Abstract
In mammals, the osmolality of the extracellular fluid is maintained near a predetermined set-point through a negative feedback regulation of thirst, diuresis, salt appetite and natriuresis. This homeostatic control is believed to be mediated by osmosensory neurones which synaptically regulate the electrical activity of command neurones that mediate each of these osmoregulatory effector responses. Our present understanding of the molecular, cellular and network basis that underlies the central control of osmoregulation is largely derived from studies on primary osmosensory neurones in the organum vasculosum lamina terminalis (OVLT) and effector neurones in the supraoptic nucleus (SON), which release hormones that regulate diuresis and natriuresis. Primary osmosensory neurones in the OVLT exhibit changes in action potential firing rate that vary in proportion with ECF osmolality. This effect results from the intrinsic depolarizing receptor potential which these cells generate via a molecular transduction complex that may comprise various members of the transient receptor potential vanilloid (TRPV) family of cation channel proteins, notably TRPV1 and TRPV4. Osmotically evoked changes in the firing rate of OVLT neurones then regulate the electrical activity of downstream neurones in the SON through graded changes in glutamate release.
Introduction: mammals tightly regulate extracellular fluid (ECF) osmolality
Acute changes in ECF osmolality cause water to flow across the plasma membrane and therefore provoke cellular swelling or shrinking. Although many types of cells are endowed with an innate ability to restore their volume following osmotic perturbations (Wehner et al. 2003), this adaptation is often incomplete and can occur with a delay of seconds or minutes (McManus et al. 1995). Fragile tissues, such as brain, can thus be significantly damaged by the mechanical impact of acute pathological osmotic perturbations (Verbalis, 2006). Fortunately, animals have evolved behavioural and physiological mechanisms that together work to maintain systemic osmolality near a stable set-point despite the episodic nature of salt and fluid intake (Bourque et al. 1994). Mammals, in particular, aggressively maintain ECF osmolality near a value of 300 mosmol kg−1. Although various species of mammals defend slightly different osmotic set-points (e.g. humans ~ 280 mosmol kg−1, rats ~295 mosmol kg−1, mice ~ 310 mosmol kg−1), individuals with free access to salt and water normally maintain ECF osmolality within 3% of their native set-point. In humans, for example, increases in plasma osmolality of about 9 mosmol kg−1 accompany a state of mild hypernatraemia (Andersen et al. 2002). Here we review our present understanding of the mechanisms by which the brain detects the body’s hydration status and initiates responses that mediate osmotic homeostasis.
Systemic osmoregulatory responses are controlled by the central nervous system
Previous studies have shown that mammals maintain osmotic homeostasis by making proportional adjustments in the intake and excretion of sodium and water when blood osmolality deviates from the set-point value by more than 1%. These adjustments are mediated largely by concerted changes in behaviour, neurohypophysial hormone release and sympathetic outflow (see below). Although the basis for this concerted regulation is unclear, the central control of body fluid balance is presumably mediated by osmotically evoked changes in the electrical activity (i.e. action potential firing rate or pattern) of distinct subsets of ‘command’ neurones that regulate each of the osmoregulatory responses.
Osmotic control of water intake
Water intake is controlled through a modulation of thirst. Specifically, hypertonic conditions enhance the cognitive sensation of thirst to promote a homeostatic increase in water intake, whereas hypotonic conditions have the reverse effect (see Bourque et al. 1994; Denton et al. 1996). Studies involving electrical stimulation of different cortical areas in animals and functional brain imaging in humans have highlighted a number of regions that may be involved in the genesis and satiation of thirst (McKinley et al. 2006). Among these, the anterior cingulate cortex (ACx) stands out as a strong candidate area for the command of thirst. The ACx is coincidentally activated and inhibited under conditions which, respectively, promote thirst and satiation (Egan et al. 2003), and stimulation of this area reliably induces drinking in monkeys (Robinson & Mishkin, 1968). Direct evidence that subsets of ACx neurones serve as command neurones for the sensation of thirst remains to be obtained.
Osmotic control of sodium intake
The control of sodium intake is achieved through a modulation of appetite for salt. Specifically, hypotonic conditions have been shown to contribute to the homeostatic enhancement of salt appetite, whereas hypertonic conditions have the reverse effect (for review see Bourque et al. 1994; Daniels & Fluharty, 2004). A variety of brain areas have been shown to play important roles in the control of salt intake under various physiological conditions, and an integrative analysis of these studies has indicated that neural pathways between forebrain and brainstem systems are likely to be key components of the circuitry that gives rise to salt appetite (Daniels & Fluharty, 2004). Unfortunately, the identity of putative command neurones for the genesis of salt appetite has remained elusive.
Osmotic control of water excretion
The osmotic control of water excretion (diuresis) is primarily achieved through changes in the plasma concentration of vasopressin (VP, the antidiuretic hormone). Specifically, systemic hypotonic conditions suppress VP release from the neurohypophysis, thus reducing the kidney’s ability to reabsorb water. Conversely, hypertonic conditions stimulate VP release, which promotes homeostatic water conservation. Vasopressin is synthesized in magnocellular neurosecretory cells (MNCs) located in the supraoptic (SON) and paraventricular (PVN) nuclei of the hypothalamus. The release of VP into the circulation occurs at the neurohypophysial axon terminals of MNCs in response to action potential discharge. Vasopressin-secreting MNCs thus represent the ‘command’ neurones that control diuresis, which varies as an inverse function of the firing rate of these neurones (Bourque et al. 1994).
Osmotic control of sodium excretion
The osmotic control of sodium excretion (natriuresis) occurs at the kidney (Andersen et al. 2002), where it is regulated by the effects of various hormones (e.g. Antunes-Rodrigues et al. 2004; Bie et al. 2004) and by innervating sympathetic fibres (e.g. DiBona, 1977). Although peripheral organs can produce hormones that can regulate natriuresis (e.g. aldosterone, angiotensin II and atrial natriuretic peptide), oxytocin (OT) released by OT-synthesizing MNCs has been shown to act as a natriuretic hormone (Verbalis et al. 1991) and to stimulate natriuresis under hypertonic conditions (Huang et al. 1996). Thus the brain can directly contribute to the osmotic control of sodium excretion via the modulation of electrical activity in OT-MNCs, which operate as command neurones that can modulate this process.
Osmosensitive neurones form the core of the osmostat
The feedback regulation of osmoregulatory responses implies the existence of an ‘osmostat’, a specialized sensory apparatus through which ECF osmolality can be measured and compared with a predetermined set-point. Since various responses are modulated by distinct populations of command neurones, the osmostat must first transduce osmotic perturbations into a neural code (i.e. a change in action potential firing rate or pattern) that is subsequently used to regulate these distributed command neurones via axonal projections and synaptic contacts. Electrophysiological studies have shown that the core function of the osmostat is performed by osmosensitive neurones, i.e. neurones that can display significant changes in their rate or pattern of action potential discharge in response to osmotic perturbations.
Osmosensitive neurones are widely distributed
In principle, the central control of systemic osmoregulation could be mediated by a single osmostat wired to neurones located in integrative centres and/or directly to command neurones located in the ‘effector’ parts of the brain. However, it is now known that neurones with intrinsic osmosensory properties can be found in various parts of the central and peripheral nervous systems, including central nuclei thought to contain sensory, integrative and effector neurones (Bourque et al. 1994).
Intrinsically osmosensitive neurones have been found in the organum vasculosum of the lamina terminalis (OVLT; Vivas et al. 1990; Ciura & Bourque, 2006), a small brain area believed to serve as the brain’s primary osmostat (Johnson et al. 1992; Denton et al. 1996; McKinley et al. 2006). Osmosensitive neurones have also been found in the subfornical organ (Anderson et al. 2000) and in the nucleus tractus solitarii (Izawa et al. 2000), central nuclei that appear to integrate osmotic information with humoral and ascending interoceptive sensory signals (Johnson et al. 1992; Daniels & Fluharty, 2004). Outputs from these nuclei may modulate osmoregulatory responses in a manner that co-ordinates osmotic homostasis with that of other cardiovascular parameters (e.g. ECF volume). Interestingly, osmotic signals can also be detected by primary chemosensory afferents (Gallego et al. 1979) and by afferent fibres in the hepatic branch of the vagus nerve (Adachi et al. 1976). Osmosensory information collected from the splanchnic mesentery is also known to be relayed to central areas via ascending projections carried in part via spinal pathways (Vallet & Baertschi, 1982; King & Baertschi, 1991). Finally, the VP and OT neurones of the hypothalamus are also intrinsically osmosensitive (Mason, 1980; Oliet & Bourque, 1993), indicating that at least some subtypes of osmoregulatory command neurones can also express the osmosensitivity phenotype.
The significance of this distributed localization of osmosensitive neurones is presently unclear. One possibility is that some of the osmosensitive cells in these different areas may be wired to each other in a manner that amplifies osmotic signalling. Indeed, the osmotic control of the firing rate of OT- and VP-releasing MNCs has been shown to result from an integration of multiple factors, including information originating from central and peripheral osmoreceptors, and the intrinsic osmosensitivity of the neurones (Russell et al. 1988; Bourque et al. 1994; Hussy et al. 2000; Voisin & Bourque, 2002). The upregulation of ‘redundant’ osmostat mechanisms might also explain why animals eventually recover from the acute osmoregulatory deficits induced by lesions of the OVLT (e.g. Carithers & Johnson, 1986), and why transgenic animals which lack molecules that are important for specific osmosensory mechanisms can still osmoregulate (e.g. Liedtke & Friedman, 2003; Ciura & Bourque, 2006; Sharif-Naeini et al. 2006). Different osmosensitive neurones might also constitute functionally distinct osmostats. For example, osmosensory neurones might differ in terms of the type of stimulus which they are able to detect (i.e. responsiveness to hypotonic or hypertonic stimuli), or in the mechanism by which they regulate downstream neurones (e.g. by providing inhibitory or excitatory signals). Indeed, as illustrated in Fig. 1, osmotic perturbations lead to the simultaneous activation and inhibition of different subsets of osmoregulatory responses. The sensory and network processes that underlie this co-ordinated regulation remain to be defined. In the remainder of this article, we review our present understanding of the mechanisms by which neurones in the OVLT operate as primary osmostats.
Figure 1. Osmostatic control of systemic osmoregulatory responses.
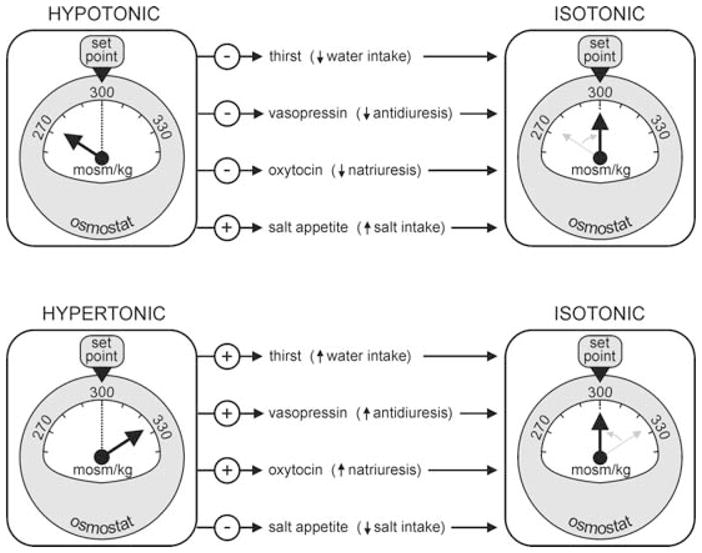
Schematic diagram illustrating some of the features of osmostat function. The dashed line shows the desired set-point (300 mosmol kg−1), and the arrow points to the prevailing ECF osmolality which is measured by the device. Arrows leading from the osmostat on the left indicate which of the effector responses are inhibited (−) or excited (+) under hypotonic (upper panel) or hypertonic conditions (lower panel) to restore homeostasis (i.e. osmostats on the right side, where ECF osmolality = set-point).
Neurones in OVLT are excited by hypertonic stimuli
Electrophysiological recordings in vivo (Honda et al. 1990) and in vitro (Sayer et al. 1984; Vivas et al. 1990; Nissen et al. 1993) have shown that a majority of neurones in the OVLT are excited by hypertonic stimuli and inhibited by hypotonicity. The functional basis for this osmoresponsiveness has remained unknown until recently. Indeed, experiments on neurones isolated from the OVLT of adult mice have provided definitive evidence that these cells are intrinsically sensitive to increases in the osmolality of the extracellular fluid (Ciura & Bourque, 2006). Hypertonic conditions were found to provoke increases in membrane non-selective cation conductance, thereby causing the generation of an inward current at normal resting potential. This inward current induces a depolarizing osmoreceptor potential that increases the probability of action potential discharge. Indeed, changes in firing rate induced by hypertonic stimuli are directly proportional to the magnitude of the depolarizing osmoreceptor potential (Ciura & Bourque, 2006). Thus, as commonly observed in cells that transduce other sensory modalities, OVLT neurones transduce osmotic signals by generating a depolarizing receptor potential. The intensity of the osmotic stimulus is encoded by the changes in firing rate that are graded in proportion with this potential.
Mechanism of osmosensory transduction in OVLT neurones
Genetic and molecular biological studies have recently highlighted the possible role of different members of the transient receptor potential vanilloid (TRPV) proteins in osmosensory transduction (Liedtke, 2006). When expressed in heterologous cells, some of the genes coding for these proteins lead to the formation of non-selective cation channels whose probability of opening can be modulated by changes in osmolality. For example, homomultimeric channels assembled from TRPV2 (Muraki et al. 2003) and TRPV4 (Liedtke et al. 2000; Strotmann et al. 2000) can be activated by hypotonic stimuli. Although it is certainly possible that hypotonicity-activated cation channels could mediate osmosensory transduction, native osmosensory neurones showing intrinsic depolarizing (i.e. excitatory) responses to hypotonic stimuli have yet to be described. Since the majority of OVLT neurones are excited by hypertonic stimuli (Sayer et al. 1984; Vivas et al. 1990; Ciura & Bourque, 2006), it seems unlikely that the cation channels transducing these effects are homomultimers of TRPV2 or TRPV4. Nonetheless, products of the Trpv2 or Trpv4 genes could still contribute to a heteromultimeric osmosensory transduction complex in OVLT neurones. Indeed, Trpv4−/− mice show impaired thirst and VP responses to systemic osmotic stimuli, and the expression of Fos protein that normally occurs in OVLT neurones under hypertonic conditions is significantly reduced in these animals (Liedtke & Friedman, 2003). Interestingly, recent studies have shown that mice lacking normal expression of the Trpv1 gene (i.e. Trpv1−/− mice) also display impaired thirst responses (Ciura & Bourque, 2006) and VP release (Sharif-Naeini et al. 2006) in response to hypertonic stimulation. Remarkably, electrophysiological analysis has shown that OVLT neurones obtained from Trpv1−/− mice lack the ability to respond to hypertonic stimuli in vitro (Ciura & Bourque, 2006). It is therefore likely that expression of the Trpv1 gene is required for the osmosensitivity of these cells, as was also shown for MNCs in the SON (Sharif-Naeini et al. 2006). In agreement with this hypothesis, the responses of wild-type (WT) OVLT neurones to hypertonic stimuli can be blocked by Ruthenium Red, a generic inhibitor of TRPV channels (Ramsey et al. 2006). The molecular composition of the osmosensory transducer expressed in OVLT neurones is therefore likely to include a product of the Trpv1 gene, and possibly other members of this family (e.g. products of Trpv4). Further studies are required to establish the molecular structure of the osmoreceptor and to establish the mechanisms by which osmotic stimuli regulate the opening probability of the transduction channel.
Neurones in OVLT modulate downstream effector neurones via glutamatergic synapses
Lesions of the OVLT have been shown to interfere with the osmotic modulation of most of the homeostatic responses illustrated in Fig. 1, and this area is now believed to be the brain’s primary osmostat (Johnson et al. 1992; Denton et al. 1996). However, it is still not known how neurones in this area mediate the co-ordinated control of all effector responses. The OVLT is a relatively heterogeneous structure that contains neurones expressing a variety of chemical neurotransmitters, including biogenic amines, amino acids and neuropeptides (Landas & Phillips, 1987). Moreover, tracing studies have shown that the OVLT sends efferent projections to a wide variety of hypothalamic and extrahypothalamic brain regions (e.g. Camacho & Phillips, 1981; Armstrong et al. 1996). A recent study combining in situ hybridization and immunohistochemical detection has revealed that the OVLT contains both GABAergic (i.e. inhibitory) and glutamatergic (i.e. excitatory) neurones (Grob et al. 2003). Surprisingly, direct evidence concerning the chemical identity of osmosensitive OVLT neurones has yet to be obtained. However, some insight into this question has been provided by anatomical and electrophysiological studies examining the functional connectivity between OVLT neurones and the MNCs in the SON. In agreement with electron microscopic analysis indicating that both GABAergic and glutamatergic OVLT neurones send monosynaptic projections to the SON (Armstrong et al. 1996), electrical stimulation of the OVLT in in vitro hypothalamic explants elicits overlapping inhibitory (IPSPs) and excitatory postsynaptic potentials (EPSPs) in MNCs of this nucleus (Yang et al. 1994). When the spontaneous electrical activity of neurones in the OVLT is depressed by the application of a local inhibitory stimulus (e.g. via local delivery of GABA onto the OVLT), the rates of spontaneous EPSPs and IPSPs detected in SON neurones are both depressed, confirming that both glutamatergic and GABAergic OVLT neurones can synaptically modulate the electrical activity of these cells (Richard & Bourque, 1995). However, when a hypertonic stimulus is applied to the OVLT, the rate of spontaneous IPSPs detected in MNCs is unaffected (Richard & Bourque, 1995), whereas that of spontaneous excitatory synaptic events is increased (Richard & Bourque, 1995; Trudel & Bourque, 2003). These observations suggest that the subset of osmosensitive OVLT neurones that project to the SON comprises exclusively glutamatergic neurones. Indeed, in hypothalamic explants, osmotically evoked changes in the rate of spontaneous EPSPs are positively correlated with the rate at which action potentials are fired by SON neurones, and the excitatory responses of SON neurones to hyperosmotic stimulation of the OVLT can be inhibited by pharmacological blockade of ionotropic glutamate receptors (Richard & Bourque, 1995).
These observations provide strong evidence indicating that osmostat signalling between the OVLT and effector (VP/OT) neurones in the SON is mediated in part by excitatory synapses. Specifically, glutamatergic neurones in the OVLT encode ECF osmolality via proportional changes in their rate of spike discharge, and this information is transmitted to MNCs in the form of a glutamate-dependent excitatory synaptic drive whose intensity varies in proportion with the firing rate of the OVLT neurone. Although the results imply that GABAergic OVLT neurones projecting to the SON are not osmosensitive, it must be cautioned that the studies cited were performed in hypothalamic explants (e.g. Richard & Bourque, 1995) or slices (e.g. Trudel & Bourque, 2003) in which the contribution of an osmosensitive GABAergic input might be impaired or absent. Thus the possibility that osmosensitive (or osmoresponsive) GABAergic neurones also participate in the osmotic control of SON neurones cannot be excluded. Indeed, previous studies have suggested that an active inhibitory process may be involved in the control of VP release under hypotonic conditions (e.g. Verbalis & Dohanics, 1991), and the osmotic control of firing rate in SON neurones in vivo appears to require a coactivation of excitatory and inhibitory inputs onto these neurones (Leng et al. 2001). The nature and origin of this putative osmotically modulated inhibitory input has yet to be identified. Whether the control of other osmoregulatory effector neurones relies on a direct excitatory modulation mediated by osmosensitive glutamatergic OVLT neurones or osmosensitive GABAergic OVLT neurones remains to be determined.
Acknowledgments
This work was supported by operating funds from the Canadian Institutes of Health Research (CIHR) and by a James McGill research Chair (JMRC) to C.W.B. Salary support to C.W.B. was also provided by a CIHR Senior Investigator Award and by the JMRC program. S.C. was recipient of a Doctoral Award from the Canada Graduate Scholarship Program, T.J.E.S. was recipient of a CIHR Doctoral Award, and E.T. and R.S.-N. were recipients of Doctoral Awards from the Heart and Stroke Foundation of Canada.
References
- Adachi A, Niijima A, Jacobs HL. An hepatic osmoreceptor mechanism in the rat: electrophysiological and behavioral studies. Am J Physiol. 1976;231:1043–1049. doi: 10.1152/ajplegacy.1976.231.4.1043. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Washburn DL, Ferguson AV. Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience. 2000;100:539–547. doi: 10.1016/s0306-4522(00)00313-4. [DOI] [PubMed] [Google Scholar]
- Andersen LJ, Andersen JL, Pump B, Bie P. Natriuresis induced by mild hypernatremia in humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1754–R1761. doi: 10.1152/ajpregu.00732.2001. [DOI] [PubMed] [Google Scholar]
- Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84:169–208. doi: 10.1152/physrev.00017.2003. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Tian M, Wong H. Electron microscopic analysis of synaptic inputs from the median preoptic nucleus and adjacent regions to the supraoptic nucleus in the rat. J Comp Neurol. 1996;373:228–239. doi: 10.1002/(SICI)1096-9861(19960916)373:2<228::AID-CNE6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bie P, Wamberg S, Kjolby M. Volume natriuresis vs. pressure natriuresis. Acta Physiol Scand. 2004;181:495–503. doi: 10.1111/j.1365-201X.2004.01323.x. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SH, Richard D. Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol. 1994;15:231–274. doi: 10.1006/frne.1994.1010. [DOI] [PubMed] [Google Scholar]
- Camacho A, Phillips MI. Horseradish peroxidase study in rat of the neural connections of the organum vasculosum of the lamina terminalis. Neurosci Lett. 1981;25:201–204. doi: 10.1016/0304-3940(81)90391-8. [DOI] [PubMed] [Google Scholar]
- Carithers J, Johnson AK. Long-term effects of lesions of the tissue surrounding the preoptic recess on paraventricular nuclei in rats. Brain Res. 1986;366:118–130. doi: 10.1016/0006-8993(86)91286-2. [DOI] [PubMed] [Google Scholar]
- Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D, Fluharty SJ. Salt appetite: a neurohormonal viewpoint. Physiol Behav. 2004;81:319–337. doi: 10.1016/j.physbeh.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Denton DA, McKinley MJ, Weisinger RS. Hypothalamic integration of body fluid regulation. Proc Natl Acad Sci U S A. 1996;93:7397–7404. doi: 10.1073/pnas.93.14.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona GF. Neurogenic regulation of renal tubular sodium reabsorption. Am J Physiol Renal Physiol. 1977;233:F73–F81. doi: 10.1152/ajprenal.1977.233.2.F73. [DOI] [PubMed] [Google Scholar]
- Egan G, Silk T, Zamarripa F, Williams J, Federico P, Cunnington R, Carabott L, Blair-West J, Shade R, McKinley M, Farrell M, Lancaster J, Jackson G, Fox P, Denton D. Neural correlates of the emergence of consciousness of thirst. Proc Natl Acad Sci U S A. 2003;100:15241–15246. doi: 10.1073/pnas.2136650100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R, Eyzaguirre C, Monti-Bloch L. Thermal and osmotic responses of arterial receptors. J Neurophysiol. 1979;42:665–680. doi: 10.1152/jn.1979.42.3.665. [DOI] [PubMed] [Google Scholar]
- Grob M, Trottier JF, Drolet G, Mouginot D. Characterization of the neurochemical content of neuronal populations of the lamina terminalis activated by acute hydromineral challenge. Neuroscience. 2003;122:247–257. doi: 10.1016/j.neuroscience.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Honda K, Negoro H, Dyball RE, Higuchi T, Takano S. The osmoreceptor complex in the rat: evidence for interactions between the supraoptic and other diencephalic nuclei. J Physiol. 1990;431:225–241. doi: 10.1113/jphysiol.1990.sp018328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Lee SL, Arnason SS, Sjoquist M. Dehydration natriuresis in male rats is mediated by oxytocin. Am J Physiol Regul Integr Comp Physiol. 1996;270:R427–R433. doi: 10.1152/ajpregu.1996.270.2.R427. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Desarmenien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62:113–134. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Izawa S, Inoue K, Adachi A, Funahashi M. Activity of neurons in the nucleus of the solitary tract of rats: effect of osmotic and mechanical stimuli. Neurosci Lett. 2000;288:33–36. doi: 10.1016/s0304-3940(00)01193-9. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Zardetto-Smith AM, Edwards GL. Integrative mechanisms and the maintenance of cardiovascular and body fluid homeostasis: the central processing of sensory input derived from the circumventricular organs of the lamina terminalis. Prog Brain Res. 1992;91:381–393. doi: 10.1016/s0079-6123(08)62357-2. [DOI] [PubMed] [Google Scholar]
- King MS, Baertschi AJ. Central neural pathway mediating splanchnic osmosensation. Brain Res. 1991;550:268–278. doi: 10.1016/0006-8993(91)91328-x. [DOI] [PubMed] [Google Scholar]
- Landas S, Phillips MI. Comparative anatomy of the organum vasculosum of the lamina terminalis. In: Gross PM, editor. Circumventricular Organs and Body Fluids. I. CRC Press, Inc; Boca Raton, FL, USA: 1987. pp. 131–155. [Google Scholar]
- Leng G, Brown CH, Bull PM, Brown D, Scullion S, Currie J, Blackburn-Munro RE, Feng J, Onaka T, Verbalis JG, Russell JA, Ludwig M. Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: an experimental and theoretical analysis. J Neurosci. 2001;21:6967–6977. doi: 10.1523/JNEUROSCI.21-17-06967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W. Transient receptor potential vanilloid channels functioning in transduction of osmotic stimuli. J Endocrinol. 2006;191:515–523. doi: 10.1677/joe.1.07000. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Denton DA, Oldfield BJ, De Oliveira LB, Mathai ML. Water intake and the neural correlates of the consciousness of thirst. Semin Nephrol. 2006;26:249–257. doi: 10.1016/j.semnephrol.2006.02.001. [DOI] [PubMed] [Google Scholar]
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med. 1995;333:1260–1266. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]
- Mason WT. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980;287:154–157. doi: 10.1038/287154a0. [DOI] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- Nissen R, Bourque CW, Renaud LP. Membrane properties of organum vasculosum lamina terminalis neurons recorded in vitro. Am J Physiol Regul Integr Comp Physiol. 1993;264:R811–R815. doi: 10.1152/ajpregu.1993.264.4.R811. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Bourque CW. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993;364:341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Richard D, Bourque CW. Synaptic control of rat supraoptic neurons during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J Physiol. 1995;489:567–577. doi: 10.1113/jphysiol.1995.sp021073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BW, Mishkin M. Alimentary responses to forebrain stimulation in monkeys. Exp Brain Res. 1968;4:330–366. doi: 10.1007/BF00235700. [DOI] [PubMed] [Google Scholar]
- Russell JA, Blackburn RE, Leng G. The role of the AV3V region in the control of magnocellular oxytocin neurons. Brain Res Bull. 1988;20:803–810. doi: 10.1016/0361-9230(88)90095-0. [DOI] [PubMed] [Google Scholar]
- Sayer RJ, Hubbard JI, Sirett NE. Rat organum vasculosum laminae terminalis in vitro: responses to transmitters. Am J Physiol Regul Integr Comp Physiol. 1984;247:R374–R379. doi: 10.1152/ajpregu.1984.247.2.R374. [DOI] [PubMed] [Google Scholar]
- Sharif-Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–98. doi: 10.1038/nn1614. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Trudel E, Bourque CW. A rat brain slice preserving synaptic connections between neurons of the suprachiasmatic nucleus, organum vasculosum lamina terminalis and supraoptic nucleus. J Neurosci Meth. 2003;128:67–77. doi: 10.1016/s0165-0270(03)00149-3. [DOI] [PubMed] [Google Scholar]
- Vallet PG, Baertschi AJ. Spinal afferents for peripheral osmoreceptors in the rat. Brain Res. 1982;239:271–274. doi: 10.1016/0006-8993(82)90850-2. [DOI] [PubMed] [Google Scholar]
- Verbalis JG. Control of brain volume during hypoosmolality and hyperosmolality. Adv Exp Med Biol. 2006;576:113–129. doi: 10.1007/0-387-30172-0_8. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Dohanics J. Vasopressin and oxytocin secretion in chronically hypoosmolar rats. Am J Physiol Regul Integr Comp Physiol. 1991;261:R1028–R1038. doi: 10.1152/ajpregu.1991.261.4.R1028. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Mangione MP, Stricker EM. Oxytocin produces natriuresis in rats at physiological plasma concentrations. Endocrinology. 1991;128:1317–1322. doi: 10.1210/endo-128-3-1317. [DOI] [PubMed] [Google Scholar]
- Vivas L, Chiaraviglio E, Carrer HF. Rat organum vasculosum laminae terminalis in vitro: responses to changes in sodium concentration. Brain Res. 1990;519:294–300. doi: 10.1016/0006-8993(90)90091-o. [DOI] [PubMed] [Google Scholar]
- Voisin DL, Bourque CW. Integration of sodium and osmosensory signals in vasopressin neurons. Trends Neurosci. 2002;25:199–205. doi: 10.1016/s0166-2236(02)02142-2. [DOI] [PubMed] [Google Scholar]
- Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol. 2003;148:1–80. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]
- Yang CR, Senatorov VV, Renaud LP. Organum vasculosum lamina terminalis-evoked postsynaptic responses in rat supraoptic neurones in vitro. J Physiol. 1994;477:59–74. doi: 10.1113/jphysiol.1994.sp020171. [DOI] [PMC free article] [PubMed] [Google Scholar]


