor 5´-AMP-activated protein kinase (AMPK) in the hypothalamus37.
Smoking cessation
Post-cessation weight gain
Widespread knowledge about the harmful effects of smoking has led to a decrease in smoking in all ethnic populations in the USA38. Statistics published in 2014 show that the proportion of adult individuals in the USA who smoke cigarettes has fallen from 20.9% in 2005 to 17.8% in 2013 (REF. 39). Superior health benefits are observed when smokers quit sooner rather than later40. Although smokers are now using tobacco products less frequently than before, the use of electronic cigarettes has greatly increased, especially among adolescents5,41. Evidence-based recommendations indicate that smoking cessation programs are useful in helping smokers to quit42, yet in the real-world setting, smoking is a very difficult addiction to break. Although ~70% of both adolescent and adult smokers state that they would like to quit smoking, only ~7% of smokers who try to quit on their own achieve long-term cessation each year43. Current marketed smoking cessation products (including medications and tobacco-replacement products) increase the chance that committed smokers can stop smoking, but their efficacy is still low, especially in the real-life setting44. The powerfully addictive nature of nicotine is the main reason smokers continue to use tobacco43. Post-cessation weight gain has been reported to be the main reason why smokers, especially women, fail to initiate smoking cessation or relapse after initiating smoking cessation45. In the remainder of this Review, therefore, we explore the metabolic effects of smoking cessation, with an emphasis on post-cessation weight gain.
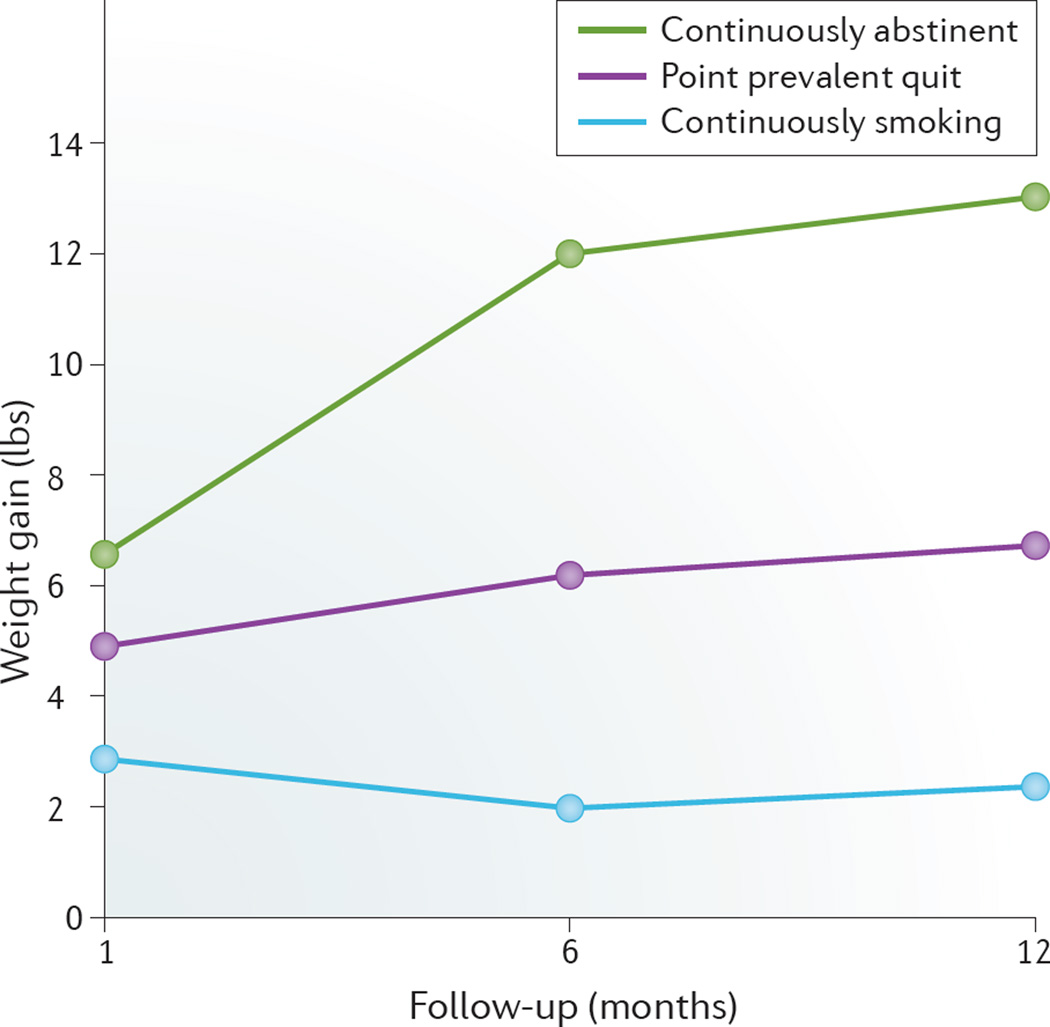
BOX 1 summarizes the results of a 2015 meta-analysis on post-cessation weight gain in adult individuals46. In another study47, an average smoker weighed 4–5 kg less than nonsmokers, but once these individuals quit, they gained on average 4.5 kg within 6–12 months and their weights returned to the same weight–age trajectory observed in nonsmokers31 (FIG. 4). This weight gain in former smokers can last for up to 10 years31,48 (FIG. 5). The mean increase in caloric intake was 227 calories per day in those who quit smoking, which explains up to 69% of the weight gained at 3 months post-cessation48. Of those who stop smoking, 13% gained >10 kg in a year, with weight gain greatest in the first few months after cessation and continuing to increase for ≥6 months48. Post-cessation weight gain leads to increased body fat31. In the USA, associated risk factors for post-cessation weight gain include: African-American ethnicity; age <55 years; a history of heavy smoking (defined as >25 cigarettes per day); lower socioeconomic status; and genetic factors (as demonstrated in twin studies)31. Additionally, many smokers are already overweight when they attempt to quit smoking33.
Box 1. Highlights of systematic review on post-smoking cessation weight gain46.
Before January 2015, five e-databases were searched to collect data. Population-based prospective cohort studies that included weight change from baseline (pre-cessation) to follow-up (≥3 months post-cessation) were included, comprising 35 studies in total that assessed 63,403 individuals who had quit smoking and 388,432 smokers.
Individuals who stopped smoking had a significant association with absolute weight gain; among these individuals the mean weight gain was 4.10 kg (95% CI 2.69–5.51; P <0.001 compared to those who continued to smoke) whereas the mean increase in BMI was 1.14 kg/m2 units over a 5-year period. (95% CI 0.50–1.79; P = 0.137 compared to those who continued to smoke).
Overall, these data showed that weight gain is a major adverse effect of smoking cessation.
However, the adverse effect of weight gain does not offset the beneficial effects of smoking cessation. Physicians should, therefore, continue to encourage their patients to pursue smoking cessation in order to become healthier and avoid or reduce negative health consequences. In addition, clinicians and researchers should focus on designing and implementing successful weight management programs for individuals who are at every stage of trying to cease smoking.
Figure 4. Weight gain within the first year of attempting to quit smoking.
Point prevalence abstinence group includes individuals who were not continuously abstinent but who were abstinent over the 7 days before testing. At baseline, the average age of participants (n = 196) was 44.5 years. Modified with permission of Wiley © Audrain-McGovern, J. & Benowitz, N. L. Clin. Pharmacol. Ther. 90, 164–168 (2011).
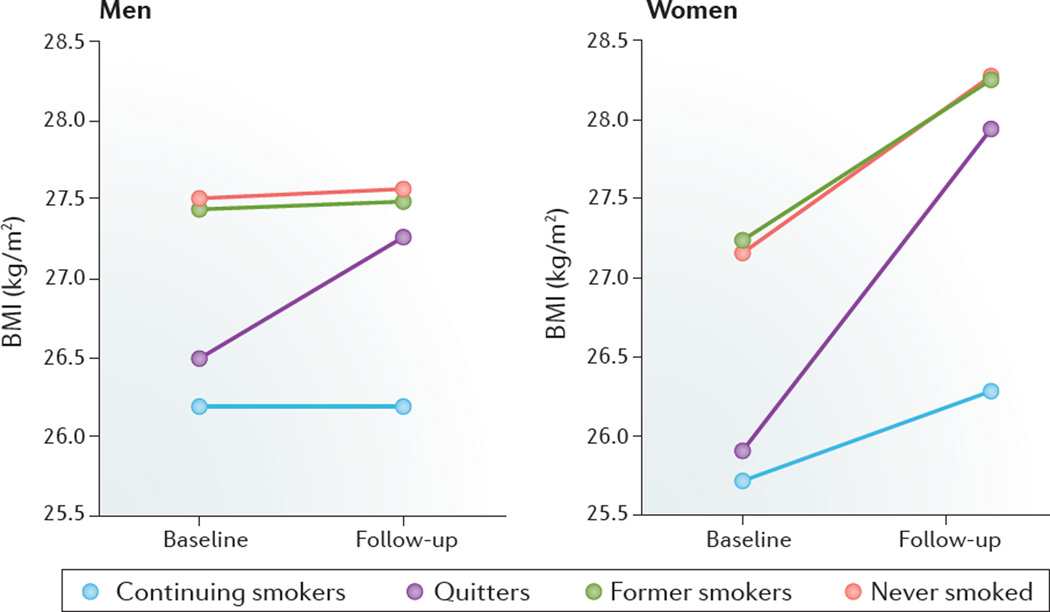
Figure 5. Changes in BMI over 10 years with smoking status.
At baseline, female participants (n = 5,639) and male participants (n = 3,365) had an average age of 47.1 years and 43.8 years, respectively. Modified with permission of Wiley © Audrain-McGovern, J. & Benowitz, N. L. Clin. Pharmacol. Ther. 90, 164–168 (2011).
Another meta-analysis that included 62 studies analysed weight gain in smokers who had not smoked for at least 12 months49. The researchers found that post-cessation weight gain was greater than previously thought. On average, those who stopped smoking and did not use nicotine replacements or other drugs gained 1.1 kg during the first month and 2.3 kg during the second month; weight gain steadily increased by up to 4.7 kg at 12 months post-cessation. Thirteen percent of individuals gained >10 kg after cessation; interestingly, 16% of those who stopped smoking lost weight after quitting49. The pattern or amount of weight gain was not reduced by pharmacotherapy. Similar weight gains occurred in individuals not receiving pharmacotherapy and in those using nicotine replacement, bupropion or varenicline.
Although on average smokers weigh less than non-smokers, the percentage of smokers who are overweight or obese (and who utilize smoking cessation quitlines50 — telephone-based advice for smokers who want to stop smoking) is reflective of that in the general population at large. In a study of 595 quitline participants, 206 had normal weight (34.6%), 182 were overweight (30.6%) and 207 had obesity (34.8%)51. More women than men had obesity and they had greater concerns about post-cessation weight gain. Individuals with obesity also expressed greater concern about post-cessation weight gain, expressed less confidence in being able to maintain their weight after stopping smoking, and were less willing to gain weight after smoking than nonobese individuals51. A 5 kg weight gain after stopping smoking can, therefore, stop and deter future cessation attempts33. However, in another study that assessed 595 quitline participants, although post-cessation weight gain occurred, no consistent association between baseline weight and cessation treatment adherence, cessation or post-cessation weight gain at 6 months was evident52.
Additionally, in the Oxfordshire (UK) general practice nicotine patch/placebo prospective cohort trial with an 8-year follow-up, those who abstained from smoking gained 8.8 kg at the 8-year follow-up53. Smokers gained 2.2 kg and relapsed smokers gained 3.3 kg; late abstainers (those who smoked after the first year but who were not smoking after 8 years) gained 8.3 kg. Smokers had a negative linear association with BMI (those with a lower BMI gained more weight than those with a higher BMI), while abstainers showed a J-shaped curve association with BMI (smoking cessation led to weight gain, with the most rapid weight gain observed during the first 6 months of cessation)53.
The influence of sex and the number of cigarettes smoked per day on weight gain 1 year post-cessation has also been investigated54. Individuals who abstained from smoking for at least 40 weeks gained 4.6 kg whereas those who abstained for <20 weeks gained 1.2 kg54. In this study, a link between weight gain and age, sex and the number of cigarettes smoked per day before quitting was also identified. Younger (lowest age tertile) participants gained more weight than older (highest age tertile) participants. For light smokers, men gained more weight than women, whereas for heavy smokers, women gained more weight than men. These findings suggest that young women who smoke heavily are at the highest risk of gaining weight after quitting smoking.
The factors associated with weight gain following smoking cessation have also been identified55. In this study, BMI significantly increased from 23.5 ± 3.6 kg/m2 at the initial consultation to 23.9 ± 3.8 kg/m2 3 months after the start of smoking-cessation therapy. Plasma levels of triglycerides and HDL cholesterol, daily cigarette consumption and the Fagerström test for nicotine dependence (FTND) score significantly correlated with the increase in BMI after initiation of cessation therapy, with the FTND score being the strongest factor. The results of this study suggest that smokers with high nicotine dependence (that is, a high FTND score) are more likely to gain weight post-cessation than those with low nicotine dependence. Predictors of weight change in sedentary smokers following smoking cessation therapy have also been identified56. Overall, weight was found to increase during the first 3 months post-cessation, and to stabilize afterwards. On average, men gained 3.9 kg and women gained 3.3 kg 1 year after smoking cessation. High nicotine dependence was also associated with the greatest weight gain after cessation56. Furthermore, men experienced more weight gain during abstinence, which contradicts other studies46,53 in which women experience more weight gain following cessation of smoking. Older age (above the median age at entry of 43 years) was associated with continuing weight gain during relapse56. Overall, this study56 suggests that older smokers, those who are men, and those with high nicotine dependence probably gain most weight after quitting smoking.
Mechanisms
Several theories have been proposed to explain increased food intake after smoking cessation. One theory is that the ability of nicotine to suppress appetite is reversed57. Substitution reinforcement, which replaces the rewards of food with the rewards of cigarettes could occur58. Nicotine absence increases the rewarding value of food. Reward circuitries in the brain, similar to those activated by smoking, are activated by increased intake of food high in sugar and fat59. Furthermore, nicotine withdrawal leads to an elevated reward threshold, which might cause individuals to eat more snacks that are high in carbohydrates and sugars60. Additionally, nicotine and/or smoking help control compulsive eating and overeating; during post-cessation these activities are inhibited. Smokers with a history of binge eating during cessation exhibit lower quit rates and gain more weight than those without a history of binge eating61. Furthermore, smoking bans, both in public spaces and in the home, are associated with smoking cessation and, indirectly, with the development of obesity as a result of nicotine being replaced by food (a compensatory behaviour)62.
Glycaemic parameters following a 3 h oral glucose tolerance test were examined in 14 individuals who achieved smoking abstinence63. Participants who stopped smoking had increased body mass (by 4 kg) and fat mass (by 22%), along with marked fasting hyperinsulinaemia and fasting insulin resistance; oral glucose insulin sensitivity was unchanged. β-cell secretion, as measured by the insulinogenic index I40, was increased by 31%. In addition, carbohydrate intake levels and fasting serum levels of neuropeptide Y increased, which suggests that neuropeptide Y functions as a hormone that mediates post-cessation weight gain. However, no change was found in oral glucose insulin sensitivity or levels of peptide YY, glucagon-like peptide 1, leptin, ghrelin or visfatin63. The authors of the study concluded that smoking cessation is associated with transient metabolic changes that include increased β-cell secretion.
A contributing social factor to post-cessation weight gain might be socioeconomic status (SES). The majority of smokers are of lower SES than nonsmokers64. In general, lower SES correlates with diminished physical activity and consumption of high-calorie and high-fat diets that can contribute to post-cessation weight gain31.
An interesting study published in 2013 found that smoking cessation led to marked changes in the composition of the intestinal microbiota in humans65. The microbiota composition of smokers undergoing cessation was compared to that of control individuals (current smokers and nonsmokers) using terminal restriction fragment length polymorphism analysis and high-throughput sequencing. A shift in the composition of the intestinal microbiota was observed in smokers undergoing cessation compared with that in control individuals; this shift was characterized by a higher proportion of Firmicutes and Actinobacteria and a lower proportion of Bacteroidetes and Proteobacteria65. Overall, increased microbial diversity was evident. The investigators followed-up this study by conducting further microbial analyses using fluorescence in situ hybridization66. Again, microbiota composition was markedly altered by smoking cessation, with an increase in the number of Firmicutes and Actinobacteria and a decrease in the number of Bacteriodetes and Proteobacteria66. These results suggest that smoking is an environmental factor that affects the composition of the gut microbiota in humans. These observed changes are similar to differences in the gut microbiota composition of obese and lean humans and mice. Changes in the composition of the gut microbiota following smoking cessation could, thus, partially explain post-cessation weight gain.
Weight management programs
No substantial data exists showing that any smoking cessation approach is effective at limiting post-cessation weight gain33. In a Cochrane Database systematic review, education about weight management was insufficient to prevent post-cessation weight gain67. However, very modest positive results were found when personalized weight-management programs that provided feedback on individual weight-management goals and individualized energy prescriptions were utilized for individuals concerned about weight gain67. Pre-smoking-cessation concerns about weight gain did not reduce overall post-cessation weight gain; less weight was gained initially (1–2 months post-cessation), but this gain disappeared 12 months after cessation45.
A 2012 meta-analysis found that weight gain after smoking cessation decreased in a minority of individuals receiving weight management education and advice, without compromising cessation success49. Weight gain after cessation of smoking was 4–5 kg at 12 months and most of this weight was gained in the first 3 months. Analysis of the data showed that up to 21% of those who had stopped smoking had lost weight at 1 year post-cessation; over the same period, 48% of those who stopped smoking gained weight in excess of 5 kg49. Importantly, these results are only from studies that included individuals who were utilizing interventions to minimize post-cessation weight gain. One hypothesis about weight management during smoking cessation is that weight loss is achieved independently after patient confidence in cessation is secured. Many individuals might be unable or unwilling to focus on any other lifestyle changes beyond smoking cessation. Successful weight management has been observed in smoking cessation programs that contain some form of dietary advice. Those undergoing smoking cessation often differ on which approach to use: cessation first and then weight management; or cessation and weight management simultaneously33.
In a pilot study that used multivariate logistic regression to control for covariates and/or factors such as age, sex, ethnicity, education level, level of nicotine dependence, BMI and weight gain concern level, attendance at the first scheduled contact increased when a weight management program was offered (88.1% versus 71.6%). Furthermore, 6-month abstinence increased (21.4% versus 10.1%)68. Individuals who were offered weight management advice were fivefold more likely to attend the first session and three times more likely to be abstinent 6 months after cessation treatment than those not offered weight management advice. Proactively informing weight-concerned smokers who are overweight or have obesity about weight management programs can incentivize them to start and complete nicotine-cessation programs.
A different approach is to address concerns about post-cessation weight gain and not weight gain itself, as concerns about weight gain are a stronger predictor of relapse than weight gain itself69. In one study, women who smoked were divided into three groups: a smoking-cessation counselling group, a smoking-cessation counselling and weight-control group, and a smoking-cessation counselling and weight-control concern reduction group70. At the 1-year follow-up, 21% of women in the weight-concern group compared with 13% in the weight-control group and 9% in the standard smoking-cessation group were abstinent from smoking70. Women in the weight-concern group also gained less weight post-cessation (2.48 kg) than women in the weight-control group (5.36 kg) and the standard smoking-cessation group (7.60 kg)70. Hence, reducing concerns about weight gain is more important than reducing weight gain before and during cessation31.
Pharmacotherapy
Several pharmacotherapies including bupropion, nicotine-replacement medications, fluoxetine and varenicline (a partial agonist of the α4β2 nicotinic acetylcholine receptor) have been investigated for preventing and reducing post-cessation weight gain71. However, these medications have been found to delay, rather than prevent, post-cessation weight gain71. On discontinuation of pharmacotherapies, individuals gain weight at the level they would have had they not taken the medication31. Nevertheless, temporary suppression of weight gain might increase smokers’ motivation to quit, thereby providing time for concerned smokers to focus first on stopping smoking and then to focus on weight management. Bupropion decreases the nicotine–reward threshold, reduces the effect of food reward and decreases weight gain72. In a 2010 study, bupropion therapy combined with a weight-gain-preventing–smoking-cessation intervention resulted in higher levels of smoking abstinence at 6 months than standard cessation counselling plus either bupropion or placebo (34%, 21% and 12%, respectively). However, no significant differences in weight gain were found in women at 3 months, 6 months or 12 months post-cessation72.
In a randomized placebo-controlled clinical trial on extended (24 weeks) versus standard (8 weeks + 16 weeks placebo) transdermal nicotine patch therapy, which was controlled for sex, baseline smoking rate and previous smoking rate, individuals who quit smoking gained 4.9–5.4 kg on average73. Compared to participants who received 8 weeks of therapy, participants in the 24-week treatment group gained less weight between pretreatment and week 24, and between week 8 and week 24; additionally, this group had greater adherence to transdermal nicotine patch therapies, which led to reduced weight gain73. Extending the duration of nicotine replacement therapy might, therefore, decrease post-cessation weight gain.
A study conducted in Japan has compared the effectiveness of varenicline and nicotine patch therapy in limiting weight gain after smoking cessation74. Participants receiving varenicline gained 0.9 kg of weight compared with 2.8 kg of weight gained by those using nicotine patch therapy. Multivariate linear regression analysis revealed that varenicline users experienced considerably less weight gain than those who used nicotine patches. These results suggest that varenicline might be more effective than nicotine patch therapy in limiting weight gain post-cessation, due to its selective activation of the α4β2 nicotinic acetylcholine receptor.
The effectiveness of osmotic-release oral system methylphenidate (known as OROS–MPH) in limiting post-cessation weight gain has been examined in adult individuals with attention-deficit/hyperactivity disorder (ADHD)75. As adult individuals with ADHD are at a higher risk of smoking and overweightness or obesity, and OROS–MPH treatment often leads to weight loss, this treatment might help limit weight gain following smoking cessation. Individuals treated with OROS–MPH lost 1.6% of their body weight on average 11 weeks post-cessation, whereas control individuals given placebo gained 1.3% of their body weight on average75.
Fruit and vegetable intake
Including the consumption of more fruits and vegetables into a nutritional advice component of smoking cessation programs might assist smoking cessation and lead to less post-cessation weight gain. In one study focusing on the diet of 373,803 participants from 10 European countries participating in the European Prospective Investigation into Cancer and Nutrition Study, after controlling for multiple covariates, an inverse relationship between baseline fruit and vegetable intake and weight change was found in individuals who ceased smoking76. A weak positive association between vegetable intake and weight change in former female smokers was found. Furthermore, a weak inverse association between fruit intake and weight change was found in women who had never smoked. Baseline fruit and vegetable intake can, therefore, help to reduce post-cessation weight gain. Fruit and vegetables might prevent weight gain due to their low-energy density and high dietary fibre content76. In the Greater Glasgow (UK) cluster randomized controlled study from January to August 2008, smokers in a nutritional intervention group gained more mean weight than a control group who received no nutritional guidance during cessation; weight gain at 8 months was 3.9 kg and 2.7 kg, respectively77. Notably, the intervention group consumed more fruits, vegetables and breakfast cereal than the control group. Furthermore, more individuals in the intervention group continued to abstain from smoking than in the control group. The nutritional intervention only improved dietary habits of participants, but did not change body weight. Improved quit rates in the intervention group might be due to contact with counsellors who reduced participants’ anxiety about weight gain and encouraged them to stop smoking despite weight gain.
Post-cessation weight control summary
During smoking cessation, a decrease in metabolic expenditure occurs without an accompanying increase in physical activity31. A positive energy balance ensues that leads to weight gain. Behavioural interventions that manage post-cessation weight gain by focusing on reducing caloric intake, increasing physical activity or both, might be helpful. Weight-control interventions have only proven beneficial in terms of cessation and weight control during the initial first 6 months. Weight management programs along with cessation programs, thus, do not hinder cessation31.
Gestational weight
Smoking cessation is associated with excessive gestational weight gain78. Not only does smoking cessation lead to weight gain in smokers, but it can also lead to weight gain in the offspring of mothers undergoing smoking cessation. A Japanese prospective cohort study of 2,663 mothers (data collected from 83.7% of participants) and their children born between 1991 and 2006 assessed the relationship between smoking status of the mother and infant and childhood weight79. Mothers who smoked during pregnancy gave birth to infants who were ~120–150 g smaller than normal. Additionally, at age 3 years, the BMI of boys born to these mothers who smoked was substantially higher than that in boys born to mothers who did not smoke. Children of mothers who ceased smoking before or during early pregnancy showed no signs of being small for gestational age or having childhood obesity79.
Data from the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development found that children whose mothers smoked within 1 year before the birth of their child were more likely to have a higher than average BMI80. Mothers who smoked 1 year before birth were also more likely to have an overweight child with a greater BMI percentile average in grades 1–6 than mothers who never smoked. Mothers’ education and the birth weight of the child were also strongly associated with children being overweight. According to these findings, women need to cease smoking at least 3 months before becoming pregnant to decrease their and their offspring’s chance of being overweight80.
Additionally, an increase in cigarette taxes has been shown to correlate with decreased rates of smoking among white and black mothers with low education; however, their babies had larger than normal birth weights81. In this study, for every US$1 tax increase, the smoking level decreased by 2.4% for white mothers and 2.1% for black mothers; furthermore, the birth weight of their infants increased by 5.4 g and 4.0 g, respectively, which might have beneficial long-term results.
Metabolic diseases
Smoking status and BMI
In a 2008–2010 cross-sectional analysis of 4,656 men from South Korea, aged 19–70 years, both current and former smokers had more visceral adipose tissue (VAT) than those who had never smoked82. Increased mean VAT was associated with higher daily pre-cessation cigarette consumption, longer smoking duration and shorter smoking abstinence. The highest mean VAT in former smokers occurred within 2 years of abstinence. No significant difference in mean VAT between individuals who had abstained from smoking for >20 years and individuals who had never smoked was found.
The Hitachi Health Study used abdominal CT to assess the prevalence of the metabolic syndrome in 5,697 male individuals from Korea83. This study used the diagnostic criteria set forth by the National Cholesterol Education Program Adult Treatment Panel III, which states that the metabolic syndrome is a combination of three of the following five conditions: insulin resistance, obesity, hyperglycaemia, atherogenic dyslipidaemia and elevated blood pressure84. After adjusting for age, alcohol intake and energy expenditure, a relationship between smoking status and the metabolic syndrome and some of its components was identified83 (TABLE 1). Ex-smokers, with the exception of individuals who abstained from smoking for ≥15 years, had more visceral adiposity, a larger ratio of visceral to subcutaneous adipose tissue and a higher incidence of elevated plasma triglyceride levels, hyperglycaemia and the metabolic syndrome than nonsmokers.
Table 1.
Relationship between smoking status and the metabolic syndrome
| Smoking status | The metabolic syndrome (OR) |
Mean visceral area (cm2) |
Ratio of visceral to subcutaneous adipose tissue |
Plasma triglyceride levels (OR) |
Hyperglycaemia (OR) |
|---|---|---|---|---|---|
| Nonsmokers | 1.00* | 123.10 | 0.95‡ | 1.00* | 1.00* |
| Current smokers | 1.02 | 120.40* | 0.98* | 1.30§ | 1.08 |
| Ex-smoker for ≤4 years |
1.33‖ | 130.60 | 1.01 | 1.26¶ | 1.44# |
| Ex-smoker for 5–9 years |
1.36‖ | 132.00** | 0.97 | 1.13 | 1.50# |
| Ex-smoker for 10–14 years |
1.40 | 131.70** | 1.00 | 1.36¶ | 1.44# |
| Ex-smoker for ≥15 years |
1.09 | 124.00 | 0.96 | 1.11 | 1.08 |
Reference population for comparison of data.
Statistically significant difference compared with reference (current smokers).
Statistically significant difference compared with reference (nonsmokers).
Statistically significant difference compared with reference (nonsmokers).
Statistically significant difference compared with reference (nonsmokers).
Statistically significant difference compared with reference (nonsmokers).
Statistically significant difference compared with reference (current smokers). Modified with permission from Wiley © Matsushita, Y et al. Obesity (Silver Spring) 19, 647–651 (2011).
These findings might be explained by adiponectin, an adipocyte-specific protein that is involved in metabolic processes such as the regulation of serum glucose levels and fatty acid catabolism85–89. An inverse association exists between levels of adiponectin and an increased risk of developing the metabolic syndrome90. In a study of 28 male smokers who successfully abstained from smoking for 2 months with the aid of transdermal nicotine treatment, individuals were divided into two groups: weight maintainers (n = 10) and weight gainers (n = 18)91. In the weight-gaining group, serum levels of adiponectin increased 1 week after initiation of transdermal nicotine therapy; at 9 weeks, levels of adiponectin were markedly decreased. Additionally, a significant increase in insulin resistance as defined by homeostatic model assessment (HOMA index; a method used to assess β-cell function and insulin resistance)92 was found in the weight-gaining group. However, no significant changes in adiponectin levels or HOMA index were found in the weight-maintaining group. Overall, the results of this study showed that post-cessation weight gain is associated with decreased adiponectin levels and increased risk of the metabolic syndrome91.
Smoking can lead to insulin resistance and T2DM, and can contribute to an atherogenic lipid profile93. Even though smokers probably weigh less than they would if they were not smokers, they still have a high ratio of visceral to subcutaneous adipose tissue82,94,95, which is a cardiovascular risk factor. Estimates suggest that 20% of smokers have obesity, although this estimate depends on the population studied. The idea of treating tobacco dependence and obesity together is a provocative one. Rimonabant, a cannabinoid receptor antagonist, has been used to treat obesity96 and nicotine dependence97, and is also effective against the metabolic syndrome98. However, rimonabant had adverse psychiatric effects and did not receive FDA approval96. The effectiveness of bupropion in smoking cessation and weight loss might be due to increased central levels of dopamine and noradrenaline99, similar to effects mediated by nicotine itself. Although nicotine controls food intake and ultimately body weight (FIG. 3), a greater understanding of how nicotine functions in the brain to control weight is required.
Insulin secretion and resistance
Nicotine might influence β-cell function directly or indirectly via the parasympathetic ganglia63. The results from animal studies and in vitro experiments show that nicotine exposure results in β-cell toxicity100–102. In rabbits, high concentrations of nicotine inhibit glucose-induced insulin secretion, whereas low doses stimulate insulin secretion103. In mice, activation of the α2 subtype of 5´-AMP-activated protein kinase in adipocytes is essential for nicotine-induced insulin resistance104.
In 2015, we used hyperinsulinaemic–euglycaemic clamps coupled with stable isotope tracers to measure hepatic glucose output and indirect calorimetry to measure substrate utilization, as part of a study that enrolled healthy smokers who smoked between half a pack and two packs per-day in an 8-week smoking cessation program of behavioural counseling plus oral bupropion (phase I)105. This phase was followed by a 16-week maintenance period without counselling or bupropion, wherein participants either remained abstinent or naturally resumed and/or increased smoking (phase II)105. Cessation (phase I) reduced the number of cigarettes smoked per day and levels of carbon monoxide and nicotine metabolites; no further changes occurred during phase II. The ratio of central to peripheral adipose tissue trended higher during phase I (inversely correlated with carbon monoxide levels), but then fell markedly during phase II. Unadjusted basal hepatic glucose output decreased over 24 weeks; changes in hepatic glucose output correlated directly with changes in carbon monoxide levels. Weight changes correlated directly with changes in levels of nicotine metabolites during phase II and overall. Over 24 weeks, changes in levels of carbon monoxide and nicotine metabolites inversely correlated with changes in several measures of glucose uptake, glucose oxidation and the respiratory quotient. This study showed that smoking cessation produces a transient worsening of central fat redistribution, which is followed by a more significant improvement in central fat redistribution, along with other net beneficial metabolic effects.
Insulin sensitivity has been shown to improve with smoking cessation that occurs in conjunction with normalization of phosphorylation of insulin receptor substrate 1 (IRS-1) at Ser636106. In cell culture studies, nicotine stimulates the two pathways known to stimulate IRS-1 (Ser636) phosphorylation (p44/42 mitogen-activated protein kinase (MAPK)) and mammalian target of rapamycin (mTOR). These findings indicate that nicotine induces insulin resistance in skeletal muscle by activating mTOR, which can be reversed by smoking cessation.
Smoking cessation can also lessen chronic damage caused by T2DM. The effect of smoking cessation on microalbuminuria (defined as a ratio of albumin to creatinine of 30–300 µg/mg) in patients with newly diagnosed T2DM has been evaluated in one study107. Of 500 individuals with newly diagnosed T2DM and microalbuminuria, 196 participants were educated about smoking cessation and weight management. During the study, ~62% of participants quit smoking and at the 1 year follow-up, the prevalence of microalbuminuria was reduced by 72.6% in the cessation population and by 22.5% in those individuals that continued to smoke107. The investigators concluded that smoking cessation in patients with newly diagnosed T2DM is associated with amelioration of metabolic parameters, blood pressure and a reduction in microalbuminuria107.
Conclusions
Smoking cessation can alleviate diseases caused by smoking and nicotine108, and can also lead to weight gain. Post-cessation weight gain is often underestimated in self-reports33. The 1997–2004 National Health Interview Survey–National Death Index Linkage study showed that normal-weight smokers had a higher mortality risk from all smoking-related diseases combined than ex-smokers who were overweight or had obesity45. Post-cessation weight gain is, therefore, less harmful than smoking. Individuals with obesity who quit smoking have the highest need for interventions to ameliorate weight gain53. Smokers with obesity, especially women, need more effective weight management treatment in conjunction with a weight gain anxiety reduction component, along with smoking cessation treatment51. To maximize smoking cessation, approaches that account for overeating during the first 6 months of cessation must be considered31.
Key points.
In general, an inverse relationship between smoking and BMI exists
Post-cessation-related obesity might contribute to insulin resistance
The number one reason for not wanting to quit smoking or quitting and then relapsing is fear of post-cessation weight gain, especially in women and in individuals with obesity
Future smoking cessation programs and therapies need to be designed with an emphasis on reducing post-cessation weight gain
The benefits of smoking cessation outweigh the risks
Review criteria.
In 2012, we published a review article on the endocrine effects of nicotine and cigarette smoke that reviewed the endocrine and metabolic effects of nicotine and/or smoke published in 2010 and earlier110. This Review, thus, included articles published in 2010 and later and focused on the metabolic effects of smoking and smoking cessation. PubMed was searched for relevant topics using the following pairs of search terms: “smoking cessation AND obesity”, “smoking cessation AND weight”, “smoking cessation AND insulin sensitivity”, “smoking cessation AND body composition”, “smoking cessation AND body mass index”, “nicotine withdrawal AND obesity” and “nicotine withdrawal AND insulin”. References cited in this Review included primarily English-language original research articles and some specific review articles. Searches were limited to articles published within the past 5 years. Articles were also included (including some older than 5 years) if they were cited by other articles and if they contributed to the topic of this Review.
Acknowledgments
Salary support for T.C.F. was provided by the Diversity-promoting Institutions Drug Abuse Research Development Program (grant R24DA017298) and the National Institute on Minority Health and Health Disparities (grant U54MD007598). The authors acknowledge the professional development core of the Charles R. Drew University Accelerating Excellence in Translational Science (AXIS) (grant U54MD007598) for help with editing the manuscript.
Footnotes
Author contributions
K.K.H., M.Z. and T.C.F. researched data for the article. K.K.H. and T.C.F. provided substantial contributions to discussions of the content. K.K.H., M.Z. and T.C.F. wrote the article and reviewed and/or edited the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
References
- 1.World Health Organization. Tobacco. Fact sheet N°339. [online] 2015 [Google Scholar]
- 2.Yuen BG, et al. Association between smoking and uveitis: results from the Pacific Ocular Inflammation Study. Ophthalmology. 2015;122:1257–1261. doi: 10.1016/j.ophtha.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roura E, et al. Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort. Int. J. Cancer. 2014;135:453–466. doi: 10.1002/ijc.28666. [DOI] [PubMed] [Google Scholar]
- 4.Jaramillo JD, et al. Reduced bone density and vertebral fractures in smokers. Men and COPD patients at increased risk. Ann. Am. Thorac. Soc. 2015;12:648–656. doi: 10.1513/AnnalsATS.201412-591OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General, 2014. 2014 [online], http://www.surgeongeneral.gov/library/reports/50-years-of-progress/
- 6.Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil. Steril. 2012;98:1400–1406. doi: 10.1016/j.fertnstert.2012.07.1146. [DOI] [PubMed] [Google Scholar]
- 7.Ruan X, Mueck AO. Impact of smoking on estrogenic efficacy. Climacteric. 2015;18:38–46. doi: 10.3109/13697137.2014.929106. [DOI] [PubMed] [Google Scholar]
- 8.Pedone C, Incalzi RA. Smoking and mortality — beyond established causes. N. Engl. J. Med. 2015;372:2169. doi: 10.1056/NEJMc1503675. [DOI] [PubMed] [Google Scholar]
- 9.Grando SA. Connections of nicotine to cancer. Nat. Rev. Cancer. 2014;14:419–429. doi: 10.1038/nrc3725. [DOI] [PubMed] [Google Scholar]
- 10.Kowall B, et al. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the KORA S4/F4 cohort study. Eur. J. Epidemiol. 2010;25:393–402. doi: 10.1007/s10654-010-9452-6. [DOI] [PubMed] [Google Scholar]
- 11.Kermah D, Shaheen M, Pan D, Friedman TC. Multivariate data analysis from the National Health and Nutrition Examination Survey (NHANES) 2001–2006 shows that second-hand smoke is associated with both obesity and diabetes mellitus. Endocrine Society. 2012 [online], http://press.endocrine.org/doi/abs/10.1210/endo-meetings.2012.DGM.5.SUN-192. [Google Scholar]
- 12.Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet. 1992;339:1128–1130. doi: 10.1016/0140-6736(92)90730-q. [DOI] [PubMed] [Google Scholar]
- 13.Assali AR, Beigel Y, Schreibman R, Shafer Z, Fainaru M. Weight gain and insulin resistance during nicotine replacement therapy. Clin. Cardiol. 1999;22:357–360. doi: 10.1002/clc.4960220512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance — a potential link with the insulin resistance syndrome. J. Intern. Med. 1993;233:327–332. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 15.Hautanen A, Adlercreutz H. Hyperinsulinaemia, dyslipidaemia and exaggerated adrenal androgen response to adrenocorticotropin in male smokers. Diabetologia. 1993;36:1275–1281. doi: 10.1007/BF00400805. [DOI] [PubMed] [Google Scholar]
- 16.Janzon L, Berntorp K, Hanson M, Lindell SE, Trell E. Glucose tolerance and smoking: a population study of oral and intravenous glucose tolerance tests in middle-aged men. Diabetologia. 1983;25:86–88. doi: 10.1007/BF00250893. [DOI] [PubMed] [Google Scholar]
- 17.Kong C, et al. Smoking is associated with increased hepatic lipase activity, insulin resistance, dyslipidaemia and early atherosclerosis in type 2 diabetes. Atherosclerosis. 2001;156:373–378. doi: 10.1016/s0021-9150(00)00664-x. [DOI] [PubMed] [Google Scholar]
- 18.Ronnemaa T, Ronnemaa EM, Puukka P, Pyorala K, Laakso M. Smoking is independently associated with high plasma insulin levels in nondiabetic men. Diabetes Care. 1996;19:1229–1232. doi: 10.2337/diacare.19.11.1229. [DOI] [PubMed] [Google Scholar]
- 19.Targher G, et al. Cigarette smoking and insulin resistance in patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1997;82:3619–3624. doi: 10.1210/jcem.82.11.4351. [DOI] [PubMed] [Google Scholar]
- 20.Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J. Intern. Med. 1992;231:25–30. doi: 10.1111/j.1365-2796.1992.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg HO, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha-Hikim I, et al. Nicotine in combination with a high-fat diet causes intramyocellular mitochondrial abnormalities in male mice. Endocrinology. 2014;155:865–872. doi: 10.1210/en.2013-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat. Rec. (Hoboken) 2008;291:684–692. doi: 10.1002/ar.20715. [DOI] [PubMed] [Google Scholar]
- 24.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim. Biophys. Acta. 2010;1801:299–310. doi: 10.1016/j.bbalip.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Liu RH, Mizuta M, Matsukura S. Long-term oral nicotine administration reduces insulin resistance in obese rats. Eur. J. Pharmacol. 2003;458:227–234. doi: 10.1016/s0014-2999(02)02726-7. [DOI] [PubMed] [Google Scholar]
- 27.Nakhate KT, Dandekar MP, Kokare DM, Subhedar NK. Involvement of neuropeptide YY1 receptors in the acute, chronic and withdrawal effects of nicotine on feeding and body weight in rats. Eur. J. Pharmacol. 2009;609:78–87. doi: 10.1016/j.ejphar.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Seeley RJ, Sandoval DA. Neuroscience: weight loss through smoking. Nature. 2011;475:176–177. doi: 10.1038/475176a. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, et al. Long-term cigarette smoke exposure increases uncoupling protein expression but reduces energy intake. Brain Res. 2008;1228:81–88. doi: 10.1016/j.brainres.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 30.Ypsilantis P, et al. Effects of cigarette smoke exposure and its cessation on body weight, food intake and circulating leptin, and ghrelin levels in the rat. Nicotine Tob. Res. 2013;15:206–212. doi: 10.1093/ntr/nts113. [DOI] [PubMed] [Google Scholar]
- 31.Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin. Pharmacol. Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bajaj M. Nicotine and insulin resistance: when the smoke clears. Diabetes. 2012;61:3078–3080. doi: 10.2337/db12-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hankey C, Leslie W. Obesity: is weight gain after smoking cessation an important concern? Nat. Rev. Endocrinol. 2012;8:630–632. doi: 10.1038/nrendo.2012.175. [DOI] [PubMed] [Google Scholar]
- 34.Clair C, et al. Dose-dependent positive association between cigarette smoking, abdominal obesity and body fat: cross-sectional data from a population-based survey. BMC Public Health. 2011;11:23. doi: 10.1186/1471-2458-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuji T, et al. Macrophage elastase suppresses white adipose tissue expansion with cigarette smoking. Am. J. Respir. Cell Mol. Biol. 2014;51:822–829. doi: 10.1165/rcmb.2014-0083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath-Morrow SA, et al. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS ONE. 2015;10:e0118344. doi: 10.1371/journal.pone.0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez de Morentin PB, et al. Nicotine induces negative energy balance through hypothalamic AMP-activated protein kinase. Diabetes. 2012;61:807–817. doi: 10.2337/db11-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenberg NE, Huang B, Seshadri S, Tucker TC. Trends in cigarette smoking and obesity in Appalachian Kentucky. South. Med. J. 2015;108:170–177. doi: 10.14423/SMJ.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 39.Jamal A, et al. Current cigarette smoking among adults — United States, 2005–2013. MMWR Morb. Mortal. Wkly Rep. 2014;63:1108–1112. [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Quitting smoking. 2015 [online], http://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/quitting/index.htm.
- 41.Bilano V, et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet. 2015;385:966–976. doi: 10.1016/S0140-6736(15)60264-1. [DOI] [PubMed] [Google Scholar]
- 42.US Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. National Center for Biotechnology Information. 2008 [online], http://www.ncbi.nlm.nih.gov/books/NBK63952/
- 43.Fiore MC, Fleming MF, Burns ME. Tobacco and alcohol abuse: clinical opportunities for effective intervention. Proc. Assoc. Am. Physicians. 1999;111:131–140. doi: 10.1046/j.1525-1381.1999.09249.x. [DOI] [PubMed] [Google Scholar]
- 44.Casella G, Caponnetto P, Polosa R. Therapeutic advances in the treatment of nicotine addiction: present and future. Ther. Adv. Chronic Dis. 2010;1:95–106. doi: 10.1177/2040622310374896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siahpush M, et al. It is better to be a fat ex-smoker than a thin smoker: findings from the 1997–2004 National Health Interview Survey-National Death Index linkage study. Tob. Control. 2014;23:395–402. doi: 10.1136/tobaccocontrol-2012-050912. [DOI] [PubMed] [Google Scholar]
- 46.Tian J, Venn A, Otahal P, Gall S. The association between quitting smoking and weight gain: a systemic review and meta-analysis of prospective cohort studies. Obes. Rev. 2015;16:883–901. doi: 10.1111/obr.12304. [DOI] [PubMed] [Google Scholar]
- 47.Klesges RC, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol. Bull. 1989;106:204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- 48.Williamson DF, et al. Smoking cessation and severity of weight gain in a national cohort. N. Engl. J. Med. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 49.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lichtenstein E, Zhu SH, Tedeschi GJ. Smoking cessation quitlines: an underrecognized intervention success story. Am. Psychol. 2010;65:252–261. doi: 10.1037/a0018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levine MD, Bush T, Magnusson B, Cheng Y, Chen X. Smoking-related weight concerns and obesity: differences among normal weight, overweight, and obese smokers using a telephone tobacco quitline. Nicotine Tob. Res. 2013;15:1136–1140. doi: 10.1093/ntr/nts226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bush TM, et al. Impact of baseline weight on smoking cessation and weight gain in quitlines. Ann. Behav. Med. 2014;47:208–217. doi: 10.1007/s12160-013-9537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lycett D, Munafò M, Johnstone E, Murphy M, Aveyard P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction. 2011;106:188–196. doi: 10.1111/j.1360-0443.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- 54.Locatelli I, Collet TH, Clair C, Rodondi N, Cornuz J. The joint influence of gender and amount of smoking on weight gain one year after smoking cessation. Int. J. Environ. Res. Public Health. 2014;11:8443–8455. doi: 10.3390/ijerph110808443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Komiyama M, et al. Analysis of factors that determine weight gain during smoking cessation therapy. PLoS ONE. 2013;8:e72010. doi: 10.1371/journal.pone.0072010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prod’hom S, et al. Predictors of weight change in sedentary smokers receiving a standard smoking cessation intervention. Nicotine Tob. Res. 2013;15:910–916. doi: 10.1093/ntr/nts217. [DOI] [PubMed] [Google Scholar]
- 57.Hur YN, Hong GH, Choi SH, Shin KH, Chun BG. High fat diet altered the mechanism of energy homeostasis induced by nicotine and withdrawal in C57BL/6 mice. Mol. Cells. 2010;30:219–226. doi: 10.1007/s10059-010-0110-3. [DOI] [PubMed] [Google Scholar]
- 58.Lerman C, et al. Changes in food reward following smoking cessation: a pharmacogenetic investigation. Psychopharmacology (Berl.) 2004;174:571–577. doi: 10.1007/s00213-004-1823-9. [DOI] [PubMed] [Google Scholar]
- 59.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Phil. Trans. R. Soc. B. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacol. Biochem. Behav. 2008;90:409–415. doi: 10.1016/j.pbb.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White MA, Masheb RM, Grilo CM. Self-reported weight gain following smoking cessation: a function of binge eating behavior. Int. J. Eat. Disord. 2010;43:572–575. doi: 10.1002/eat.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brook JS, Zhang C, Brook DW, Finch SJ. Voluntary smoking bans at home and in the car and smoking cessation, obesity, and self-control. Psychol. Rep. 2014;114:20–31. doi: 10.2466/18.13.PR0.114k16w4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stadler M, et al. Effects of smoking cessation on β-cell function, insulin sensitivity, body weight, and appetite. Eur. J. Endocrinol. 2014;170:219–227. doi: 10.1530/EJE-13-0590. [DOI] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. Vital signs: nonsmokers’ exposure to secondhand smoke — United States, 1999–2008. MMWR Morb. Mortal. Wkly. Rep. 2010;59:1141–1146. [PubMed] [Google Scholar]
- 65.Biedermann L, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE. 2013;8:e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biedermann L, et al. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm. Bowel Dis. 2014;20:1496–1501. doi: 10.1097/MIB.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 67.Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst. Rev. 2012;1:CD006219. doi: 10.1002/14651858.CD006219.pub3. [DOI] [PubMed] [Google Scholar]
- 68.Love SJ, et al. Offer of a weight management program to overweight and obese weight-concerned smokers improves tobacco dependence treatment outcomes. Am. J. Addict. 2011;20:1–8. doi: 10.1111/j.1521-0391.2010.00091.x. [DOI] [PubMed] [Google Scholar]
- 69.Perkins KA, et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J. Consult. Clin. Psychol. 2001;69:604–613. [PubMed] [Google Scholar]
- 70.Meyers AW, et al. Are weight concerns predictive of smoking cessation? A prospective analysis. J. Consult. Clin. Psychol. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- 71.Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst. Rev. 2012;1:CD006219. doi: 10.1002/14651858.CD006219.pub3. [DOI] [PubMed] [Google Scholar]
- 72.Levine MD, et al. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Arch. Intern. Med. 2010;170:543–550. doi: 10.1001/archinternmed.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schnoll RA, Wileyto EP, Lerman C. Extended duration therapy with transdermal nicotine may attenuate weight gain following smoking cessation. Addict. Behav. 2012;37:565–568. doi: 10.1016/j.addbeh.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taniguchi C, et al. Varenicline is more effective in attenuating weight gain than nicotine patch 12 months after the end of smoking cessation therapy: an observational study in Japan. Nicotine Tob. Res. 2014;16:1026–1029. doi: 10.1093/ntr/ntu045. [DOI] [PubMed] [Google Scholar]
- 75.Heffner JL, Lewis DF, Winhusen TM. Osmotic release oral system methylphenidate prevents weight gain during a smoking-cessation attempt in adults with ADHD. Nicotine Tob. Res. 2013;15:583–587. doi: 10.1093/ntr/nts152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vergnaud AC, et al. Fruit and vegetable consumption and prospective weight change in participants of the European Prospective Investigation into Cancer and Nutrition-Physical Activity, Nutrition, Alcohol, Cessation of Smoking, Eating Out of Home, and Obesity study. Am. J. Clin. Nutr. 2012;95:184–193. doi: 10.3945/ajcn.111.019968. [DOI] [PubMed] [Google Scholar]
- 77.Leslie WS, et al. Changes in body weight and food choice in those attempting smoking cessation: a cluster randomised controlled trial. BMC Public Health. 2012;12:389. doi: 10.1186/1471-2458-12-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet. Gynecol. 2015;125:773–781. doi: 10.1097/AOG.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki K, et al. Effect of maternal smoking cessation before and during early pregnancy on fetal and childhood growth. J. Epidemiol. 2014;24:60–66. doi: 10.2188/jea.JE20130083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Mamudu HM, Wu T. The impact of maternal prenatal smoking on the development of childhood overweight in school-aged children. Pediatr. Obes. 2013;8:178–188. doi: 10.1111/j.2047-6310.2012.00103.x. [DOI] [PubMed] [Google Scholar]
- 81.Hawkins SS, Baum CF, Oken E, Gillman MW. Associations of tobacco control policies with birth outcomes. JAMA Pediatr. 2014;168:e142365. doi: 10.1001/jamapediatrics.2014.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee K, et al. Associations of smoking and smoking cessation with CT-measured visceral obesity in 4,656 Korean men. Prev. Med. 2012;55:183–187. doi: 10.1016/j.ypmed.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 83.Matsushita Y, et al. Associations of smoking cessation with visceral fat area and prevalence of metabolic syndrome in men: the Hitachi health study. Obesity (Silver Spring) 2011;19:647–651. doi: 10.1038/oby.2010.237. [DOI] [PubMed] [Google Scholar]
- 84.Huang PL. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes. Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 86.Maeda K, et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1). 1996. Biochem. Biophys. Res. Commun. 2012;425:556–559. doi: 10.1016/j.bbrc.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 87.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr. Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 88.Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 89.Chen J, et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49:1292–1302. doi: 10.1007/s00125-006-0194-7. [DOI] [PubMed] [Google Scholar]
- 90.Kaur J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Inoue K, et al. Early effects of smoking cessation and weight gain on plasma adiponectin levels and insulin resistance. Intern. Med. 2011;50:707–712. doi: 10.2169/internalmedicine.50.4600. [DOI] [PubMed] [Google Scholar]
- 92.Matthews DR, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 93.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog. Cardiovasc. Dis. 2003;46:91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 94.Nakanishi K, Nishida M, Ohama T, Moriyama T, Yamauchi-Takihara K. Smoking associates with visceral fat accumulation especially in women. Circ. J. 2014;78:1259–1263. doi: 10.1253/circj.cj-13-1134. [DOI] [PubMed] [Google Scholar]
- 95.Yun JE, Kimm H, Choi YJ, Jee SH, Huh KB. Smoking is associated with abdominal obesity, not overall obesity, in men with type 2 diabetes. J. Prev. Med. Public Health. 2012;45:316–322. doi: 10.3961/jpmph.2012.45.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kirilly E, Gonda X, Bagdy G. CB1 receptor antagonists: new discoveries leading to new perspectives. Acta Physiol. (Oxf.) 2012;205:41–60. doi: 10.1111/j.1748-1716.2012.02402.x. [DOI] [PubMed] [Google Scholar]
- 97.Gamaleddin IH, et al. Role of the endogenous cannabinoid system in nicotine addiction: novel insights. Front. Psychiatry. 2015;6:41. doi: 10.3389/fpsyt.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Silvestri C, Di Marzo V. Second generation CB1 receptor blockers and other inhibitors of peripheral endocannabinoid overactivity and the rationale of their use against metabolic disorders. Expert Opin. Investig. Drugs. 2012;21:1309–1322. doi: 10.1517/13543784.2012.704019. [DOI] [PubMed] [Google Scholar]
- 99.Garwood CL, Potts LA. Emerging pharmacotherapies for smoking cessation. Am. J. Health Syst. Pharm. 2007;64:1693–1698. doi: 10.2146/ajhp060427. [DOI] [PubMed] [Google Scholar]
- 100.Bruin JE, Gerstein HC, Morrison KM, Holloway AC. Increased pancreatic β-cell apoptosis following fetal and neonatal exposure to nicotine is mediated via the mitochondria. Toxicol. Sci. 2008;103:362–370. doi: 10.1093/toxsci/kfn012. [DOI] [PubMed] [Google Scholar]
- 101.Yoshikawa H, Hellstrom-Lindahl E, Grill V. Evidence for functional nicotinic receptors on pancreatic β cells. Metabolism. 2005;54:247–254. doi: 10.1016/j.metabol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 102.Woynillowicz AK, Raha S, Nicholson CJ, Holloway AC. The effect of smoking cessation pharmacotherapies on pancreatic β-cell function. Toxicol. Appl. Pharmacol. 2012;265:122–127. doi: 10.1016/j.taap.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tjalve H, Popov D. Effect of nicotine and nicotine metabolites on insulin secretion from rabbit pancreas pieces. Endocrinology. 1973;92:1343–1348. doi: 10.1210/endo-92-5-1343. [DOI] [PubMed] [Google Scholar]
- 104.Wu Y, et al. Activation of AMPKα2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat. Med. 2015;21:373–382. doi: 10.1038/nm.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsia S, DesNoyers M, Lee ML, Goldstein C, Friedman TC. Metabolic effects of smokers undergoing smoking cessation. Endocrine Society. 2015 [online], https://endo.confex.com/endo/2015endo/webprogram/Paper21451.html. [Google Scholar]
- 106.Bergman BC, et al. Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes. 2012;61:3156–3166. doi: 10.2337/db12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Voulgari C, Katsilambros N, Tentolouris N. Smoking cessation predicts amelioration of microalbuminuria in newly diagnosed type 2 diabetes mellitus: a 1-year prospective study. Metabolism. 2011;60:1456–1464. doi: 10.1016/j.metabol.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 108.Athyros VG, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP. Effect of tobacco smoking and smoking cessation on plasma lipoproteins and associated major cardiovascular risk factors: a narrative review. Curr. Med. Res. Opin. 2013;29:1263–1274. doi: 10.1185/03007995.2013.827566. [DOI] [PubMed] [Google Scholar]
- 109.Legislative Analyst’s Office. 2011 Cal Facts. California’s economy and budget in perspective. 2011 [online], http://www.lao.ca.gov/reports/2011/calfacts/calfacts_010511.aspx.
- 110.Tweed JO, Hsia SH, Lutfy K, Friedman TC. The endocrine effects of nicotine and cigarette smoke. Trends Endocrinol. Metab. 2012;23:334–342. doi: 10.1016/j.tem.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]