Abstract
The regulated movement of monovalent cations such as H+, Li+, Na+ and K+ across biological membranes influences a myriad of cellular processes and is fundamental to all living organisms. This is accomplished by a multiplicity of ion channels, pumps and transporters. Our insight into their molecular, cellular and physiological diversity has increased greatly in the past few years with the advent of genome sequencing, genetic manipulation and sophisticated imaging techniques. One of the revelations from these studies is the emergence of novel alkali cation/protons exchangers that are present in endomembranes, where they function to regulate not only intraorganellar pH but also vesicular biogenesis, trafficking and other aspects of cellular homeostasis.
Introduction
Ion transport across the cell surface and organellar membranes is essential for cellular survival and proliferation. Inorganic ions play a defining role in cytoplasmic and organellar pH and volume homeostasis, in the provision of energy, thetranslocation of organic solutesandin cellular excitability and contractility. Much is known about transporters on the plasma membrane, which have been studied extensively by isotopic or electrophysiological means, or by employing ion-specific fluorescent probes that can be selectively targeted to the cytoplasm. By contrast, endomembrane compartments are highly dynamic structures that are not readily accessible and therefore much less amenable to study. As a consequence, our understanding of organellar ion transport is at present rudimentary. In some cases, the same or related transporters operate at both the surface and internal membranes. In these instances, lessons learned from studying plasmalemmal function can be extrapolated to infer the function of the organellar transporters. Alkali cation/proton exchangers (or antiporters), commonly referred to as Na+/H+ exchangers (NHEs), are one such example.
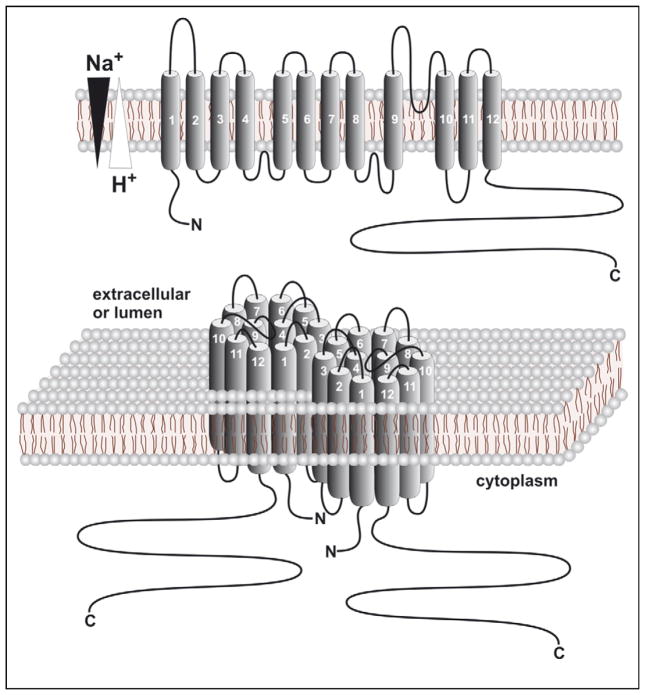
Mammalian NHEs are integral membrane proteins with a proposed secondary structure of 12 transmembrane segments at their N-terminus, followed by a hydrophilic C-terminus that is oriented towards the cytoplasm and the target for various regulatory molecules [1–3] (illustrated in Figure 1). Though their tertiary and quaternary structures have yet to be elucidated, biochemical analyses of plasmalemmal NHEs suggest that they assemble and function as homodimers [4,5•], a structure that compares favourably with the recent crystal structure of the more distantly related bacterial Escherichia coli Na+/H+ antiporter NhaA [6•]. However, unlike its bacterial homologues that operate as electrogenic transporters, the mammalian NHEs are generally thought to catalyze the electroneutral exchange of alkali cations (Li+, Na+ or K+) for protons, though their cation selectivity can differ amongst isoforms. The basic transport kinetic properties are known with certainty for the isoforms resident on the plasmalemma [4] but are not fully established for the endomembrane isoforms, which have remained refractory to direct measurements for technical and topological reasons. The objective of this review is to summarize our current understanding of the distribution and function of members of the mammalian NHE family, with particular emphasis on those present in internal membrane compartments.
Figure 1.
Predicted membrane topology and higher ordered structure of mammalian alkali cation/proton exchangers.
Genetic and subcellular diversity
Mammalian NHEs constitute a family of nine related gene products (NHE1–9) that share ~25–70% amino acid identity overall (Figure 2a) [2,7••]. In addition to the NHE gene family, mammals also possess another novel cluster of distant NHE-related genes, termed NHA1 and NHA2 on the basis of their closer homology to the fungal/plant NHA1 and bacterial NhaA Na+/H+ antiporters [7••]. Their functional and physiological properties, however, are presently unknown.
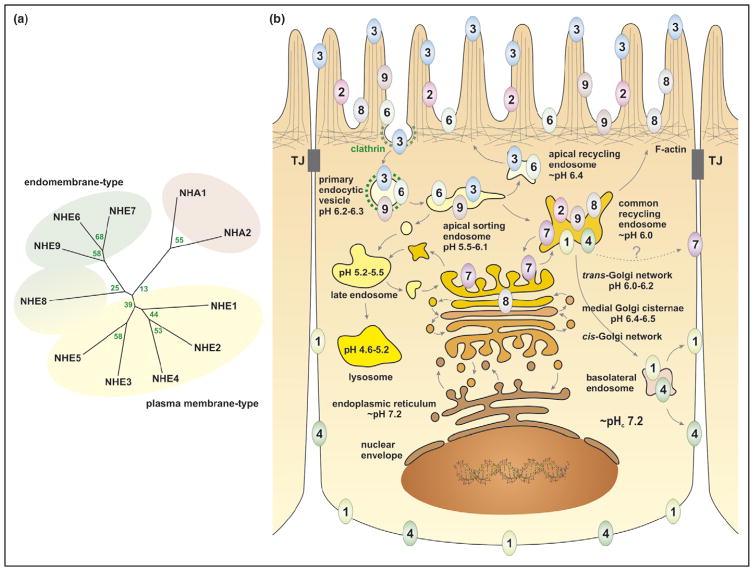
Figure 2.
Genetic diversity and membrane distribution of mammalian alkali cation/proton exchangers. (a) Phylogenetic relationships of human alkali cation/proton exchangers were determined by multiple sequence alignments using the CLUSTAL W algorithm [68] and the radial tree was drawn using TreeView [69]. The GenBank™ accession numbers for the various NHEs are as follows: NHE1 to NHE9: NM_003047, NM_003048, NM_004174, NM_001011552, NM_004594, NM_006359, NM_032591, NM_015266, NM_173653, respectively; NHA1, NM_139173 and NHA2, NM_178833. (b) The diagram displays a composite subcellular distribution of the mammalian Na+(K+)/H+ exchangers (NHE1–4, 6–9) in a prototypical epithelial cell. In native epithelia, these isoforms exhibit cell-specific expression. NHE5 is not depicted since it is found predominantly in neuronal cells but exhibits a subcellular distribution analogous to that of NHE3. The various biosynthetic and endocytic routes are shown, along with approximate values for the luminal pH of the various endomembrane compartments, as extrapolated from published data of different cell types.
The recognized members of the NHE family can be broadly classified into two major subgroups on the basis of their primary structure similarity and principal subcellular location, that is plasma-membrane-type or endomembrane-type transporters (Figure 2a). NHE1 through NHE5 (~40–60% identity) are all targeted to the plasma membrane, but isoforms such as NHE3 and NHE5 can also enter a recycling endosomal pool (Figure 2b). NHE6 to NHE9 are thought to reside predominantly, though not exclusively, in endomembrane compartments. This latter group can be further subdivided into the endosomal/trans-Golgi network (TGN) cluster (encompassing NHE6, 7 and 9, which share ~60–70% identity) and NHE8 that exhibits the least similarity (25% identity) to other NHEs [2,7••]. Orthologues for the mammalian endosomal/TGN NHE cluster can be found in all eukaryotes, the best studied being the yeast Saccharomyces cerevisiae Nhx1 that resides in the late endosomal/prevacuolar compartment [7••]. By contrast, NHE8 is seemingly restricted to animal cells, suggesting a more recent emergence in evolution to satisfy a particular physiological need.
Plasma-membrane-type NHEs
The plasma-membrane-type NHEs (NHE1–5) preferentially catalyze the electroneutral exchange of one extracellular Na+ for one cytosolic H+. Li+ is also a transportable substrate, but its rate of translocation is generally slower than that of Na+. NHE1 is by far the best studied prototypical isoform. It has a ubiquitous tissue distribution, resides only at the plasma membrane (basolateral membrane in polarized epithelia) and is thought to be a ‘housekeeping’ enzyme responsible for maintaining cytoplasmic pH and controlling cellular volume. In fibroblasts and possibly other cell types, the carboxy-terminal cytoplasmic tail of NHE1 also serves as a scaffold for the assemblage of various actin-binding proteins (ezrin, radixin and moesin) and signalling complexes, transmitting signals from activated growth factor and integrin receptors to pathways regulating cell proliferation, shape, adhesion and migration (summarized in Table 1) [3,8,9].
Table 1.
Characteristics of mammalian alkali cation/proton exchangers
| Isoform | Tissue distribution | Membrane location | Main functions | Interacting partners | References |
|---|---|---|---|---|---|
| NHE1 | Ubiquitous |
|
|
|
[2,3,8,9,60–63]; and references therein |
| NHE2 | Gastrointestinal tract > skeletal muscle ⋙ kidney, brain, uterus, testis ≫ heart |
|
|
|
[60,61] |
| NHE3 | Kidney, intestines (other epithelia) |
|
|
|
[2,10,23–25,32–37,60,61, 64–66] |
| NHE4 | Stomach ≫ ≫ kidney |
|
|
|
[60] |
| NHE5 | Brain (neurons) |
|
|
|
[39•,67] |
| NHE6 | Ubiquitous |
|
|
? | |
| NHE7 | Ubiquitous |
|
|
|
[41,55•] |
| NHE8 | Ubiquitous |
|
|
? | |
| NHE9 | Ubiquitous |
|
|
? |
CHP, calcineurin B-homologous protein; ERK, extracellular signal-regulated protein kinase; MAPK, mitogen-activated protein kinase; p90rsk, p90 ribosomal S6 kinase, p160ROCK/ROCK1, Rho-associated, coiled-coil containing protein kinase 1; NIK, Nck-interacting kinase; PIP2, phosphatidylinositol (4,5)-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; DPP-IV, dipeptidyl peptidase IV; NHERF, Na+/H+ exchanger regulatory factor; PDZK1, PDZ domain-containing protein highly homologous to rat Diphor-1; MAST205, microtubule-associated serine/threonine kinase-205 kDa that contains a Ser/Thr kinase domain and a PDZ domain; Shank2, SH3 and multiple ankyrin repeat domains 2; RACK1, receptor for activated protein kinase C; SCAMP, secretory carrier membrane protein.
The other members of this subgroup, NHE2–5, have a more limited tissue distribution and, in some polarized cell types, are confined to discrete regions of the plasma membrane, suggestive of more specialized functions. For instance, both NHE2 and NHE3 are localized at the apical surface of renal and gastrointestinal epithelial cells as well as other epithelia and play important roles in salt, bicarbonate and fluid (re)absorption. Unlike NHE2, however, NHE3 (and the neuronal NHE5) is further sorted to the recycling endosomal pathway.
Roles in recycling endosomes
Accumulation of NHE3 in recycling endosomes may serve as an important regulated reservoir of functional transporters. It constitutes a sizeable source of spare exchangers when their abundance at the surface needs to be increased acutely. Conversely, internalization into endomembrane storage sites serves to downregulate the number of active surface transporters in a rapid and reversible manner. Delivery to the internal pool seems to follow clathrin-dependent endocytosis. NHE3 has been detected in clathrin-coated pits at the base of microvilli [10] and expression of a dominant-negative allele of dynamin interfered with its internalization [11]. It is noteworthy that dynamin is required not only for clathrin-mediated endocytosis but also for caveolar uptake and that extraction of cholesterol with cyclodextrin, which disrupts rafts and caveolae, also impaired NHE3 endocytosis [12]. However, cyclodextrin non-selectively also affects clathrin-mediated internalization, which is the predominant pathway for apical endocytosis and the likely mechanism utilized by NHE3.
There is evidence that NHE3 is functional in endocytic and endosomal membranes. If operating in the same direction as it does at the surface, NHE3 would move H+ into the vesicular lumen while delivering Na+ to the cytosol (illustrated in Figure 3). This would result in a luminal acidification, which has been detected not only after heterologous overexpression in fibroblastic cells [13] but also in native systems [14,15]. Indeed, there is compelling evidence that the presence and activity of NHE3 in endosomes affect some aspects of epithelial physiology. Specifically, Gekle et al. [14,16,17,18•,19] have demonstrated that a functional NHE3 is required for optimal albumin uptake and subsequent delivery to lysosomes in renal cells (Figure 3). Importantly, the activity of the exchangers in endocytic vesicles, and not at the surface membrane, was found to be the crucial parameter. Inhibition of NHE3 activity significantly attenuated the rate of fusion of plasma-membrane-derived albumin-laden vesicles with early (sorting) endosomes, thereby blocking subsequent trafficking and processing of albumin [16]. Acidification of the endosomal lumen is known to be required for proper routing of internalized cargo [20], but it is not clear whether this is the mechanism whereby NHE3 contributes to albumin uptake and traffic. Indeed, the contribution of ‘forward’ Na+/H+ exchange to endosomal acidification can only be small and transient (i.e. during the formation of the primary endocytic vesicle), as Na+ is predicted to be depleted rapidly from the lumen, unless continuously restored by active transporters like the Na+/K+-ATPase. The latter, however, is not expected to be found in apical endosomes, as it is restricted to the basolateral membrane. Moreover, it is well established that vacuolar-type H+-pumps (V-ATPases), in conjunction with electrogenic chloride transporters of the CLC family, are the primary means of endosomal acidification [21,22•]. Accordingly, apical endosomes of epithelial cells were found to be only marginally more acidic than cells devoid of NHE3 (6.20 ± 0.02 in wild type versus 6.30 ± 0.03 in NHE3-null cells) [17]. The fact that ATP-dependent H+-pumping is predominant raises the possibility that the acidification generated by the V-ATPases, together with the depletion of luminal Na+, may eventually reverse the direction of the combined Na+/H+ gradient that drives NHE activity. In this event, NHE3 or other endosomal NHEs may in fact function to dissipate the acidification generated by the H+-pumps and serve to regulate the pH setpoint of these compartments.
Figure 3.
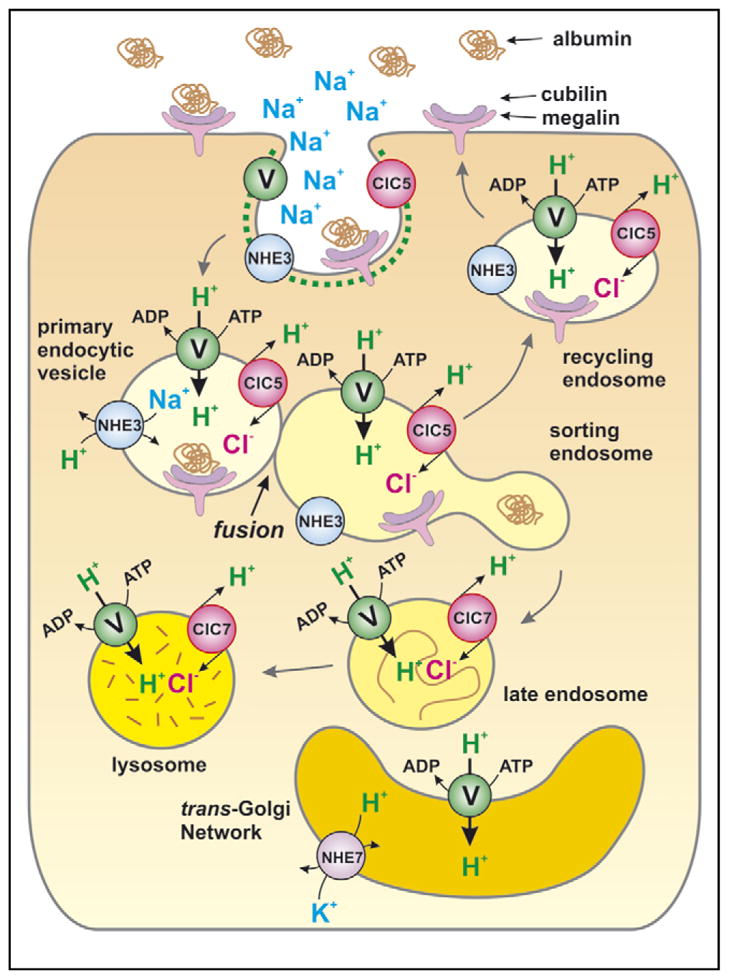
Involvement of the apical Na+/H+ exchanger NHE3 in endosomal pH and reabsorption of albumin in renal proximal tubules. Acidification of intracellular compartments is driven primarily by the vacuolar-type (V) H+-ATPase pumps (V-ATPases) [21]. In addition, vesicle acidification is facilitated by counterion Cl− influx pathways that minimize the generation of a large inside-positive voltage that would otherwise impede V-ATPase function. In proximal tubular epithelia, the Cl− conductance in apical early and recycling endosomes is mediated by ClC-5, an electrogenic 2Cl−/H+ exchanger of the CLC family of channels/transporters [22]. Acidification of the late endosomal and lysosomal compartments is aided by another CLC member, ClC-7; depicted as an exchanger though its precise mode of operation (exchanger or channel) is uncertain. The apical Na+/H+ exchanger isoform 3 (NHE3) also contributes to acute acidification of early/recycling endosomes. The glomerular filtrate in the proximal tubule, which contains plasma-levels of Na+, is internalized into primary endocytic vesicles upon their formation. This establishes an outwardly directed Na+ gradient that is used by NHE3 to propel the inward movement of H+; a process that terminates upon dissipation of the Na+ gradient. Disruption of vesicle acidification by pharmacological inhibition or gene knockout of either the V-ATPase, ClC-5 or NHE3 impedes endocytosis of albumin by the megalin-cubilin scavenger receptor complex and its subsequent degradation in lysosomes (reviewed in reference [19]). The molecular mechanisms underlying this pH-associated effect are ill defined, but may result from impaired dissociation of the albumin-receptor complex and/or biogenesis of recycling vesicles.
Regulation
The apical membrane and subapical vesicular pools of NHE3 appear to be in dynamic equilibrium, regulated by a bewildering assortment of agents and conditions. These include aldosterone, glucocorticoids, lysophosphatidic acid, albumin, extracellular acidity and glucose-induced cell swelling, all of which upregulate the number of apical surface transporters, as well as cholinergic agonists, dopamine, TNF and elevated blood pressure, which reduce transporter density (see reference [23] for review). The number and variety of signalling pathways that have been invoked in this regulation – either directly or indirectly – is perplexing, including an array of serine/threonine kinases [10,24–26], non-receptor tyrosine kinases [26,27], phospholipases [28] and other lipid-metabolizing enzymes [29,30]. Of particular interest is the observation that a large fraction of the apical NHE3 is firmly anchored along the sides and near the tip of microvilli, which restricts the mobility of the exchangers and presumably retains them on the apical membrane. NHE3 is thought to attach to actin filaments that are formed and/or stabilized by Rho-family GTPases [31•]. Bridging between the integral membrane and cytoskeletal proteins is thought to be mediated by PDZ-based adaptor proteins (NHERF1, NHERF2, PDZK1, MAST205, Shank2) and/or ezrin (Table 1) [32–36]. Substantive internalization may require detachment of NHE3 from such anchorage sites, making the latter prime targets for the regulation of traffic between surface and endomembrane compartments. Despite the mechanistic uncertainty of this step, recent analyses of the later phases of endocytosis of NHE3 induced in response to elevated levels of cAMP and Ca2+ have identified a crucial role for synaptotagmin I in the binding of NHE3 and subsequent recruitment of the adaptor protein complex AP2 and clathrin [37].
Of all the NHE isoforms, NHE5 bears the highest similarity to NHE3. It was therefore not unexpected to find NHE5 not only on the plasmalemma but also in an endomembrane compartment. This observation was first made following ectopic expression in fibroblasts, but was subsequently validated in PC12 neuroendocrine cells and in primary hippocampal neurons. In the heterologous expression system, NHE5 was also internalized via clathrin-coated structures in a dynamin-dependent fashion [38]. The adaptor/scaffolding protein, β-arrestin, probably mediates the association between NHE5 and clathrin [39•]. The two proteins interact directly both in vitro and in vivo, and overexpression of β-arrestin redirected NHE5 from the surface to endomembranes. The linkage with β-arrestin raises the possibility that both NHE5 and neurotransmitter-activated G-protein-coupled receptors, which are also internalized upon association with β-arrestin, may co-localize within the same clathrin-coated pits. Such an assembly has the potential to promote more rapid vesicle acidification, ligand dissociation and recycling of the receptors.
Endomembrane-type NHEs
Compared with the mammalian plasma-membrane-type NHEs, considerably less is known about the function, regulation and trafficking of the endomembrane transporters NHE6–9. These isoforms are widely expressed and appear to share distinct but overlapping compartments along the secretory and endocytic pathways (see Figure 2).
Subcellular distribution
NHE7 is located largely in the trans-Golgi network (TGN) and perinuclear recycling vesicles when expressed stably in CHO and MCF-7 cells [40,41]. Like other TGN-resident proteins such as TGN38, a minor fraction of NHE7 also appears to transiently shuttle to the cell surface, at least in non-polarized cells [41]. NHE8 accumulates within the mid-cisternae to trans-cisternae of the Golgi complex when heterologously expressed in COS7 cells [42]. However, in renal proximal tubule and intestinal epithelial cells, native NHE8 localizes predominantly at the microvillar membrane surface and to a lesser extent in intermicrovillar-coated pits and/or subapical vesicles [43], suggesting that it may undergo recycling to and from the apical surface. By comparison, the distributions of NHE6 and NHE9 overlap considerably with markers of the recycling endosomal pathway, though they tend to partition differentially to early and recycling endosomes, respectively [42]. Again, their relative distribution may also be influenced by the cell type in which they are expressed. A striking example is found in the sensory hair cells of the vestibular system, where NHE6 and NHE9 are concentrated not only in intracellular vesicles of the cell bodies but also apically at the tips of stereocilia—the sensory organelles that mediate mechano-electrical transduction [44]. It thus appears that the membrane sorting of these isoforms is dependent on the cell context (i.e. cell-specific adaptors, scaffolding or anchoring proteins, cytoskeletal elements), forming a mosaic wherein individual isoforms address specialized needs both within vesicles and at the cell surface.
Kinetic features
A distinguishing kinetic feature of the endomembrane class of NHEs is their ability to mediate not only Na+ (or Li+) but also K+ in exchange for H+, which contrasts with the high selectivity for Na+ displayed by their plasma-membrane-type counterparts. This was shown initially for NHE7 [40] and has since been demonstrated directly or inferred indirectly for other mammalian endomembrane isoforms [42,44], as well as for their orthologues in yeast (i.e. Nhx1) [45•] and plants [46]. Since K+ is the major cytoplasmic alkali cation, and most endomembrane compartments are acidic, the prevailing view is that these transporters use the combined electrochemical ion gradients to direct the inward movement of K+ in exchange for luminal H+, thereby serving as an electroneutral alkalinizing mechanism. However, because of the crudeness of the ion transport assays used to date – usually involving intact or permeabilized cells – the precise cation selectivity and energetics of these transporters are ill defined. A precise characterization awaits more refined measurements in purified reconstituted in vitro systems.
Roles and regulation
The physiological importance of NHEs to endosomal function is not well understood, but insights from the study of the S. cerevisiae Nhx1 suggest that alkali cation/ proton exchange may influence not only luminal ion homeostasis but also vesicle biogenesis and protein trafficking. Initially, Nhx1 was found to protect yeast cells from the cytotoxic effects of high saline environments by sequestering excess cytoplasmic Na+ into endosomes [47]. However, subsequent analyses revealed an important role of Nhx1 in the trafficking of proteins from the late endosome to the vacuole. Null (nhx1Δ) or functionally inactive mutants of Nhx1 displayed an atypical distension of the prevacuolar compartment that was accompanied by aberrant accumulation and processing of vacuolar-targeted proteins originating either from the late Golgi (e.g. carboxypeptidase Y) or internalized from the cell surface (e.g. the G protein-coupled receptor Ste3p) [48]. This effect was relatively specific, as sorting of other vacuolar-targeted proteins (e.g. alkaline phosphatase) that did not pass through the prevacuolar compartment was unaffected.
The molecular basis for these observed defects is unclear but is probably linked to disruption in K+-dependent and/ or pH-dependent endosomal processes. Though not examined in the context of Nhx1 function, K+ is known to act as a regulatory co-factor of the Kex2/furin family of endoproteases required for maturation of newly synthesized proteins in the secretory pathway of yeast as well as animal cells [49]. Hence, alterations in intraorganellar K+ concentrations could conceivably compromise proper protein processing. Disturbances of intravesicular K+ concentration are also likely to have an impact on organellar volume.
By contrast, acidification of endomembrane compartments is well recognized as a crucial determinant of protein processing and trafficking along the biosynthetic and endocytic pathways [50–52]. Indeed, recent analyses of nhx1Δ mutant cells revealed that the endosomal (and, unexpectedly, also the cytoplasmic) compartments were more acidic than those of wild type cells and that treatment with weak bases could alleviate the severe defect in vacuolar protein trafficking [45]. These data support the notion that endomembrane Nhx/NHEs serve as a crucial alkalinizing mechanism to finely control intraorganellar pH that, by an ill-defined mechanism, modulates endosome biogenesis and trafficking.
While the available evidence indicates that endomembrane Nhx/NHEs influence organellar function through ionic/pH changes, they may also exert other effects by interacting with proteins required for normal membrane traffic. In this regard, Gyp6, a specific GTPase-activating protein (GAP) for Ypt6, the orthologue for the mammalian Rab6-GTPase, was found to bind to the hydrophilic C-terminal segment of Nhx1 and to co-localize with the transporter at the prevacuolar compartment [53•]. This is an intriguing observation since Ypt6 is implicated in endosome to Golgi membrane trafficking. Additional genetic manipulations supported a functional interrelationship between Gyp6, Nhx1 and Ypt6, with Gyp6 acting as a negative regulator of both Nhx1 and Ypt6 both to control intraendosomal pH and retrograde traffic from the endosome to the late Golgi. However, interpretation of these data is complicated by membrane topological studies of Nhx1 showing that its C-terminus (or a portion thereof) lies within the endosomal lumen [54], whereas Gyp6 is cytoplasmic or tethered to the outer leaflet of the endosomal membrane. Hence, the precise nature of these molecular interactions, linking Nhx1-regulated endosomal ion homeostasis to membrane traffic, remains obscure.
The factors that govern the trafficking and regulation of mammalian endomembrane NHEs are also largely unknown. Recently, a yeast two-hybrid screen identified members of the secretory carrier membrane protein family (i.e. SCAMP 1, 2 and 5) as interacting partners of NHE7 [55•]. SCAMPs are transmembrane proteins localized in the Golgi and post-Golgi compartments and thought to be involved in membrane traffic, though their precise roles are not well defined [56]. Consistent with this view, a dominant-negative deletion mutant of SCAMP2 caused NHE7 (as well as wild type SCAMP2) to redistribute to recycling endosomes, suggesting that it plays a role in the retrieval of NHE7 from recycling endosomes to the TGN [55•]. Recently, SCAMP2 was found to bind to the small GTPase ADP-ribosylation factor 6 (Arf6) and to phospholipase D1 in PC12 cells [57], indicating that it may facilitate recruitment of vital elements of the trafficking machinery involved in vesicle formation and fusion [58]. This raises the prospect that NHE7 may form a macromolecular complex that includes not only SCAMP2 but also Arf6. Such an assembly could provide a potential link between endomembrane NHEs and vesicle trafficking along the TGN/endosome pathway. Intriguingly, in a recent study Hurtado-Lorenza et al. [59••] showed that Arf6 and its associated guanine-nucleotide-exchange factor ARNO also interacted with the integral membrane c-subunit and a2-subunit, respectively, of the V-ATPase in early endosomes. Significantly, the association of ARNO with the a2-subunit was dependent on endosomal acidification and necessary for subsequent vesicle traffic between early and late endosomes. Thus, the V-ATPase, in addition to pumping H+, appears capable of sensing the optimal intraorganellar pH and transmitting that signal across the membrane, possibly by a pH-dependent conformational change in the a2-subunit. In turn, the ‘activated’ a2-subunit becomes competent to recruit cytoplasmic proteins required for vesicle maturation along the degradative pathway. It would be interesting to determine whether this molecular paradigm also applies to NHE7 as well as other endomembrane NHEs, whereby intraorganellar acidification to a ‘defined’ pH setpoint – regulated partly by the NHEs themselves – triggers a conformational change in the transporters that allows for the scaffolding of other proteins important for membrane traffic.
Despite its predominance in endomembranes, a minor fraction of NHE7 also shuttles to and from the plasma membrane, analogous to the plasma membrane/recycling-type NHEs (i.e. NHE3 and 5). In MCF-7 cells, surface NHE7 concentrates in caveolae/lipid-enriched rafts [41]. This association is mediated through direct binding of the cytoplasmically oriented C-terminal tail of NHE7 with caveolin-1, perhaps stabilizing the transporter at the cell surface [41]. However, internalization of NHE7 appears to occur exclusively through the clathrin-mediated pathway, suggesting that NHE7 may exist in dynamic equilibrium between caveolae/lipid rafts and non-caveolae/lipid rafts. Curiously, disruption of the interaction with dominant-negative mutants of caveolin-1, despite shifting NHE7 to non-caveolae/lipid raft fractions, did not appreciably affect its subcellular distribution. Hence, the functional significance of this association remains obscure.
The presence of endomembrane NHEs at the cell surface raises the possibility that they may also fulfil more specialized roles in regulating cytoplasmic ion homeostasis. For instance, in vestibular hair cells that express NHE6 and NHE9 in their stereocilia, the apical endolymph fluid lacks extracellular Na+ and instead is highly enriched in K+. Hill et al. [44] provided intriguing evidence that these NHEs use the high extracellular K+ concentration to expel intracellular H+ generated by the Ca2+/H+ exchange activity of the apical plasma membrane Ca2+-ATPase isoform 2 (PMCA2). PMCA2 is required to extrude sufficient Ca2+ into the surrounding endolymph to generate otoconia, the calcium carbonate crystals embedded in the otolithic membrane that shifts in response to gravity or linear acceleration, thereby stimulating the hair cells. These data suggest that NHE6 and NHE9 are functionally coupled with PMCA2 to maintain the pH of hair bundles while supporting robust extrusion of Ca2+.
Conclusions
While the contributions of plasma-membrane-type NHEs to cellular and systemic physiology are well documented, the observation that some of these isoforms (i.e. NHE3 and NHE5) can be internalized into recycling endosomes and the discovery of novel isoforms (NHE6–9) localized to discrete compartments along the exocytic and endocytic pathways highlights broader roles of these transporters in cell function. The endomembrane-type transporters appear to operate physiologically as K+/H+ exchangers, but there is a need to better define their kinetic properties (cation selectivity, affinities, energetics) under more defined experimental conditions. Two-hybrid screening has begun to identify interacting proteins that underlie their membrane sorting and regulation. The combination of RNAi methodologies, powerful imaging techniques and deployment of existing as well as novel organelle-specific, cation-sensitive, fluorescent probes should help to distinguish their roles in intraorganellar ion homeostasis and as scaffolds for recruitment of proteins involved in carrier vesicle coat formation and traffic. This could be particularly challenging, as the endomembrane isoforms appear to share overlapping compartments; thus, selection of suitable cell model systems will be crucial. A key question is whether their abilities to modulate organellar pH is functionally coupled with the recruitment of trafficking machinery, or whether these processes are regulated independent of each other. These approaches should help to provide a more complete understanding of the roles of alkali cation/ proton exchangers in cellular/organellar homeostasis.
Acknowledgments
Original work in the authors’ laboratories is supported by the Canadian Institutes for Health Research, the Heart and Stroke Foundation and the Canadian Cystic Fibrosis Foundation. JO is the current holder of a James McGill Professorship. SG is the current holder of the Pitblado Chair in Cell Biology.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Wakabayashi S, Pang T, Su X, Shigekawa M. A novel topology model of the human Na+/H+ exchanger isoform 1. J Biol Chem. 2000;275:7942–7949. doi: 10.1074/jbc.275.11.7942. [DOI] [PubMed] [Google Scholar]
- 2.Orlowski J, Grinstein S. Diversity of the mammalian sodium/ proton exchanger SLC9 gene family. Pflügers Arch—Eur J Physiol. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 3.Slepkov ER, Rainey JK, Sykes BD, Fliegel L. Structural and functional analysis of the Na+/H+ exchanger. Biochem J. 2007;401:623–633. doi: 10.1042/BJ20061062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacroix J, Poet M, Maehrel C, Counillon L. A mechanism for the activation of the Na/H exchanger NHE-1 by cytoplasmic acidification and mitogens. EMBO Rep. 2004;5:91–96. doi: 10.1038/sj.embor.7400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Hisamitsu T, Ammar YB, Nakamura TY, Wakabayashi S. Dimerization is crucial for the function of the Na+/H+ exchanger NHE1. Biochemistry. 2006;45:13346–13355. doi: 10.1021/bi0608616. This paper provides the first evidence that dimerization of the exchanger is functionally important for setting the resting pH sensitivity of the transporter in the neutral pH range. [DOI] [PubMed] [Google Scholar]
- 6•.Hunte C, Screpanti E, Venturi M, Rimon A, Padan E, Michel H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature. 2005;435:1197–1202. doi: 10.1038/nature03692. This paper provides the first high-resolution crystal structure of a Na+/H+ exchanger isolated from bacteria. [DOI] [PubMed] [Google Scholar]
- 7••.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. This paper provides a very comprehensive and authoritative review of the phylogenetic and functional diversity of prokaryotic and eukaryotic sodium/proton exchangers. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner M, Patel H, Barber DL. Na+/H+ exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Cell Physiol. 2004;287:C844–C850. doi: 10.1152/ajpcell.00094.2004. [DOI] [PubMed] [Google Scholar]
- 9.Patel H, Barber DL. A developmentally regulated Na-H exchanger in Dictyostelium discoideum is necessary for cell polarity during chemotaxis. J Cell Biol. 2005;169:321–329. doi: 10.1083/jcb.200412145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol. 2005;289:F249–F258. doi: 10.1152/ajprenal.00082.2004. [DOI] [PubMed] [Google Scholar]
- 11.Chow CW, Khurana S, Woodside M, Grinstein S, Orlowski J. The epithelial Na+/H+ exchanger, NHE3, is internalized through a clathrin-mediated pathway. J Biol Chem. 1999;274:37551–37558. doi: 10.1074/jbc.274.53.37551. [DOI] [PubMed] [Google Scholar]
- 12.Murtazina R, Kovbasnjuk O, Donowitz M, Li X. Na+/H+ exchanger NHE3 activity and trafficking are lipid Raft-dependent. J Biol Chem. 2006;281:17845–17855. doi: 10.1074/jbc.M601740200. [DOI] [PubMed] [Google Scholar]
- 13.D’Souza S, Garcia-Cabado A, Yu F, Teter K, Lukacs GL, Skorecki K, Moore HP, Orlowski J, Grinstein S. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem. 1998;273:2035–2043. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- 14.Gekle M, Drumm K, Mildenberger S, Freudinger R, Gassner B, Silbernagl S. Inhibition of Na+-H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. J Physiol (Lond) 1999;520:709–721. doi: 10.1111/j.1469-7793.1999.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akhter S, Kovbasnjuk O, Li X, Cavet M, Noël J, Arpin M, Hubbard AL, Donowitz M. Na+/H+ exchanger 3 is in large complexes in the center of the apical surface of proximal tubule-derived OK cells. Am J Physiol Cell Physiol. 2002;283:C927–C940. doi: 10.1152/ajpcell.00613.2001. [DOI] [PubMed] [Google Scholar]
- 16.Gekle M, Freudinger R, Mildenberger S. Inhibition of Na+-H+ exchanger-3 interferes with apical receptor-mediated endocytosis via vesicle fusion. J Physiol (Lond) 2001;531:619–629. doi: 10.1111/j.1469-7793.2001.0619h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gekle M, Serrano OK, Drumm K, Mildenberger S, Freudinger R, Gassner B, Jansen HW, Christensen EI. NHE3 serves as a molecular tool for cAMP-mediated regulation of receptor-mediated endocytosis. Am J Physiol Renal Physiol. 2002;283:F549–F558. doi: 10.1152/ajprenal.00206.2001. [DOI] [PubMed] [Google Scholar]
- 18•.Gekle M, Volker K, Mildenberger S, Freudinger R, Shull GE, Wiemann M. NHE3 Na+/H+ exchanger supports proximal tubular protein reabsorption in vivo. Am J Physiol Renal Physiol. 2004;287:F469–F473. doi: 10.1152/ajprenal.00059.2004. This paper provides a physiologically relevant extension of earlier in vitro studies of cultured renal cell lines by demonstrating, using whole animals (wild type and NHE3−/− knockout mice), that NHE3 is crucial for efficient receptor-mediated endocytosis of albumin from the lumen of the proximal tubule. [DOI] [PubMed] [Google Scholar]
- 19.Gekle M. Renal tubule albumin transport. Annu Rev Physiol. 2005;67:573–594. doi: 10.1146/annurev.physiol.67.031103.154845. [DOI] [PubMed] [Google Scholar]
- 20.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 21.Inoue T, Wang Y, Jefferies K, Qi J, Hinton A, Forgac M. Structure and regulation of the V-ATPases. J Bioenerg Biomembr. 2005;37:393–398. doi: 10.1007/s10863-005-9478-8. [DOI] [PubMed] [Google Scholar]
- 22•.Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol. 2007;578:633–640. doi: 10.1113/jphysiol.2006.124719. This paper provides a thorough review of the CLC family of chloride channels and transporters and their importance in acidification of intracellular compartments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bobulescu IA, Moe OW. Na+/H+ exchangers in renal regulation of acid–base balance. Semin Nephrol. 2006;26:334–344. doi: 10.1016/j.semnephrol.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiederkehr MR, Zhao H, Moe OW. Acute regulation of Na/H exchanger NHE3 activity by protein kinase C: role of NHE3 phosphorylation. Am J Physiol Cell Physiol. 1999;276:C1205–C1217. doi: 10.1152/ajpcell.1999.276.5.C1205. [DOI] [PubMed] [Google Scholar]
- 25.Lee-Kwon W, Kim JH, Choi JW, Kawano K, Cha B, Dartt DA, Zoukhri D, Donowitz M. Ca2+-dependent inhibition of NHE3 requires PKCα which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am J Physiol Cell Physiol. 2003;285:C1527–C1536. doi: 10.1152/ajpcell.00017.2003. [DOI] [PubMed] [Google Scholar]
- 26.Tsuganezawa H, Sato S, Yamaji Y, Preisig PA, Moe OW, Alpern RJ. Role of c-SRC and ERK in acid-induced activation of NHE3. Kidney Int. 2002;62:41–50. doi: 10.1046/j.1523-1755.2002.00418.x. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Sato S, Yang X, Preisig PA, Alpern RJ. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest. 2004;114:1782–1789. doi: 10.1172/JCI18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JW, Lee-Kwon W, Jeon ES, Kang YJ, Kawano K, Kim HS, Suh PG, Donowitz M, Kim JH. Lysophosphatidic acid induces exocytic trafficking of Na+/H+ exchanger 3 by E3KARP-dependent activation of phospholipase C. Biochim Biophys Acta. 2004;1683:59–68. doi: 10.1016/j.bbalip.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Kurashima K, Szabó EZ, Lukacs GL, Orlowski J, Grinstein S. Endosomal recycling of the Na+/H+ exchanger NHE3 isoform is regulated by the phosphatidylinositol 3-kinase pathway. J Biol Chem. 1998;273:20828–20836. doi: 10.1074/jbc.273.33.20828. [DOI] [PubMed] [Google Scholar]
- 30.Lee-Kwon W, Johns DC, Cha BY, Cavet M, Park J, Tsichlis P, Donowitz M. Constitutively active phosphatidylinositol 3-kinase and AKT are sufficient to stimulate the epithelial Na+/H+ exchanger 3. J Biol Chem. 2001;276:31296–31304. doi: 10.1074/jbc.M103900200. [DOI] [PubMed] [Google Scholar]
- 31•.Alexander RT, Furuya W, Szaszi K, Orlowski J, Grinstein S. Rho GTPases dictate the mobility of the Na/H exchanger NHE3 in epithelia: role in apical retention and targeting. Proc Natl Acad Sci USA. 2005;102:12253–12258. doi: 10.1073/pnas.0409197102. This paper reveals that the subcellular distribution of NHE3 is complex and can be resolved into four distinct regulated subcompartments: an immobile subpopulation that is retained on the apical microvillar membrane by interaction with the actin cytoskeleton in a manner that depends on the activity of Rho GTPases; a mobile subpopulation on the apical membrane, which can be readily internalized; two intracellular compartments that can be differentiated by their rate of exchange with the apical pool of NHE3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinman EJ, Cunningham R, Shenolikar S. NHERF and regulation of the renal sodium-hydrogen exchanger NHE3. Pflugers Arch. 2005;450:137–144. doi: 10.1007/s00424-005-1384-8. [DOI] [PubMed] [Google Scholar]
- 33.Thomson RB, Wang T, Thomson BR, Tarrats L, Girardi A, Mentone S, Soleimani M, Kocher O, Aronson PS. Role of PDZK1 in membrane expression of renal brush border ion exchangers. Proc Natl Acad Sci USA. 2005;102:13331–13336. doi: 10.1073/pnas.0506578102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Lee HJ, Cooper DS, Cebotaro L, Walden PD, Choi I, Yun CC. Coexpression of MAST205 inhibits the activity of Na+/ H+ exchanger NHE3. Am J Physiol Renal Physiol. 2006;290:F428–F437. doi: 10.1152/ajprenal.00161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han W, Kim KH, Jo MJ, Lee JH, Yang J, Doctor RB, Moe OW, Lee J, Kim E, Lee MG. Shank2 associates with and regulates Na+/H+ exchanger 3. J Biol Chem. 2006;281:1461–1469. doi: 10.1074/jbc.M509786200. [DOI] [PubMed] [Google Scholar]
- 36.Cha B, Tse M, Yun C, Kovbasnjuk O, Mohan S, Hubbard A, Arpin M, Donowitz M. The NHE3 juxtamembrane cytoplasmic domain directly binds ezrin: dual role in NHE3 trafficking and mobility in the brush border. Mol Biol Cell. 2006;17:2661–2673. doi: 10.1091/mbc.E05-09-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musch MW, Arvans DL, Walsh-Reitz MM, Uchiyama K, Fukuda M, Chang EB. Synaptotagmin I binds intestinal epithelial NHE3 and mediates cyclic AMP- and Ca++-induced endocytosis by recruitment of AP2 and clathrin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1549–G1558. doi: 10.1152/ajpgi.00388.2006. [DOI] [PubMed] [Google Scholar]
- 38.Szászi K, Paulsen A, Szabó EZ, Numata M, Grinstein S, Orlowski J. Clathrin-mediated endocytosis and recycling of the neuron-specific Na+/H+ exchanger NHE5 isoform: regulation by phosphatidylinositol 3′-kinase and the actin cytoskeleton. J Biol Chem. 2002;277:42623–42632. doi: 10.1074/jbc.M206629200. [DOI] [PubMed] [Google Scholar]
- 39•.Szabo EZ, Numata M, Lukashova V, Iannuzzi P, Orlowski J. β-Arrestins bind and decrease cell-surface abundance of the Na+/H+ exchanger NHE5 isoform. Proc Natl Acad Sci USA. 2005 doi: 10.1073/pnas.0407444102. This study revealed a novel role for β-arrestin, a specialized scaffolding/ adaptor molecule involved in desensitization and internalization of G protein-coupled receptors, in clathrin-mediated endocytosis of the plasma membrane NHE5 isoform. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Numata M, Orlowski J. Molecular cloning and characterization of a novel (Na+, K+)/H+ exchanger localized to the trans-Golgi network. J Biol Chem. 2001;276:17387–17394. doi: 10.1074/jbc.M101319200. [DOI] [PubMed] [Google Scholar]
- 41.Lin PJ, Williams WP, Kobiljski J, Numata M. Caveolins bind to (Na+, K+)/H+ exchanger NHE7 by a novel binding module. Cell Signal. 2007;19:978–988. doi: 10.1016/j.cellsig.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/ H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280:1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 43.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol. 2004;288:F530–F538. doi: 10.1152/ajprenal.00229.2004. [DOI] [PubMed] [Google Scholar]
- 44.Hill JK, Brett CL, Chyou A, Kallay LM, Sakaguchi M, Rao R, Gillespie PG. Vestibular hair bundles control pH with (Na+,K+)/ H+ exchangers NHE6 and NHE9. J Neurosci. 2006;26:9944–9955. doi: 10.1523/JNEUROSCI.2990-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Brett CL, Tukaye DN, Mukherjee S, Rao R. The yeast endosomal Na+(K+)/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell. 2005;16:1396–1405. doi: 10.1091/mbc.E04-11-0999. This paper provides key evidence linking Nhx1-regulated endosomal pH to the control of vesicle trafficking out of the endosome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardo JM, Cubero B, Leidi EO, Quintero FJ. Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot. 2006;57:1181–1199. doi: 10.1093/jxb/erj114. [DOI] [PubMed] [Google Scholar]
- 47.Nass R, Cunningham KW, Rao R. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J Biol Chem. 1997;272:26145–26152. doi: 10.1074/jbc.272.42.26145. [DOI] [PubMed] [Google Scholar]
- 48.Bowers K, Levi BP, Patel FI, Stevens TH. The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:4277–4294. doi: 10.1091/mbc.11.12.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rockwell NC, Fuller RS. Specific modulation of Kex2/furin family proteases by potassium. J Biol Chem. 2002;277:17531–17537. doi: 10.1074/jbc.M111909200. [DOI] [PubMed] [Google Scholar]
- 50.Mellman I. The importance of being acid: the role of acidification in intracellular membrane traffic. J Exp Biol. 1992;172:39–45. doi: 10.1242/jeb.172.1.39. [DOI] [PubMed] [Google Scholar]
- 51.Yamashiro DJ, Maxfield FR. Regulation of endocytic processes by pH. Trends Pharmacol Sci. 1988;9:190–193. doi: 10.1016/0165-6147(88)90078-8. [DOI] [PubMed] [Google Scholar]
- 52.Sun-Wada GH, Wada Y, Futai M. Diverse and essential roles of mammalian vacuolar-type proton pump ATPase: toward the physiological understanding of inside acidic compartments. Biochim Biophys Acta. 2004;1658:106–114. doi: 10.1016/j.bbabio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 53•.Ali R, Brett CL, Mukherjee S, Rao R. Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J Biol Chem. 2004;279:4498–4506. doi: 10.1074/jbc.M307446200. This paper provides intriguing data showing that the yeast endosomal Nhx1 is physically and/or functionally coupled to proteins involved in retrograde vesicle trafficking (Gyp6 GAP and its target, the Ypt6 Rab-GTPase) between the endosome and late Golgi compartments. Significantly, Gyp6 was found to modulate endosome pH by negatively regulating Nhx1. [DOI] [PubMed] [Google Scholar]
- 54.Wells KM, Rao R. The yeast Na+/H+ exchanger Nhx1 is an N-linked glycoprotein—topological implications. J Biol Chem. 2001;276:3401–3407. doi: 10.1074/jbc.M001688200. [DOI] [PubMed] [Google Scholar]
- 55•.Lin PJ, Williams WP, Luu Y, Molday RS, Orlowski J, Numata M. Secretory carrier membrane proteins interact and regulate trafficking of the organellar (Na+,K+)/H+ exchanger NHE7. J Cell Sci. 2005;118:1885–1897. doi: 10.1242/jcs.02315. This paper identifies an important role of SCAMPs in the trafficking of NHE7 between the trans-Golgi network and recycling vesicles. [DOI] [PubMed] [Google Scholar]
- 56.Castle A, Castle D. Ubiquitously expressed secretory carrier membrane proteins (SCAMPs) 1–4 mark different pathways and exhibit limited constitutive trafficking to and from the cell surface. J Cell Sci. 2005;118:3769–3780. doi: 10.1242/jcs.02503. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, Liao H, Castle A, Zhang J, Casanova J, Szabo G, Castle D. SCAMP2 interacts with Arf6 and phospholipase D1 and links their function to exocytotic fusion pore formation in PC12 cells. Mol Biol Cell. 2005;16:4463–4472. doi: 10.1091/mbc.E05-03-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 59••.Hurtado-Lorenzo A, Skinner M, El AJ, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol. 2006;8:124–136. doi: 10.1038/ncb1348. This excellent study is a ‘tour-de-force’ of biochemistry and cell biology that provide compelling evidence that the V-ATPase acts as a scaffold to recruit elements of the trafficking machinery in a pH-dependent manner and that this interaction is crucial for protein trafficking between early and late endosomes. The results shed significant mechanistic insight into the poorly understood relationship between organellar acidification and vesicle trafficking. [DOI] [PubMed] [Google Scholar]
- 60.Pang T, Su X, Wakabayashi S, Shigekawa M. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J Biol Chem. 2001;276:17367–17372. doi: 10.1074/jbc.M100296200. [DOI] [PubMed] [Google Scholar]
- 61.Pang T, Wakabayashi S, Shigekawa M. Expression of calcineurin B homologous protein 2 protects serum deprivation-induced cell death by serum-independent activation of Na+/H+ exchanger. J Biol Chem. 2002;277:43771–43777. doi: 10.1074/jbc.M208313200. [DOI] [PubMed] [Google Scholar]
- 62.Mailander J, Muller-Esterl W, Dedio J. Human homolog of mouse tescalcin associates with Na+/H+ exchanger type-1. FEBS Lett. 2001;507:331–335. doi: 10.1016/s0014-5793(01)02986-6. [DOI] [PubMed] [Google Scholar]
- 63.Li X, Liu Y, Alvarez BV, Casey JR, Fliegel L. A novel carbonic anhydrase II binding site regulates NHE1 activity. Biochemistry. 2006;45:2414–2424. doi: 10.1021/bi051132d. [DOI] [PubMed] [Google Scholar]
- 64.Di Sole F, Cerull R, Babich V, Quinones H, Gisler SM, Biber J, Murer H, Burckhardt G, Helmle-Kolb C, Moe OW. Acute regulation of Na/H exchanger NHE3 by adenosine A1 receptors is mediated by calcineurin homologous protein. J Biol Chem. 2004;279:2962–2974. doi: 10.1074/jbc.M306838200. [DOI] [PubMed] [Google Scholar]
- 65.Biemesderfer D, Nagy T, DeGray B, Aronson PS. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J Biol Chem. 1999;274:17518–17524. doi: 10.1074/jbc.274.25.17518. [DOI] [PubMed] [Google Scholar]
- 66.Girardi AC, Knauf F, Demuth HU, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol. 2004;287:C1238–C1245. doi: 10.1152/ajpcell.00186.2004. [DOI] [PubMed] [Google Scholar]
- 67.Onishi I, Lin PJ, Diering GH, Williams WP, Numata M. RACK1 associates with NHE5 in focal adhesions and positively regulates the transporter activity. Cell Signal. 2007;19:194–203. doi: 10.1016/j.cellsig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Page RDM. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]