Abstract
Object recognition is an important visual process. We are not only required to recognize objects across a variety of lighting conditions and variations in size, but also across changes in viewpoint. It has been shown that reaction times in object matching increase as a function of increasing angular disparity between two views of the same object, and it is thought that this is related to the time it takes to mentally rotate an object. Recent studies have shown that object rotations for familiar objects affect older subjects differently than younger subjects. To investigate the general normalization effects for recognizing objects across different viewpoints regardless of visual experience with an object, in the current study we used novel 3D stimuli. Older and younger subjects matched objects across a variety of viewpoints along both in-depth and picture-plane rotations. Response times (RTs) for in-depth rotations were generally slower than for picture plane rotations and older subjects, overall, responded slower than younger subjects. However, a male RT advantage was only found for objects that differed by large, in-depth rotations. Compared to younger subjects, older subjects were not only slower but also less accurate at matching objects across both rotation axes. The age effect was primarily due to older male subjects performing worse than younger male subjects, whereas there was no significant age difference for female subjects. In addition, older males performed even worse than older females, which argues against a general male advantage in mental rotations tasks.
Keywords: Aging, Object recognition, Mental rotation, Novel objects, Viewpoint
1. Introduction
Despite the apparent ease with which we identify or categorize objects in the environment, object recognition is a demanding task for our visual system. An object is rarely seen twice under the same illumination, from the same viewing distance, or the same viewpoint. Consequently, depending on these viewing conditions, the same object can project drastically different two-dimensional (2D) images onto the retina. For example, if an object is rotated in depth by a large angle from one viewpoint to another, relative to a stationary observer, that person will see very different surfaces, features, and parts of that object from the two viewpoints. Still, our visual system seems to be able to compensate for the tremendous changes in visual information due to changes in viewpoints.
Previous studies have shown that there is a performance cost associated with matching 2D images of the same object across different viewing conditions. For example, reaction time (RT) or errors in matching tasks typically increase as a function of increasing angular disparity between two views of the same object (Tarr & Bülthoff, 1998). This viewpoint effect has been found for rotations in depth and rotations in the picture plane (e.g., Biederman & Gerhardstein, 1993; Bülthoff & Edelman, 1992; Cooper, 1975; Edelman & Bülthoff, 1992; Shepard & Metzler, 1971; Tarr & Bülthoff, 1995). The increase in RTs with increasing angular disparity may reflect the time it takes to mentally rotate an object to achieve a match between the stored mental representation and the retinal input (Jolicoeur, 1985; Shepard & Metzler, 1971; Tarr & Pinker, 1989). Other researchers have suggested that the increase in RTs may be caused by other normalization mechanisms such as view interpolation (Poggio & Edelman, 1990; Ullman, 1998) or evidence accumulation (Perrett, Oram, & Ashbridge, 1998).
Some previous studies on age-related changes in mental rotation abilities have shown that older subjects have difficulty matching objects across in-depth and picture-plane rotations (e.g., Cerella, Poon, & Fozard, 1981; Dror, Schmitz-Williams, & Smith, 2005; Gaylord & Marsh, 1975; Hertzog, Vernon, & Rypma, 1993; Jansen & Heil, 2010; Lee, Harris, & Calvert, 1998; Sharps & Gollin, 1987). It seems, however, that these age differences depend not only on the complexity or familiarity of the objects used (Dror et al., 2005; Jacewicz & Hartley, 1979) but also on whether speeded responses were or were not required (Hertzog et al., 1993; Sharps & Gollin, 1987). It has been suggested that age-related differences in mental rotation tasks are related to general slowing of cognitive and motor functions (e.g., Gaylord & Marsh, 1975; Jacewicz & Hartley, 1979; Salthouse & Somberg, 1982). More recently, though, Habak, Wilkinson, and Wilson (2008) found that older subjects performed as well as younger subjects at matching faces shown from the same view, regardless of stimulus duration. However, older subjects’ performance was significantly worse when faces had to be matched across different viewpoints, and did not improve with increased stimulus duration. Habak et al. (2008)’s results suggest that a slowing of cognitive and motor functions cannot account entirely for age-related deficits in mental rotation tasks.
Moreover, under some conditions, older subjects can compensate for drastic changes in object appearance caused by changes in viewing conditions. In a study by Dror et al. (2005), for example, older and younger subjects had to match line drawings of objects that varied in complexity (as calculated by the compactness of drawings) across three rotations in the picture-plane (Dror et al., 2005). For simple objects, both age groups showed the same relative increase in reaction time (RT) as a function of increasing change in viewpoint. For more complex objects, the relative increase in RT with changes in viewpoint was smaller in older subjects than younger subjects. Finally, older subjects were, overall, slower at recognizing both simple and complex objects across views. Dror et al. (2005) interpreted their results as showing that older subjects use the same holistic processing strategies for simple and complex objects, whereas younger subjects use featural or piecemeal strategies for more complex objects and rely on holistic processing for simple objects.
However, the objects used in Dror et al.’s study were highly familiar real-world objects, and stimuli were degraded in ways that might affect older and younger subjects’ ability to match them across viewpoints differently. Habak et al. (2008) on the other hand, measured matching performance across only two viewpoints and only for faces, which have been suggested to be a special category of object processing (Farah, 1996; Farah, Wilson, Drain, & Tanaka, 1998; Maurer, Grand, & Mondloch, 2002; Mondloch, Maurer, & Ahola, 2006); but also see (Gauthier & Tarr, 1997; Gauthier, Skudlarski, Gore, & Anderson, 2000; Gauthier & Bukach, 2007), and only two levels of comparison were included in that study: a complete image match (same viewpoint), and a non-match (different 3D viewpoints).
In the current study we tested matching performance of older and younger adults across a broader range of viewpoints than has been done before. In addition, to understand how general normalization mechanisms are affected by aging, we used a set of stimuli that neither age group had seen before, and is different to the previous studies mentioned above. Therefore, we used a set of non-degraded novel three-dimensional (3D) objects. We also compared matching performance for in-depth rotations, which changes the visible features and parts of objects, with rotations in the picture-plane, for which the same features and object parts are visible all the time.
2. Methods
2.1. Subjects
Fourteen younger (M = 23.21; range = 19–31; seven male) and 14 older subjects (M = 68.35; range = 60–75; seven male) participated in the experiment. All subjects were naïve as to the purpose of the experiment, and all had normal or corrected-to-normal visual acuity. A general health questionnaire was administered prior to testing, and none of the subjects reported having any visual disorders or major health problems. All subjects had visited an ophthalmologist or an optometrist within the past 3 years and were free of glaucoma, strabismus, amblyopia, macular degeneration, and cataracts. None of the subjects was aphakic. Older subjects also completed the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) to assess their cognitive abilities. All scores were within the normal ranges for their age and education levels (Crum, Anthony, Bassett, & Folstein, 1993). Subjects were paid $10 per hour for their participation in the experiment.
2.2. Stimuli
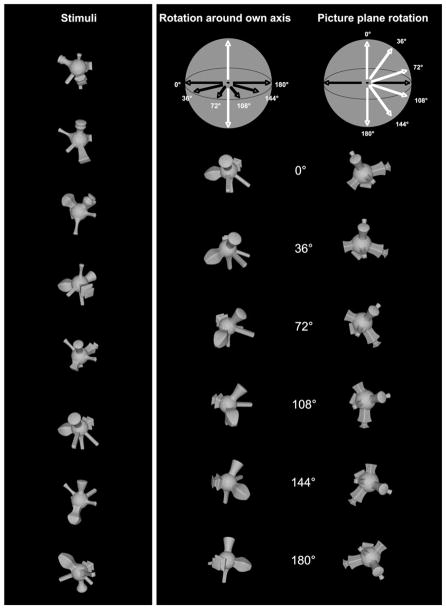
The stimuli used in the present study were nine novel amoebalike objects described by Vuong and Tarr (2004). Each object comprised a central sphere with six parts randomly distributed across the sphere’s surface and placed at arbitrary depths along the surface normal. We placed a virtual camera in the scene and arbitrarily fixed the 3D pose of each object relative to the camera. This initial pose was designated as the 0° viewpoint for each object. We then rotated each object at 0°, 36°, 72°, 108°, 144° and 180° clockwise around the vertical axis (i.e., in-depth relative to the initial 3D pose), and rendered images from these six viewpoints. The 0° image of each object served as its upright orientation. We then took the 0° image from each object, and rotated them 0°, 36°, 72°, 108°, 144°, 180° clockwise in the picture-plane. All objects were modeled in 3D Studio Max 4.0 (Discreet, Montreal, Quebec), and were illuminated by an ambient light source so that all surface features were uniformly visible. The rendered images were 256-level greyscale bitmap images. Fig. 1b shows example objects for indepth (top row) and picture-plane (bottom row) rotations. The objects subtended, on average 8.5° × 8.5° of visual angle and were of high contrast (see Fig. 1).
Fig. 1.
Stimuli as used in the current experiment (left). Example stimuli depict in-depth rotations (middle) and picture-plane rotations (right).
2.3. Apparatus
The experiment was conducted on a Macintosh G5 computer under the control of the Video and Psych ToolBox extensions for MAT-LAB (Brainard, 1997; Pelli, 1997). Stimuli were presented on a 20 in. Apple Studio Display (model M6204), with a resolution of 1024 × 864 pixels and a refresh rate of 75 Hz. Each subject was seated in a darkened room, and viewed the stimuli binocularly with a chin/forehead rest stabilizing the subject’s head. At the viewing distance of 60 cm, the entire display subtended 37° × 28° of visual angle.
2.4. Procedure
The paradigm was a sequential matching task in which subjects were shown two stimuli and judged, as quickly and as accurately as possible, whether the two stimuli were the same object or different objects. On each experimental trial subjects saw the first stimulus for 600 ms, followed by a blank inter-stimulus interval (ISI) of 300 ms, followed by a second stimulus, which stayed on the screen until the subject responded. Half the trials were same object trials, the other half different object trials.
The viewpoint (in depth) or orientation (in the picture plane) of the first stimulus was always chosen to be the frontal view of the object. The second object was rotated by 0° (same view), 36°, 72°, 108°, 144°, 180°. The experiment consisted of two blocks: in one block the object was rotated in the picture plane (picture-plane rotation), and in the other block the object was rotated around its own axis (in-depth rotation). Block order was randomized for each subject. For each block, eight of the nine objects were randomly chosen as stimuli. In each block of the experiment there were 12 different conditions (2 trial types (same/different)) × 6 angles (0°, 36°, 72°, 108°, 144°, 180°). Each subject performed 24 trials per condition (four repetitions per object), resulting in a total of 288 trials per block and 576 trials for the whole experiment. Within each block, the trials were randomized. For different trials, a random object was chosen from the remaining seven objects.
3. Results
3.1. Reaction times
An initial 2(age) × 2(sex) × 2(rotation axis) × 2(trial type) × 6(rotation angle) ANOVA of RTs of correct responses found significant differences between same and different trials (F(1,24) = 34.4, p < 0.001). Because same and different trials are likely to represent different processes in a matching task, and we were mainly interested in the results given by the same trials, we analyzed RTs of correct responses on same and different trials separately. A 2(age) × 2(sex) × 2(rotation axis) × 6(rotation angle) ANOVA on different trials found significant main effects of age (F(1,24) = 38, p < 0.001) and rotation axis (F(1,24) = 2.7, p < 0.001) indicating that older subjects were generally slower than younger subjects and RTs on picture-plane rotations were generally faster than for indepth rotations. There were no further main effects or interactions for different trials.
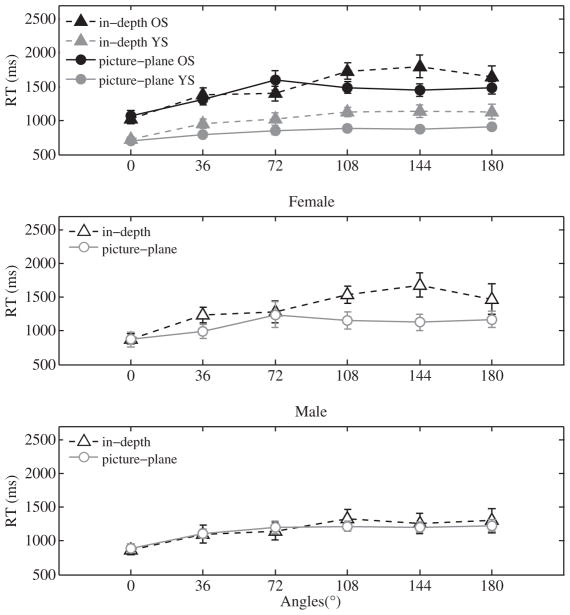
The RTs from same trials are shown in Fig. 2. A 2(age) × 2(sex) × 2(rotation axis) × 6(rotation angle) ANOVA of RTs on same trials found significant main effects of rotation angle (F(5,120) = 41.88, p < 0.001) and rotation axis (F(1,24) = 7.30, p < 0.05), indicating that RTs increased with increasing rotation angle and that RTs were longer for in-depth rotations. There also was a significant rotation angle × axis interaction (F(5,120) = 6.12, p < 0.001), which reflected the fact that the slope of the RT-vs.-angle function was steeper for in-depth rotation than picture-plane rotation (t(27) = 3.4, p < 0.001; in-depth: M = 110 ms/deg, SD = 70 ms/deg; picture-plane: M = 50 ms/deg, SD = 50 ms/deg). The main effect of age was significant (F(1,24) = 32.25, p < 0.001), indicating that older subjects generally were slower than younger subjects. However, that main effect was tempered by a significant age × rotation angle interaction (F(5,120) = 3.98, p < 0.01). Inspection of Fig. 2(top panel) suggests that the difference between older and younger subjects increased with rotation angle. This observation was confirmed by a comparison of slopes of the RT-vs.-angle functions measured in the two groups, which found that the slope was significantly steeper in older subjects (t(13) = 2.7, p < 0.01; older: M = 100 ms/deg, SD = 40 ms/deg; younger: M = 60 ms/deg, SD = 30 ms/deg). None of the other interactions with age were significant
Fig. 2.
RTs for correct responses on same trials for older subjects (OS) and younger subjects (YS) plotted separately for both axes of rotation at all rotation angles (top panel). Data from female and male subjects in both age groups are plotted separately in the middle and bottom panels, respectively. Error bars represent ±SEM.
The ANOVA also found a significant sex × rotation axis interaction (F(1,24) = 4.86, p < 0.05), as well as a significant sex × rotation axis × rotation angle interaction (F(5,120) = 2.41, p < 0.05). To assess the effect of rotation axis and rotation angle on sex in further detail, we performed separate 2(rotation axis) × 6(rotation angle) ANOVAs on female and male subjects.
The middle and bottom panels in Fig. 2 show RTs for older and younger subjects (collapsed across both rotation axes) at all rotation angles separately for female (middle panel) and male subjects (bottom panel). The ANOVA on data collected from male subjects found a significant main effect of rotation angle (F(5,60) = 18.89, p < 0.001) but no other significant effects. For female subjects, the ANOVA found main effects of rotation axis (F(1,12) = 16.18, p < 0.01) and rotation angle (F(5,60) = 23.64, p < 0.001), as well as a rotation axis × angle interaction (F(5,60) = 5.43, p < 0.001). Hence, the sex × rotation axis interaction reflected the fact that, regardless of age, female subjects (but not male subjects) showed a bigger effect of viewpoint for in-depth rotations than picture-plane rotations (t(13) = 5.4, p < 0.01; picture-plane: M = 50 ms/deg, SD = 40ms/deg; in-depth: M = 120 ms/deg, SD = 80 ms/deg). Finally, the three-way interaction between sex, rotation axis, and rotation angle reflects the fact that, in female subjects, the difference between in-depth and picture plane rotations was greater at larger angels, particularly 108° and 144°, than smaller angles. Finally, we directly compared RTs in males and females in 2(sex) × 6(rotation angle) ANOVAs separately for in-depth and picture-plane rotations. For picture-plane rotations, the main effect of sex (F(1,26) = 0.32, p = 0.58) and the sex × angle interaction (F(5,130) = 1.06, p = 0.38) were not significant. For in-depth rotations the main effect of sex was also not significant (F(1,26) = 0.48, p = 0.49) but there was a significant sex × angle interaction (F(5,130) = 3.3, p < 0.01), which reflected the fact that RTs were longer in females than males when the rotation angle was large.
In summary, RTs for in-depth rotations were longer than for picture-plane rotations. In general, older subjects responded more slowly than younger subjects. This age difference increased with increasing rotation angle. In addition, we found that RTs were significantly greater in female subjects only in conditions that used objects that differed by large, in-depth rotations.
3.2. D-prime
Mean response accuracy is shown in Table 1. Previous studies have shown that, at least in some tasks, older subjects exhibit different response biases than younger subjects (Flicker, Ferris, Crook, & Bartus, 1989; Konar, Bennett, & Sekuler, 2010; Naveh-Benjamin et al., 2009). Therefore, we used our accuracy measures to compute d′, a measure of sensitivity, which is less affected by response bias, and submitted this measure to an ANOVA. All subjects performed well above chance: The general accuracy level for older subjects was 72% and 78% for younger subjects. In general, observers reported that the task was much easier for picture-plane rotations.
Table 1.
Accuracy (percent correct) for same and different trials for both young and old subjects. Standard deviations in brackets. Chance performance on the task was 50%.
| Angles | 0° | 36° | 72° | 108° | 144° | 180° |
|---|---|---|---|---|---|---|
| Younger | ||||||
| Picture-plane rotations | ||||||
| Same trials | 98(2) | 95(4) | 89(8) | 93(9) | 87(9) | 85(10) |
| Different trials | 79(11) | 79(9) | 78(8) | 77(10) | 79(11) | 79(11) |
| In-depth rotations | ||||||
| Same trials | 99(2) | 84(13) | 76(16) | 68(15) | 65(15) | 57(15) |
| Different trials | 68(13) | 67(11) | 64(17) | 61(17) | 64(11) | 71(10) |
| Older | ||||||
| Picture-plane rotations | ||||||
| Same trials | 98 (3) | 89(10) | 83(12) | 85(11) | 81(11) | 79(11) |
| Different trials | 75(15) | 68(17) | 68(16) | 71(12) | 68(14) | 65(13) |
| In-depth rotations | ||||||
| Same trials | 97(4) | 80(9) | 69(14) | 55(16) | 61(14) | 55(15) |
| Different trials | 62(18) | 65(16) | 61(20) | 64(16) | 62(18) | 63(19) |
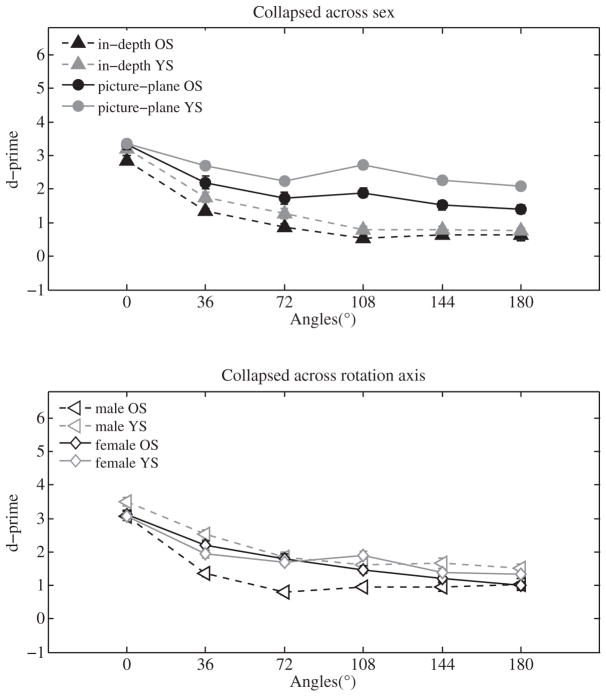
Fig. 3 shows d′ for all conditions. A 2(age) × 2(rotation axis) × 6(rotation angle) ANOVA found main effects of age (F(1,24) = 5.70, p < 0.05), rotation axis (F(1,24) = 34.70, p < 0.001), and rotation angle (F(5,120) = 80.35, p < 0.001), indicating that sensitivity was lower for older than for younger subjects, was generally lower for in-depth than picture-plane rotations, and decreased with increasing angular rotations. There also was a significant rotation axis × rotation angle interaction (F(5,120) = 6.72, p < 0.001), which reflected the fact that the sensitivity-vs.-angle function was steeper for in-depth rotations (t(13) = 3.0, p < 0.01; in-depth: M = −0.4 (dp/ms), SD = 0.1 (dp/ms); picture-plane: M = −0.3 (dp/ms), SD = 0.1 (dp/ms)).
Fig. 3.
D-primes for older subjects (OS) and younger subjects (YS) for both axes of rotation at all rotation angles for both male and female subjects (top panel), and collapsed across rotation axis (bottom panel). Error bars represent ±SEM. In cases where the error bars are not visible, the standard error was smaller than the width of the symbols.
The ANOVA also found significant sex × rotation angle (F(5,120) = 2.52, p < 0.05) and age × sex × rotation angle (F(5,120) = 2.61, p < 0.05) interactions. To assess these effects in further detail, we performed separate 2(age) × 6(rotation angle) ANOVAs for male and female subjects.
For male subjects, our analyses revealed significant main effects of age (F(1,12) = 8.63, p < 0.05), and rotation angle (F(1,12) = 12, p < 0.01) The interaction was not significant. In other words, sensitivity was lower in older males than younger males across rotation angle, and the age difference did not vary significantly with rotation angle. For female subjects, the main effect of rotation angle (F(5,60) = 32.5, p < 0.001) was significant. The main effect of age was not significant (F(1,12) = 0.1, p = 0.7), and there was no significant interaction.
In addition, we tested for differences between older and younger male and female subjects in the sensitivity-vs.-angle function. We found that this function was steeper for older female subjects than older male subjects (t(12) = 1.7, p < 0.05; older female: M = −0.3 (dp/ms), SD = 0.26 (dp/ms); older male: M = −0.04 (dp/ms), SD = 0.23 (dp/ms)), which was probably due to older female subjects performing better at smaller angular deviations than male subjects (Fig. 3, bottom panel). The function was not different for younger male and younger female subjects (t(12) = 0.79, p = 0.4), younger and older female subjects (t(12) = 1.79, p = 0.08), older and younger male subjects (t(12) = 1.7, p = 0.09), younger female and older male subjects (t(12) = 0.75, p = 0.5), or older male and younger female subjects (t(12) = 1.3, p = 0.2).
In summary, sensitivity was lower for in-depth than picture plane rotations and lower for older than younger subjects. This age effect was primarily due to older male subjects performing worse than younger male subjects, whereas there was no significant age difference for female subjects. However, male subjects showed shallower slopes in the sensitivity-vs.-slope functions, which was due to older females performing better than older males at smaller angular deviations.
4. Discussion
In the current study, older and younger subjects matched novel 3D objects across in-depth and picture-plane rotations, and we investigated the effects of age on reaction times and d′. Our results generally are consistent with previous studies that measured the effects of picture-plane and in-depth rotation on object recognition in younger subjects (e.g., Gauthier et al., 2002; Lacey, Peters, & Sathian, 2007; Logothetis, Pauls, & Poggio, 1995; Perrett et al., 1985; Shepard & Metzler, 1971; Tarr & Pinker, 1989), and extend those findings to older subjects. In both age groups, RTs increased, and d′ decreased, with increasing rotation angle. Older subjects, in general, were slower than younger subjects, and this age difference in RT increased with increasing rotation angle. We also found that d′ was lower overall in older subjects, although subsequent analyses revealed that this age difference was significant only in males.
In addition, across both age groups, female subjects, but not male subjects, had longer RTs for in-depth rotations than picture-plane rotations. Sex differences in mental rotation have been reported previously. Jansen and Heil (2010), for example, investigated sex differences in mental rotation tasks in three age groups (20–30, 40–50, 60–70) and found that males were more accurate than females in all conditions. In the current study, however, RTs were longer in female subjects only for large, in-depth rotations; we found no evidence of a sex difference in RTs when objects were rotated in the picture plane. We also found no evidence of a general male advantage for accuracy as measured using d′
Therefore, the current study does not support a general male advantage for mental rotation tasks in the picture-plane as suggested previously (e.g., Astur, Tropp, Sava, Constable, & Markus, 2004; Crucian & Berenbaum, 1998; Jansen & Heil, 2010; Tapley & Bryden, 1977). On the other hand, the RT-based sex difference for in-depth rotations was rather strong, and is consistent with the view that men have an advantage over woman for processing 3D information and perform better in tasks involving spatial memory (e.g., Astur et al., 2004; Crucian & Berenbaum, 1998; Voyer, Voyer, & Bryden, 1995; Peters et al., 1995; Wolbers & Hegarty, 2010).
It has been suggested that age differences in many tasks – including mental rotation tasks (e.g., Gaylord & Marsh, 1975; Jacewicz & Hartley, 1979) – are related to a general slowing of perceptual and cognitive operations in the aging brain (Salthouse & Somberg, 1982). The overall effects of age on RTs measured in the current study seem consistent with this hypothesis. However, the steeper slopes for older subjects’ RTs as a function of angular deviation compared to younger subjects, as well as the interactions between sex and age, indicate that the age difference is not solely due to a generalized slowing of information processing in older subjects. In addition, the d′ analysis found that older men were similarly impaired compared to younger men for both picture-plane and in-depth rotations, whereas there was no age-difference for female subjects.
Results from previous studies indicate that there might be more to the age difference in recognizing objects across viewpoints. Habak et al. (2008), for example, suggested that the age-related deterioration of discriminating faces across viewpoints was related to the fact that populations of neurons in the aging visual system saturated earlier when accumulating useful information compared to populations of neurons in the younger visual system (also see Perrett et al., 1998). Their experiment measured facial identity discrimination thresholds and showed that for faces shown from the same viewpoint thresholds were similar in older and younger subjects and did not change with increased exposure duration. For faces shown from different viewpoints, however, thresholds degraded with age, and exposure duration only improved performance for younger but not for older subjects, which suggests that generalized slowing alone cannot explain their age effects (but see Dey, Pachai, Bennett, & Sekuler (2010) for comparison). In the current study, viewing time was always the same for all observers and both rotation axes. However, older subjects’ RTs increased significantly more with increasing rotation angle than younger subjects’ RTs, which indicates that older subjects accumulate information slower than younger subjects, and supports results from Habak et al.
Unlike the studies mentioned above that used familiar objects such as faces or real world objects, for which older and younger subjects might have different levels of expertise, we used novel 3D objects that both older and younger adults had no previous exposure to. Hence, we can rule out familiarity as an interacting factor for the observed age differences.
Our analyses of RTs found a general age effect, but there was no evidence that the effect of age differed between males and females. Analyses of d′, however, found a significant effect of age, but only in male subjects.
Older women even seemed to outperform older men at small angular deviations as suggested by Fig. 3 and the fact that the sensitivity-vs.-angle function was steeper for older women than older men. This finding is particularly interesting given that men have been found to generally perform better than women in tasks involving spatial-ability such as mental rotation (Astur et al., 2004; Crucian & Berenbaum, 1998; Tapley & Bryden, 1977). The reasons for such previously suggested male advantage are not entirely clear. One hypothesis is that it is related to differences between the sexes in hemispheric functioning: mental rotation seems to rely on right hemispheric processing mechanisms and males typically perform better than females on tasks involving the right hemisphere (Levy & Reid, 1978; Klinteberg, Levander, & Schalling, 1987). This hypothesis, however, is still controversial, and seems to depend strongly on the task and stimulus (Cohen & Polich, 1989). Also, differences in mental rotation tasks due to differences in hemispheric functioning cannot necessarily account for the age effects presented in the current paper.
Another hypothesis that has been put forward in the context of sexual differences in mental rotation tasks is that testosterone plays a crucial role for the observed male advantage in spatial tasks (e.g., Liben et al., 2002; Hooven, Chabris, Ellison, & Kosslyn, 2004). For example, Hooven et al. (2004) found that testosterone facilitates mental rotation by influencing the encoding, comparison or decision process of mental rotation. Also, the administration of testosterone in younger females has been shown to increase performance on mental rotation tasks (Aleman, Bronk, Kessels, Koppeschaar, & van Honk, 2004), suggesting a role of testosterone on spatial tasks.
An explanation of differences in mental rotation tasks due to effects of testosterone could also account for the age and sex differences observed in the current paper. It has been shown previously that aging reduces testosterone levels in men (Davidson et al., 1983; Nankin & Calkins, 1986; Vermeulen, 1991). Considering the findings on the relation between testosterone levels and performance in mental rotation tasks, it seems plausible to assume that decreased levels of testosterone are related to a decreased performance in mental rotation tasks. It has been previously suggested that reduced testosterone levels affect spatial cognitive abilities in older men (Janowsky, Oviatt, & Orwoll, 1994; Van Strien, Weber, Burdorf, & Bangma, 2009). Therefore, the observed deficits of older men in the current study might be related to reduced testosterone levels. However, the role of testosterone for spatial cognitive functioning is still debated (e.g., Aleman et al., 2004; Falter, Arroyo, & Davis, 2006; Hooven et al., 2004; Liben et al., 2002; Puts et al., 2010), and the exact relationship between testosterone levels and performance on mental rotation tasks and aging has yet to be defined.
4.1. Conclusion
Theories of object recognition propose that our visual system utilizes certain normalization mechanisms to compensate for changes in viewing conditions (e.g., Jolicoeur, 1985; Perrett et al., 1998; Poggio & Edelman, 1990; Shepard & Metzler, 1971; Tarr & Pinker, 1989; Ullman, 1998). It is often assumed that these mechanisms are independent of other cognitive systems such as attention or memory (see also Gauthier et al., 2002). In support of this assumption, we have found that healthy aging can affect mechanisms that generalize across changes in viewpoint during object recognition independently of general cognitive and motor decline associated with healthy aging (e.g., Bayen, Phelps, & Spaniol, 2000; Li, Lindenberger, & Sikstrom, 2001; Salthouse & Somberg, 1982; Smith, Lozito, & Bayen, 2005). Furthermore, we found differential effects of rotation angle on males and females in aging, which could be explained by the idea that testosterone levels play a role in differences in spatial cognitive abilities in men and women.
Acknowledgments
This work was funded by the Canadian Institute of Health Research (A.B.S. and P.J.B.). The authors thank Donna Waxman for her valuable help running subjects for the experiment described here.
References
- Aleman A, Bronk E, Kessels RPC, Koppeschaar HPF, van Honk J. A single administration of testosterone improves visuospatial ability in young women. Psychoneuroendocrinology. 2004;29(5):612–617. doi: 10.1016/S0306-4530(03)00089-1. [DOI] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual morris water task, a virtual radial arm maze, and mental rotation. Behavioural Brain Research. 2004;151(1–2):103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Bayen UJ, Phelps MP, Spaniol J. Age-related differences in the use of contextual information in recognition memory: A global matching approach. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2000;55(3):P131–P141. doi: 10.1093/geronb/55.3.p131. [DOI] [PubMed] [Google Scholar]
- Biederman I, Gerhardstein PC. Recognizing depth-rotated objects: evidence and conditions for three-dimensional viewpoint invariance. Journal of Experimental Psychology: Human Perception and Performance. 1993;19(6):1162–1182. doi: 10.1037//0096-1523.19.6.1162. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Bülthoff HH, Edelman S. Psychophysical support for a two-dimensional view interpolation theory of object recognition. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(1):60–64. doi: 10.1073/pnas.89.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerella J, Poon LW, Fozard JL. Mental rotation and age reconsidered. Journal of Gerontology. 1981;36(5):620–624. doi: 10.1093/geronj/36.5.620. [DOI] [PubMed] [Google Scholar]
- Cohen W, Polich J. No hemispheric-differences for mental rotation of letters and polygons. Bulletin of the Psychonomic Society. 1989;27(1):25–28. [Google Scholar]
- Cooper LA. Mental rotation of two-dimensional shapes. Cognitive Psychology. 1975;7:20–43. [Google Scholar]
- Crucian GP, Berenbaum SA. Sex differences in right hemisphere tasks. Brain and Cognition. 1998;36(3):377–389. doi: 10.1006/brcg.1998.0999. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Davidson JM, Chen JJ, Crapo L, Gray GD, Greenleaf WJ, Catania JA. Hormonal changes and sexual function in aging men. Journal of Clinical Endocrinology and Metabolism. 1983;57(1):71–77. doi: 10.1210/jcem-57-1-71. [DOI] [PubMed] [Google Scholar]
- Dey AK, Pachai MV, Bennett PJ, Sekuler AB. The effects of aging and stimulus duration on face identification accuracy with differing viewpoints. Journal of Vision. 2010;10(7):568. [Google Scholar]
- Dror IE, Schmitz-Williams IC, Smith W. Older adults use mental representations that reduce cognitive load: mental rotation utilizes holistic representations and processing. Experimental Aging Research. 2005;31(4):409–420. doi: 10.1080/03610730500206725. [DOI] [PubMed] [Google Scholar]
- Edelman S, Bülthoff HH. Orientation dependence in the recognition of familiar and novel views of three-dimensional objects. Vision Research. 1992;32(12):2385–2400. doi: 10.1016/0042-6989(92)90102-o. [DOI] [PubMed] [Google Scholar]
- Falter CM, Arroyo M, Davis GJ. Testosterone: Activation or organization of spatial cognition? Biological Psychology. 2006;73(2):132–140. doi: 10.1016/j.biopsycho.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Farah MJ. Is face recognition ’special’? Evidence from neuropsychology. Behavioural Brain Research. 1996;76(1–2):181–189. doi: 10.1016/0166-4328(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychological Review. 1998;105(3):482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Crook T, Bartus RT. Age differences in the vulnerability of facial recognition memory to proactive interference. Experimental Aging Research. 1989;15(3–4):189–194. doi: 10.1080/03610738908259774. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Bukach C. Should we reject the expertise hypothesis? Cognition. 2007;103(2):322–330. doi: 10.1016/j.cognition.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Hayward WG, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Bold activity during mental rotation and viewpoint-dependent object recognition. Neuron. 2002;34(1):161–171. doi: 10.1016/s0896-6273(02)00622-0. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience. 2000;3(2):191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ. Becoming a “greeble” expert: exploring mechanisms for face recognition. Vision Research. 1997;37(12):1673–1682. doi: 10.1016/s0042-6989(96)00286-6. [DOI] [PubMed] [Google Scholar]
- Gaylord SA, Marsh GR. Age differences in the speed of a spatial cognitive process. Journal of Gerontology. 1975;30(6):674–678. doi: 10.1093/geronj/30.6.674. [DOI] [PubMed] [Google Scholar]
- Habak C, Wilkinson F, Wilson HR. Aging disrupts the neural transformations that link facial identity across views. Vision Research. 2008;48(1):9–15. doi: 10.1016/j.visres.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Vernon MC, Rypma B. Age differences in mental rotation task performance: The influence of speed/accuracy tradeoffs. Journal of Gerontology. 1993;48(3):P150–P156. doi: 10.1093/geronj/48.3.p150. [DOI] [PubMed] [Google Scholar]
- Hooven CK, Chabris CF, Ellison PT, Kosslyn SM. The relationship of male testosterone to components of mental rotation. Neuropsychologia. 2004;42(6):782–790. doi: 10.1016/j.neuropsychologia.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Jacewicz MM, Hartley AA. Rotation of mental images by young and old college students: The effects of familiarity. Journal of Gerontology. 1979;34(3):396–403. doi: 10.1093/geronj/34.3.396. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behavioral Neuroscience. 1994;108(2):325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Jansen P, Heil M. Gender differences in mental rotation across adulthood. Experimental Aging Research. 2010;36(1):94–104. doi: 10.1080/03610730903422762. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. The time to name disoriented natural objects. Memory and Cognition. 1985;13(4):289–303. doi: 10.3758/bf03202498. [DOI] [PubMed] [Google Scholar]
- Klinteberg BA, Levander SE, Schalling D. Cognitive sex differences: Speed and problem-solving strategies on computerized neuropsychological tasks. Perceptual and Motor Skills. 1987;65(3):683–697. doi: 10.2466/pms.1987.65.3.683. [DOI] [PubMed] [Google Scholar]
- Konar Y, Bennett PJ, Sekuler AB. Face identification and the evaluation of holistic indexes: CFE and the whole-part task. Journal of Vision. 2010:10. [Google Scholar]
- Lacey S, Peters A, Sathian K. Cross-modal object recognition is viewpoint-independent. PLoS One. 2007;2(9):e890. doi: 10.1371/journal.pone.0000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Calvert JE. Impairments of mental rotation in parkinson’s disease. Neuropsychologia. 1998;36(1):109–114. doi: 10.1016/s0028-3932(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Levy J, Reid M. Variations in cerebral organization as a function of handedness, hand posture in writing, and sex. Journal of Experimental Psychology: General. 1978;107(2):119–144. doi: 10.1037//0096-3445.107.2.119. [DOI] [PubMed] [Google Scholar]
- Li S, Lindenberger U, Sikstrom S. Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences. 2001;5(11):479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Liben LS, Susman EJ, Finkelstein JW, Chinchilli VM, Kunselman S, Schwab J, et al. The effects of sex steroids on spatial performance: A review and an experimental clinical investigation. Developmental Psychology. 2002;38(2):236–253. [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Poggio T. Shape representation in the inferior temporal cortex of monkeys. Current Biology. 1995;5(5):552–563. doi: 10.1016/s0960-9822(95)00108-4. [DOI] [PubMed] [Google Scholar]
- Maurer D, Grand RL, Mondloch CJ. The many faces of configural processing. Trends in Cognitive Science. 2002;6(6):255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Maurer D, Ahola S. Becoming a face expert. Psychological Science. 2006;17(11):930–934. doi: 10.1111/j.1467-9280.2006.01806.x. [DOI] [PubMed] [Google Scholar]
- Nankin HR, Calkins JH. Decreased bioavailable testosterone in aging normal and impotent men. Journal of Clinical Endocrinology and Metabolism. 1986;63(6):1418–1420. doi: 10.1210/jcem-63-6-1418. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Shing YL, Kilb A, Werkle-Bergner M, Lindenberger U, Li SC. Adult age differences in memory for name–face associations: The effects of intentional and incidental learning. Memory. 2009;17(2):220–232. doi: 10.1080/09658210802222183. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Perrett DI, Oram MW, Ashbridge E. Evidence accumulation in cell populations responsive to faces: An account of generalisation of recognition without mental transformations. Cognition. 1998;67(1–2):111–145. doi: 10.1016/s0010-0277(98)00015-8. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Potter DD, Mistlin AJ, Head AS, Milner AD, et al. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proceedings of the Royal Society of London Series B – Biological Sciences. 1985;223(1232):293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Peters M, Laeng B, Latham K, Jackson M, Zaiyouna R, Richardson C. A redrawn Vandenberg and Kuse mental rotations test: Different versions and factors that affect performance. Brain and Cognition. 1995;28(1):39–58. doi: 10.1006/brcg.1995.1032. [DOI] [PubMed] [Google Scholar]
- Poggio T, Edelman S. A network that learns to recognize three-dimensional objects. Nature. 1990;343(6255):263–266. doi: 10.1038/343263a0. [DOI] [PubMed] [Google Scholar]
- Puts DA, Cárdenas RA, Bailey DH, Burriss RP, Jordan CL, Breedlove SM. Salivary testosterone does not predict mental rotation performance in men or women. Hormones and Behavior. 2010;58(2):282–289. doi: 10.1016/j.yhbeh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Somberg BL. Isolating the age deficit in speeded performance. Journal of Gerontology. 1982;37(1):59–63. doi: 10.1093/geronj/37.1.59. [DOI] [PubMed] [Google Scholar]
- Sharps MJ, Gollin ES. Speed and accuracy of mental image rotation in young and elderly adults. Journal of Gerontology. 1987;42(3):342–344. doi: 10.1093/geronj/42.3.342. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171(972):701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Smith RE, Lozito JP, Bayen UJ. Adult age differences in distinctive processing: The modality effect on false recall. Psychology and Aging. 2005;20(3):486–492. doi: 10.1037/0882-7974.20.3.486. [DOI] [PubMed] [Google Scholar]
- Tapley SM, Bryden MP. An investigation of sex differences in spatial ability: Mental rotation of three-dimensional objects. Canadian Journal of Psychology. 1977;31(3):122–130. doi: 10.1037/h0081655. [DOI] [PubMed] [Google Scholar]
- Tarr MJ, Bülthoff HH. Is human object recognition better described by geon structural descriptions or by multiple views? Comment on Biederman and Gerhardstein (1993) Journal of Experimental Psychology: Human Perception and Performance. 1995;21(6):1494–1505. doi: 10.1037//0096-1523.21.6.1494. [DOI] [PubMed] [Google Scholar]
- Tarr MJ, Bülthoff HH. Image-based object recognition in man, monkey and machine. Cognition. 1998;67(1–2):1–20. doi: 10.1016/s0010-0277(98)00026-2. [DOI] [PubMed] [Google Scholar]
- Tarr MJ, Pinker S. Mental rotation and orientation-dependence in shape recognition. Cognitive Psychology. 1989;21(2):233–282. doi: 10.1016/0010-0285(89)90009-1. [DOI] [PubMed] [Google Scholar]
- Ullman S. Three-dimensional object recognition based on the combination of views. Cognition. 1998;67(1–2):21–44. doi: 10.1016/s0010-0277(98)00013-4. [DOI] [PubMed] [Google Scholar]
- Van Strien JW, Weber RFA, Burdorf A, Bangma C. Higher free testosterone level is associated with faster visual processing and more flanker interference in older men. Psychoneuroendocrinology. 2009;34(4):546–554. doi: 10.1016/j.psyneuen.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. Clinical review 24: Androgens in the aging male. Journal of Clinical Endocrinology and Metabolism. 1991;73(2):221–224. doi: 10.1210/jcem-73-2-221. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Vuong QC, Tarr MJ. Rotation direction affects object recognition. Vision Research. 2004;44(14):1717–1730. doi: 10.1016/j.visres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Hegarty M. What determines our navigational abilities? Trends in Cognitive Sciences. 2010;14(3):138–146. doi: 10.1016/j.tics.2010.01.001. [DOI] [PubMed] [Google Scholar]