Abstract
Introduction
The delivery of nucleic acids such as DNA and short interfering RNA (siRNA) is promising for the treatment of many diseases, including cancer, by enabling novel biological mechanisms of action. Non-viral nanoparticles are a promising class of nucleic acid carriers that can be designed to be safer and more versatile than traditional viral vectors.
Areas covered
In this review, recent advances in the intracellular delivery of DNA and siRNA are described with a focus on non-viral nanoparticle-based delivery methods. Material properties that have enabled successful delivery are discussed as well as applications that have directly been applied to cancer therapy. Strategies to co-deliver different nucleic acids are highlighted, as are novel targets for nucleic acid co-delivery.
Expert opinion
The treatment of complex genetically-based diseases such as cancer can be enabled by safe and effective intracellular delivery of multiple nucleic acids. Non-viral nanoparticles can be fabricated to deliver multiple nucleic acids to the same cell simultaneously to prevent tumor cells from easily compensating for the knockdown or overexpression of one genetic target. The continued innovation of new therapeutic modalities and non-viral nanotechnologies to provide target-specific and personalized forms of gene therapy hold promise for genetic medicine to treat diseases like cancer in the clinic.
Keywords: cancer, DNA, gene delivery, gene therapy, nanoparticle, polymer, siRNA
1. Introduction
The discovery that exogenous DNA introduced into isolated nuclei can be transcribed into mRNA [1] and lead to protein expression [2, 3] created the promise of gene therapy as a modality capable of treating myriad diseases. siRNA is a more recently discovered therapeutic modality and can be used to knock down gene expression through the RNA interference (RNAi) pathway. RNAi was initially discovered in C. elegans as a gene silencing pathway used as a natural mechanism for viral defense [4]. A strand of long, double-stranded RNA (dsRNA) is cleaved by the Dicer protein into 21–25 bp siRNAs [5]. siRNA is then incorporated into the RNA-induced silencing complex (RISC) where the sense strand is removed. The antisense strand is then used as a template for complementary mRNA. mRNA that pairs with siRNA-RISC is cleaved, thus preventing translation and thereby gene expression. (For review, see Hannon [6].)
Early delivery methods of these nucleic acids often involved introducing nucleic acids by mechanical disruption of the cell membrane or direct injection [7, 8]. However, these methods are laborious and not clinically translatable. Viral methods of DNA and siRNA delivery are effective [9], yet often induce immunogenicity or tumorigenicity and are therefore limited for clinical translation [10]. Non-viral nucleic acid delivery has traditionally been considered less effective [11], but can be designed to avoid tumorigenesis and immune stimulation. Recent advances in nanoparticle vectors for nucleic acid delivery have continued to improve delivery efficacy while minimizing toxicity, but several obstacles remain that make successful delivery an ongoing challenge.
1.1 Obstacles to intracellular delivery
Due to their size and negative charge, nucleic acids cannot readily pass through the cell membrane to their intracellular sites of action (Fig. 1). Nanocarriers can encapsulate nucleic acids to not only to promote successful delivery into cells, but also to protect them from degradation by extracellular nucleases. Nanocarriers for nucleic acid delivery include liposomes that hold DNA and siRNA within their aqueous interiors [12–14], cationic polymers that bind anionic nucleic acids to form polyplexes [15, 16] and solid nanoparticles that can carry nucleic acids via covalent linkages [17]. For nanocarriers that electrostatically bind to nucleic acids, special considerations must be taken for short oligonucleotides like siRNA, which are much shorter and stiffer than plasmid DNA, and are therefore often harder to complex into nanoparticles [18, 19]. To prevent unwanted non-specific interactions between nanoparticles and biomolecules and cells, nanoparticles are frequently coated with hydrophilic polymers, such as polyethylene glycol (PEG) [20].
Figure 1.
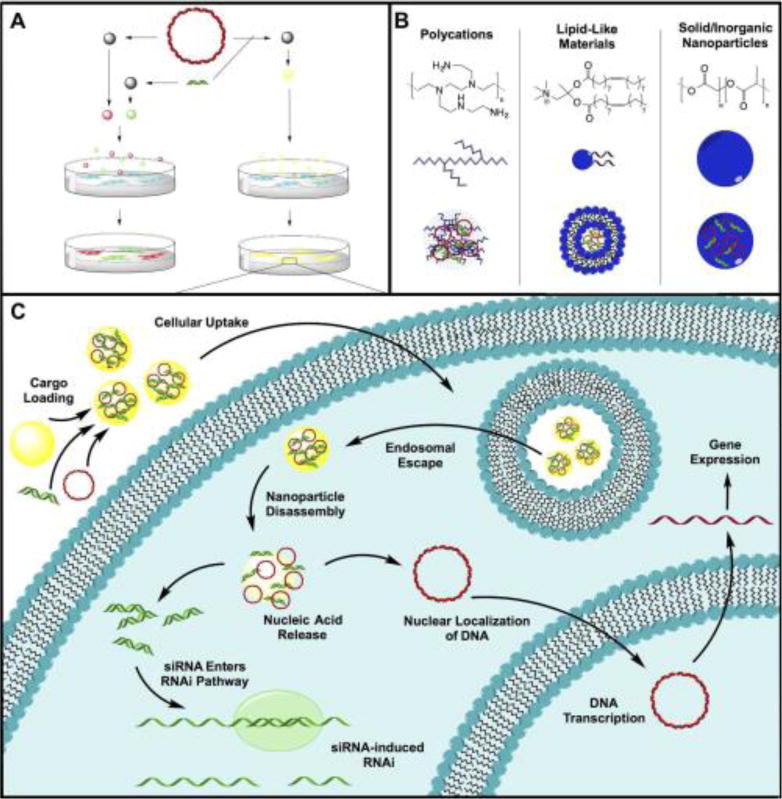
Overview of nanoparticle codelivery of DNA and siRNA. (A) Nanoparticles carrying DNA and siRNA cargoes separately yield little codelivery, while nanoparticles carrying both nucleic acids optimize coexpression. (B) There are multiple classes of non-viral nanoparticles for gene delivery. Each class with its own representative chemical structure (top), nanoparticle structure (center), and method of carrying nucleic acid (bottom). (C) There are multiple steps for successfulintracellular delivery of DNA and siRNA.
For intracellular delivery, cells must take up the nucleic acid carrying nanoparticles. To enable cellular uptake, cell-penetrating peptides can be used to promote internalization directly through the cell membrane [21–23], or cationic nanoparticles can nonspecifically interact with the negatively charged cell surface to promote endosomal uptake [24–27]. If internalized via endosomes, the nanoparticle must escape the endosome to prevent degradation in lysosomes, prevent recycling out of the cell, and to promote cytosolic delivery. This can be achieved with hydrophobic or amphiphilic biomaterials that can destabilize the endosomal membrane [28, 29]. Endosomal escape can also be achieved using the proton sponge mechanism, in which a nanomaterial may act as a buffer against endosomal acidification and eventually result in endosomal lysis. Although this mechanism has been challenged [30], it is the most widely accepted hypothesis to explain successful transfection when utilizing nanomaterials with titratable amines [31, 32].
For siRNA delivery, the nanocarrier must release its contents at the site of RNAi in the cytosol [33]. Several polymeric materials degrade hydrolytically and can thereby release siRNA as the polymer degrades [34, 35]. As the cytosol is approximately 1000 times more reducing than the extracellular space [36], nanomaterials may also employ bioreducible disulfide bonds to promote release targeted specifically to the cytosol. (For review, see Son et al.[37]) Nanocarriers delivering DNA may need to remain intact longer, as naked DNA is slow to diffuse in the cytosol and may be degraded by cytosolic nucleases on its way to the nucleus [38, 39]. Nuclear penetration is an additional major bottleneck to gene delivery. It has been shown that actively dividing cells are easier to transfect [40], and this can be an avenue to increase transfection in cancer cells compared to non-cancerous slower growing cells. In non-mitotic cells, attaching a nuclear localization signal peptide sequence to DNA is a strategy that improves nuclear penetration by using the cell’s own nuclear import machinery [41]. Complexing DNA within a polymeric nanocarrier has been shown to increase nuclear association and permeability [42]. For all of these steps, nanomaterial properties are key in order to achieve intracellular nanoparticle-based DNA and siRNA delivery (Table 1). Table 1 also illustrates the evolution of nanomaterials used for non-viral gene delivery from readily available off-the-shelf chemicals to custom biomaterials designed specifically for intracellular nucleic acid delivery.
Table 1.
Key nanomaterials listed in chronological order of their initial investigation to illustrate the evolution of nanomaterials used for non-viral gene delivery. Representative cancer types that have been investigated using each material for therapeutic gene delivery are also listed.
| Non-viral Vector | Key Characteristics | Early Investigation | Representative Cancer Types |
|---|---|---|---|
| Calcium phosphate | Co-precipitate nucleic acids with calcium phosphate to form nanocrystals | 1973[171] | Melanoma and breast [172] cancer ; nasopharyngeal carcinoma[173] |
| Liposomes | Encapsulate nucleic acid cargo in aqueous interior | 1980[174] | Colorectal cancer and breast cancer[175]; pancreatic islet cell tumors[176]; Lewis lung carcinoma[48] |
| PLL | Cationic polypeptide for nucleic acid binding | 1987[16] | Lung cancer[177]; bladder cancer[178] |
| Gold | Chemically inert, easily functionalized; can be used for theranostic purposes | 1990[179] | Breast cancer[180, 181] |
| Dendrimers | Highly branched polymers with greater shape control and end group density | 1993 (PAA)[182]; 1999 (PPI)[183] | Breast cancer[184]; ovarian cancer[185]; epidermoid carcinoma and glioblastoma[186] |
| PEI | Titratable amines facilitate endosomal escape | 1995[16, 99] | Neuroblatoma[149]; glioma and medulloblastoma[187]; glioma and hepatoma[150] |
| PLGA | Encapsulates nucleic acids through double emulsion process | 1997[182, 183, 188] | Lung cancer[92]; prostate cancer[184–186, 189] |
| Cyclodextrin | Water-soluble polysaccharides that can complex with nucleic acids | 1999[190] | Hepatoma[114]; leukemia[151]; breast and ovarian cancer[191] |
| Mesoporous silica | Solid material with porous structure allowing cargo adsorption on the outer surface and inside pores | 2000[83] | Lung cancer[89]; ovarian cancer[87]; breast cancer[192] |
| PBAE | Contains hydrolizable ester bonds for greater biocompatability | 2000[122] | Glioblastoma[125, 126, 170]; melanoma[129]; small cell lung cancer[135]; hepatoma[136]; prostate cancer[193] |
2. Nanoparticles for DNA and siRNA delivery
2.1 Liposomes and lipid-based materials
Lipid-based nanoparticles are the most commonly used non-viral chemical method of intracellular nucleic acid delivery. Several commercially available transfection reagents including Lipofectamine® 2000 [43], 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) [44], RNAifect [44], TransIT-TKO and TransIT-siQuest [45] are all lipid-based. For DNA delivery, the molecular structure of the cationic lipid is an important factor in transfection efficacy as it determines how the liposome interacts with the cell membrane [46, 47]. These liposomes can be modified by conjugating targeting ligands on the surface [48] or adding cholesterol to improve cell binding and uptake [49]. In liposomes delivering siRNA, cholesterol is often added to formulations to increase membrane fluidity and thereby increase cell membrane fusion and cellular uptake [50, 51]. Other lipid-based approaches utilize modified structures of individual lipids to make it energetically easier for the liposome to leave the lamellar phase and disrupt the endosome, thus releasing nucleic acid cargo into the cytoplasm and preventing lysosomal degradation [24, 52, 53]. Liposomally delivered siRNA cargo simultaneously escapes the endosome and is released from its carrier into the cytosol, its site of action. Lipid hydrocarbon tail properties such as chain length and saturation have been shown to play a role in cell membrane fusogenicity and can be optimized to promote cellular uptake [54].
Factors other than efficacy must be considered when designing nanoparticle systems. For example, DOTAP forms stable nanoparticles that protect DNA from degradation [55] but has been shown to promote strong interferon responses in mice [44]. Chono et al. modified DOTAP with hyaluronic acid and were able to demonstrate reduced immunotoxicity as measured by inflammatory cytokine expression [56]. Functionalizing liposomes with a hydrophilic polymer poly(ethylene glycol) (PEG) has also been shown to reduce immune stimulation [57]. Semple et al. were able to show successful intravenous administration of siRNA using PEGylated lipids in non-human primates [58]. Interestingly, some groups have been able to take advantage of the immunogenic properties of DOTAP. Ott et al. showed that DOTAP nanoparticles in a DNA vaccine formulation induced greater antibody response compared to naked DNA [59]. Thus, DOTAP could potentially be used as an adjuvant as well as a carrier in DNA vaccines.
2.2 Inorganic nanoparticles
Calcium phosphate (CaP) nanoparticles enable DNA delivery via co-precipitation of CaP and DNA into nanoscale crystals [60–62]. Sokolova et al. synthesized nanoparticles with a CaP core and alternating DNA and CaP shells that protected DNA from degradation and improved transfection [63]. Methods to optimize CaP for siRNA delivery have often employed polymers. Polymethacrylate-PEG (PMA-PEG) block copolymers were coated onto the surface of CaP/siRNA nanoparticles and assisted in endosomal escape [64]. To improve loading of siRNA into CaP, Zhang et al. covalently functionalized siRNA with PEG and then co-precipitated siRNA and CaP [65].
Gold nanoparticles are advantageous for several types of gene delivery because they are safe, easy to chemically functionalize, and have the potential for diagnostic as well as therapeutic use [66, 67]. The particle surface can be modified by cationic groups such as quaternary ammonium salts to increase DNA binding [68]. Alternatively, anti-sense DNA oligonucleotides have been covalently linked to the surface of nanoparticles to induce gene knockdown [69]. Spherical nucleic acids, oligonucleotides arranged in a dense, oriented, and spherical configuration, have shown promise for intracellular delivery in multiple applications and are often designed by conjugation to an inorganic nanoparticle core, such as thiolated nucleic acids conjugated to gold nanoparticles [70, 71]. The covalent linker can also be modified to allow greater control of DNA release. Han et al. used a photoactive o-nitrobenzyl ester linker to control the spatial and temporal release of DNA by applying a near-UV light [72]. Alternative approaches to deliver siRNA using inorganic nanoparticles include combination with polymers. siRNA can be non-covalently layered onto gold nanoparticles by alternating layers of siRNA with the cationic polymer poly(ethylene imine) (PEI) [73]. A combinatorial approach was designed by Lee et al. in which siRNA was covalently linked to gold nanoparticles via disulfide bonds and then electrostatically coated with PBAEs in order to promote cell uptake and endosomal buffering [74].
Quantum dots such as CdSe/ZnS nanoparticles can be used as fluorophores as well as nucleic acid delivery vehicles [75–77]. DNA can be covalently conjugated onto the quantum dot using a peptide nucleic acid linker [78], or through non-covalent association with cationic polymers that are capped on the quantum dot surface [79]. Methods for siRNA delivery commonly employ covalently linking siRNA to the quantum dot surface, often with a polymeric spacer [80, 81].
Mesoporous silicas are solid materials that have a honeycomb-like porous structure with empty channels (mesopores) that can encapsulate bioactive molecules.[82] This unique porous structure provides an inner and an outer surface onto which cargo can adsorb. Silica has been shown to have high affinity for the head groups of phospholipids that promotes its association with the cell membrane, enhancing cellular uptake through physical concentration of mesoporous silica nanoparticles (MSNs) on the cell surface [83, 84]. MSNs for DNA delivery require surface modification with cationic groups for DNA binding [85]. Similarly, MSNs have been coated with cationic polymers such as PEI [86] and PAA [87] to facilitate siRNA binding. In addition to nucleic acid binding on the outside, the internal surfaces of MSN mesopores have been used to encapsulate fluorescent dyes for intracellular tracking [88] or anticancer drugs for multimodal therapies [87]. Li et al. designed an MSN modified with PEI and the fusogenic peptide KALA encapsulating siRNA targeting vascular endothelial growth factor receptor [89]. When these particles were injected intratumorally into mice that had been subcutaneously inoculated with human lung cancer cells, they significantly inhibited tumor growth through the suppression of tumor neovascularization. These results demonstrate the potential of MSNs delivering nucleic acids as powerful anti-cancer therapies.
2.3 Polymeric nanoparticles
Poly(lactic-co-glycolic acid) (PLGA) nanoparticles are advantageous for several types of drug delivery due to their biodegradability and safety, and PLBA as a biomaterial has already been used in a number of FDA-approved devices. PLGA particles are typically synthesized via a simple emulsion-solvent evaporation process [90] and have a readily functionalizable surface chemistry that allows easy attachment of molecules to promote delivery functions like tissue homing and cellular uptake [91]. DNA can be encapsulated within PLGA particles through a double emulsion process or adsorbed onto the particle surface after surface treatment with bioadhesive agents such as Carbopol, a polyacrylic acid-based polymer [92]. Cationic polymers such as chitosan or spermidine can be blended into PLGA particles, the latter of which was used by Woodrow et al. for intravaginal siRNA delivery [93].
More commonly employed polymers for polymeric gene delivery nanoparticles are often cationic and electrostatically interact with nucleic acids to form polyplexes. Early gene delivery strategies often employed poly(L-lysine) (PLL) due to its cationic nature [15, 16]. PLL particles delivering DNA have been modified by adding PEG groups to prevent particle aggregation in serum [94] and Kim et al. created a terplex system with stearyl-PLL, low density lipoprotein, and DNA that increased particle compactness and improved DNA binding [95]. PEG-PLL block copolymers have also been used for siRNA delivery [96], but were often so stable that the siRNA cargo could not be released. Miyata et al. enabled PEG-PLL nanoparticles to undergo cytoplasmic siRNA release by crosslinking PLL end chains with bioreducible disulfides [97]. A similar delivery system was later modified with the RGD integrin recognition peptide to promote in vivo tissue targeting [98].
PLL nanoparticles often are unable to escape the endosome. This realization led to the exploration of other cationic polymers such as PEI, a polymer that contains primary, secondary, and tertiary amines to enable both nucleic acid binding and more efficient endosomal escape [99]. Godbey et al. used extensive confocal microscopy experiments to elucidate the intracellular fate of PEI-DNA nanoparticles. They found that PEI particles aggregate in discrete patches on the cell surface before being internalized through endocytic vesicles; some particles then escape through lysed endosomes and localize to the nucleus [100]. The polymer structure can be tuned to modulate gene delivery as low molecular weight PEI cannot condense DNA as well as its high molecular weight counterpart but is less toxic than higher molecular weight PEI [101]. Modifications with targeting ligands and PEGylation have also been shown to improve particle stability and in vivo transfection [102]. PEI analogs optimized for siRNA delivery frequently employ lower molecular weight linear PEIs linked with disulfide bonds to enable degradation and siRNA release, as PEI itself contains no biodegradable moieties [103].
Similar polymeric materials such as poly(amido amine)s (PAAs) and poly(amido ethyleneimine)s (PAEIs) have been designed that have buffering capacities in the endosomal pH range superior to PEI [104]. Partial degradation of PAA dendrimers by heat treatment increases the dendrimer flexibility and has been shown to lead to better transfection [105]. PEGylation further increases transfection efficacy and decreases toxicity [106]. PAA modifications for siRNA delivery employ disulfides linkages in the polymer backbone, and have shown superior siRNA delivery compared to PEI even in cells with comparable nanoparticle uptake [107–109]. This is likely due to the enhanced cytoplasmic siRNA release enabled by the inclusion of bioreducible disulfides. Modification of disulfide containing PAAs with PEG has shown a reduction in hemolysis and particle aggregation in vivo, but with reduced particle stability and decreases in gene knockdown [110].
Cyclodextrin-based nanoparticles are effective and have reached clinical trials. Cycldextrins are a class of water-soluble molecules of 6–9 glucose units that form a cone-shaped structure with a hydrophobic interior that can complex with various molecules, including nucleic acids [111]. Nanoparticles for DNA delivery can be made by conjugating cyclodextrin with polymers including PEI [112] and PAA [113], which condense DNA through electrostatic interactions. DNA can also be covalently linked to cyclodextrin via cationic adamantyl linkers [114]. For siRNA delivery, self-assembled nanoparticles can be made from cyclodextrin polymer, siRNA, and adamantane-PEG conjugates [115, 116]. These nanoparticles were used to deliver siRNA targeting the M2 subunit of ribonucleotide reductase in a Phase 1a/1b clinical trial which demonstrated siRNA activity in humans [116, 117].
Dendrimers are polymer structures that consist of a central core molecule from which highly branched arms extend out in an ordered and symmetric fashion. The stepwise method of dendrimer synthesis lends greater control of polymer size while the branching structure results in a higher density of terminal groups, offering unique surface characteristics and additional attachment sites for drugs or targeting moieties [118]. Two dendrimers that have been used for gene delivery are the PAAs mentioned above and polypropylenimine (PPI). PPI dendrimers with a butylenediamine (DAB) core have been shown to increase DNA binding with increasing dendrimer generations, with generation 2 providing the optimal balance between nucleic acid binding and toxicity [119]. Arginine has been conjugated to the terminal ends to increase membrane permeability and improve nuclear localization [120]. PPI nanoparticles for siRNA delivery have been modified with a disulfide crosslinking molecular cage on the surface to increase particle stability [121].
2.4 Poly(beta-amino ester) (PBAE) nanoparticles for DNA and siRNA delivery to cancer
PBAEs are a class of polymer that contains tertiary amines and ester bonds along the polymer backbone. These chemical moieties provide positive charge for nucleic acid binding, buffering to promote endosomal release, and hydrolytic degradability for cargo release [122]. PBAEs have been well studied for DNA delivery, and have been designed to deliver DNA more efficiently and with less cytotoxicity than commercially available reagents such as PEI and Lipofectamine® 2000 in several cell types [123, 124]. By changing the chemical properties of the PBAE, it is possible to design nanoparticles that selectively deliver DNA to certain cell types, while avoiding delivery to others [124]. PBAEs can also be designed to deliver DNA to cancer while avoiding healthy cells, thereby allowing for the delivery of cell-killing genes to tumor cells without off-target effects [125–127]. Additionally, PBAE-DNA nanoparticles can be fabricated, dried, and stored as a powder for at least two years at −20°C without losing function, highlighting their translational potential [125, 127].
Various modifications to the PBAE backbone have been made to allow controlled DNA release from PBAE nanoparticles. A light-responsive 2-nitrobenzene moiety was added to the PBAE backbone to allow quick and controlled DNA release upon UV radiation (Fig. 2) [128]. Gu et al. electrostatically linked pH-sensitive carboxymethyl poly(L-histidine) groups to PBAE-DNA nanoparticles to neutralize the particle’s positive surface charge and increase buffering capacity; this modification decreased erythrocyte agglutination and enhanced the particle’s tumor targeting capabilities after intravenous injection [129].
Figure 2.
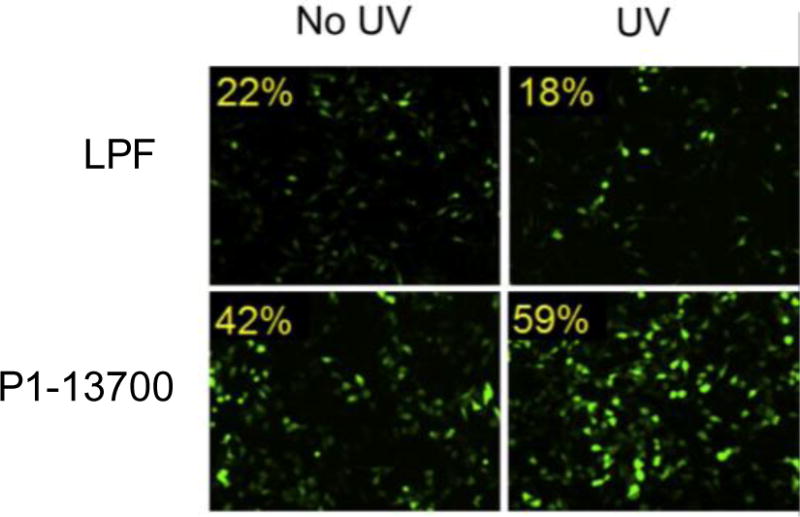
Light-responsive PBAE nanoparticles (P1-13700) delivering an EGFP plasmid to Hela cells with or without 2 minutes of UV irradiation; Lipofectamine™ 2000 (LPF) was used as a control. PBAE transfection efficacy increased with UV treatment, which broke 2-nitrobenzene linkers in the polymer backbone and allowed controlled DNA release. Reproduced with permission [128].
Due to the physical differences between DNA and siRNA described above, PBAE-based siRNA delivery had initially been difficult without the addition of other delivery vectors such as gold nanoparticles [74]. Hong et al. took the approach of modifying the siRNA itself. They designed complementary DNA/siRNA strands that self-assembled to form a dendrimeric siRNA structure; these siRNA dendrimers had higher charge density and structural flexibility, which allowed them to form stable particles with PBAE formulations that had been optimized for DNA delivery [130]. Tzeng et al. modified the polymer structure by end-capping traditional PBAEs with a disulfide-containing small molecule and showed successful siRNA delivery to both cancer cells and mesenchymal stem cells [131, 132]. The addition of the degradable disulfide moiety enabled this polymer structure to form polyplexes at a higher polymer:siRNA mass ratio (wt/wt) without causing significant toxicity, even though the disulfide bonds were only at the polymer end-caps. Building on this work, Kozielski et al. designed a novel disulfide containing monomer to form disulfide bonds within every repeat unit [133]. This monomer, 2,2′-disulfanediylbis(ethane-2,1-diyl) diacrylate, was referred to as “BR6” as it was the reducible form of a well-established PBAE monomer known as “B6,” hexane-1,6-diyl diacrylate [134]. PBAE nanoparticles made from BR6 were shown to bind siRNA with the same strength as particles made from its non-reducible analog but quickly released its siRNA cargo in a reducing environment, unlike the conventional non-bioreducible PBAEs. Furthermore, these particles achieved gene knockdown in vitro that was significantly higher than that achieved by Lipofectamine® 2000 (Fig. 3) and was shown to preferentially deliver siRNA to brain cancer cells while avoiding delivery to healthy brain cells.
Figure 3.
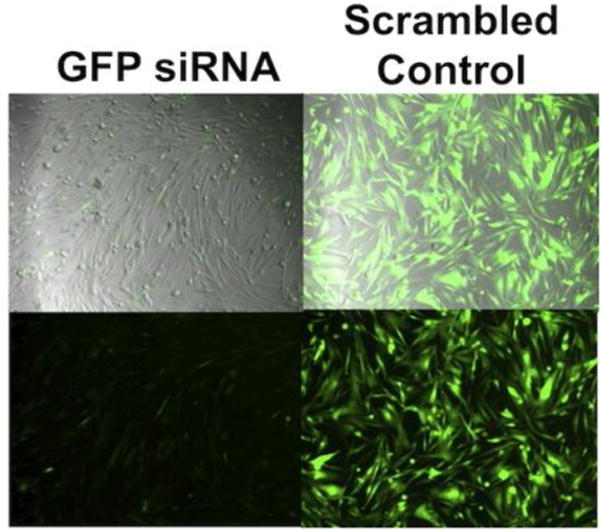
Phase contrast (top) and fluorescence (bottom) images of GFP+ GBM cells with bioreducible PBAE (R647) nanoparticles delivering either an siRNA targeting GFP (left) or a scrambled control RNA (right) [170].
The ability to effectively bind and deliver both DNA and siRNA make PBAEs an attractive option for gene delivery in cancer therapies. PBAE nanoparticles delivering the p53 tumor suppressor gene inhibited tumor growth in a small cell lung cancer mouse model after intratumoral injection [135]. A nanoparticle with a PBAE-DNA core and a pullulan-methotrexate shell showed enhanced circulation time and targeted delivery to hepatoma cells in vivo, with high levels of particle accumulation and transfection in the tumor [136]. For more controlled long-term release, Segovia et al. encapsulated PBAE-siRNA particles in a PAA-dextran aldehyde hydrogel; when implanted in a breast cancer model, they saw a high level of knockdown even after 7 days [137]. Our group is particularly interested in the use of PBAE nanoparticles for the treatment of glioblastoma. Glioblastoma (GBM), a grade IV glioma, is one of the most deadly human cancers with a median survival of only 15 months following treatments such as tumor resection, chemotherapy and radiotherapy [138–140]. Polymeric nanoparticles for the intracellular delivery of nucleic acids enable new modalities of treatment for many cancer cell types, including GBM. By tuning the PBAE polymer structure, we were able to form PBAE nanoparticles that preferentially delivered nucleic acids to brain tumor initiating cells, a cell population that is believed to be responsible for tumor recurrence [127]. High levels of transfection were seen when PBAE particles delivering a GFP reporter gene were injected into an orthotopic GBM murine model [125]. In addition, these particles were able to achieve a therapeutic effect. PBAE nanoparticles delivering DNA encoding the herpes simplex virus-derived thymidine kinase (HSVtk) were injected intracranially in a rat GBM model while the ganciclovir pro-drug was administered systemically [126]. The PBAE nanoparticles penetrated through the whole brain tumor volume (a length of approximately 2 mm) and HSVtk catalyzed the phosphorylation of ganciclovir into its active form to enable killing of brain cancer cells, resulting in significant survival benefits [126].
Further modifications of PBAEs such as synthesis of dendrimeric versions of the polymers are interesting future directions for enhanced nucleic acid delivery. Cutlar et al. synthesized a highly branched PBAE that showed higher transfection efficacy when compared to linear counterparts as they could better condense their DNA cargo [141]. Zhou et al. synthesized a dendrimeric ester nanoparticle that successfully delivered microRNAs to a liver cancer model and achieved significant survival benefits [142]. The authors hypothesized that the increased nucleic acid binding capacity and degradability of these polyester dendrimers contributed to successful RNA delivery while maintaining low hepatotoxicity. Indeed, dendrimeric PBAEs may produce smaller, more compact nanoparticles that contain more polymer end groups, which could increase biomaterial-mediated cell specificity. This may be especially relevant for cancer therapy, including brain cancer therapy, where smaller particle sizes can increase particle penetration and transport.
2.5 Methods for DNA and RNA co-delivery
Despite the physical differences between DNA and RNA that present different challenges for their intracellular delivery, several strategies have been developed for co-delivery in order to achieve novel therapeutic goals. In designing nanoparticle formulations, it is particularly important to also ensure that each of the different nucleic acids to be delivered reaches the target cells at the desired ratios. To enable co-delivery to the same cell, loading different nucleic acids into the same particles (rather than delivering a combination of particles, each with its own cargo) has been show to increase the co-expression of delivered nucleic (Fig. 4) [143]. As polyplexes are formed through self-assembly between cationic polyelectrolytes and anionic polyelectrolytes, with larger more multivalent polyelectrolytes leading to enhanced stability, carrier DNA can be complexed into the same polyplexes as siRNA as a strategy to stabilize the particle for enhanced siRNA delivery [144]. This can be an effective way to achieve gene knockdown and expression in the same cell to achieve synergistic therapeutic effects [145, 146]. Another way to complex multiple nucleic acids in the same particle is through layer-by-layer (LbL) assembly. Elbakry et al. used LbL to synthesize a particle with a gold core, 11-mercaptoundecanoic acid coating, and PEI-siRNA layers to condense siRNA into a particle and achieve effective knockdown [73]. Bishop et al. adopted a similar strategy but added DNA, siRNA, and PBAE layers [147]. This strategy can be used to deliver multiple nucleic acid cargos as well as control their relative release times.
Figure 4.
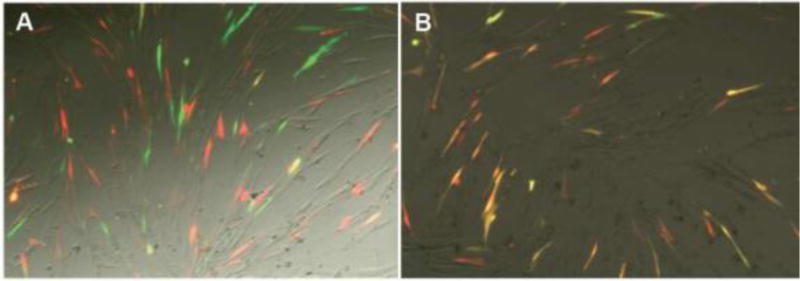
(A) Nanoparticles carrying either GFP or DsRed plasmid DNA are blended following nanoparticle fabrication, resulting in particles containing only one type of plasmid. Transfection of IMR90 human fibroblasts with this nanoparticle combination yields little codelivery, as indicated by few cells coexpressing GFP and DsRed (yellow cells). (B) Nanoparticles formed using a blend of GFP and DsRed plasmids yield particles containing both plasmids, and coexpression is high. Reproduced with permission [143].
3. Conclusion
Non-viral nanoparticle technologies for DNA and siRNA delivery have advanced rapidly, with many complementary biomaterial and particle designs. Several promising delivery platforms involving lipid-based, inorganic, and polymeric nanocarriers have been developed with strong in vivo efficacies, some of which have entered clinical trials. The interest in these technologies is due to the large potential for gene delivery and siRNA-induced gene knockdown to treat diseases caused by aberrant gene expression, such as cancer, and the need to obtain safe and effective delivery methods. Non-viral nanoparticles have the potential to fulfill this promise. Continuing to investigate the barriers to intracellular delivery as well as to innovate the nanotechnologies capable of overcoming these barriers may one day allow genetic medicine to clinically treat genetically based diseases such as cancer.
4. Expert Opinion
As polymeric nanoparticle-based gene therapy shows increasing promise against cancer in vitro and for local administration in vivo, increasing attention is being turned towards strategies to allow the systemic delivery of these particles to treat metastatic cancer. A common method employs PEGylation, which shields the particles from interacting with serum proteins or off-target cells. For example, PEGylation of PLL, PEI, and PAA-based nanoparticles has been shown to enhance their circulation time and reduce hemolysis and serum-induced aggregation [110, 148]. Such a strategy could greatly enhance the ability of newer types of non-viral nanoparticles, such as PBAE-based nanoparticles, to enable them to circulate effectively and diffuse through tissue, improving their translational potential for use in cancer applications.
PEG can also be used as a linking molecule onto which targeting ligands may be conjugated to enable nanoparticle targeting to cellular receptors. Ligands that have successfully been conjugated to PEGylated nanocarriers for cancer targeting include the RGD peptide sequence targeting integrins in tumor vasculature [149] as well as folate [150] and transferrin [151], molecules whose receptors are overexpressed in many cancer cell types. This strategy takes advantage of PEG’s ability to increase nanocarrier serum stability, reduce non-specific uptake, and better enable the display of targeting moieties on the nanoparticle surface, resulting in higher particle accumulation in the tumor. However, one potential concern with PEGylated electrostatic polyplex nanocarrier systems is that while the charge masking properties of PEG have been shown to increase nanoparticle colloidal stability in serum, they may also decrease particle complexation stability. Kichler et al. showed that in a PEI polymer covalently endcapped with high molecular weight PEG, the resulting nanoparticles could not protect their DNA cargo from nuclease degradation and resulted in poor transfection when compared to un-PEGylated PEI [152]. Similarly, Mao et al. showed that in PEI-PEG block copolymers, formulations with lower molecular weight PEG at higher substitution levels resulted in large, loosely structured particles that could not effectively condense siRNA and resulted in poor knockdown [153]. The charge shielding capability of PEG molecules protect cationic polymers from serum aggregation but also reduce their ability to electrostatically bind to nucleic acid cargos. To create a PEGylated polymer for successful nanoparticle formation, it is crucial to balance these opposing forces, such as through the addition of crosslinks or by adding non-PEGylated polymers to the co-complex to increase its stability.
Another strategy for systemic delivery is coating the particle with peptides to decrease toxicity, enhance circulation time, and enable particle targeting to specific organs or tumors [154]. Simberg et al. coated a peptide sequence to iron oxide particles that targeted clotted plasma proteins in leaky tumor vasculature [155]. These particles in turn induced more clots and amplified the effect. PBAE nanoparticles electrostatically coated with poly(glutamic acid) based peptide sequences reduced in vivo toxicity and could enable targeting to specific organs based on peptide sequence differences and nanoparticle properties [156]. Peptide coating can allow nanoparticles to remain in circulation for longer periods of time and can enhance particle targeting and uptake through ligand-mediated endocytosis. An important area for future research in the field is the investigation of new types of nanoparticle coatings that enable greater specific intracellular delivery to on-target cancer cells (perhaps in a manner specific to the receptors on a patient’s particular tumor), while preventing intracellular delivery to off-target cells. In addition, such next generation coating must enable prolonged circulation times, including resistance to clearance by neutralizing antibodies, even after multiple previous treatments of the next-generation nanoparticles.
The clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) system is a recently discovered genome editing tool with many potential therapeutic applications. The CRISPR/Cas9 system derives from a bacterial defense mechanism, where foreign DNA segments are transcribed into a dual-RNA complex and used to recognize and silence invading targets through double-stranded breaks (DSB) induced by the Cas9 endonuclease [157]. A single chimeric sgRNA was recently developed that can activate site-specific cleavage by Cas9 [158]. The resulting DSB can be repaired through error-prone non-homologous end joining, leading to indels that knock out gene function, or homology-directed repair upon introduction of a donor repair template [159]. The CRISPR/Cas9 system has been successfully used in human cells to introduce permanent changes in the genome [159, 160], making it a powerful tool for gene editing to treat diseases like cancer.
Only a handful of studies have been done using non-viral methods to deliver the CRISPR/Cas9 system. Jinek et al. constructed a plasmid encoding Cas9 and the sgRNA and delivered it using commercial transfection agents. However, they were only able to achieve editing efficiencies in the range of 6–8% [161]. Sun et al. constructed DNA nanoclews by packing sgRNA and the Cas9 protein into DNA strands that are partially complementary to the sgRNA and coating the outside with PEI (Fig. 5); the DNA nanoclew system achieved a 36% editing efficiency [162]. The low editing efficiency seen by many groups may be due to poor delivery efficacy of the Cas9 plasmid or sgRNA. Co-delivery of the two using non-viral nanoparticles, such as biodegradable polymeric nanoparticles, is a potential way to increase expression and gene editing efficacy. Strategies such as a layer-by-layer approach can be used to package the Cas9 plasmid and sgRNA using polymers that are suitable for each and to control their intracellular temporal release.
Figure 5.
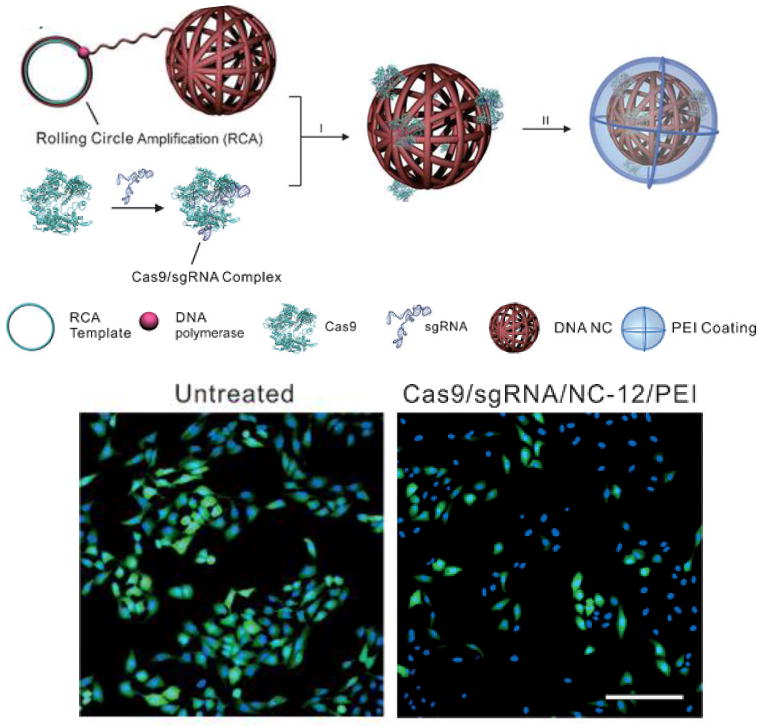
(Top) Assembly schematic of DNA nanoclew (DNA-NC) carrying Cas9 and sgRNA. Cas9 and sgRNA were incubated together and complexed with the DNA-NC; a PEI coating was then applied to the outside to facilitate endosomal escape. (Bottom) Fluorescence microscopy images of EGFP+ U2OS cells with or without treatment with DNA-NC delivering Cas9 and EGFP-specific sgRNA. Cas9-induced DNA cleavage resulted in significant EGFP gene knockout. Reproduced with permission [162].
Another interesting target for the co-delivery of DNA and siRNA is the TNF-related apoptosis-inducing ligand (TRAIL) system. TRAIL induces apoptosis in many transformed cell lines by binding to the death receptors DR4 and DR5 on the cell surface [163, 164]. Its apoptotic function is selective for transformed and tumor cells [165], and exhibits a bystander effect [166]. These properties make TRAIL an attractive delivery target for cancer treatment as it can produce a cancer-specific, self-amplifying apoptotic effect. However, it has been shown that many cancer cell types resist TRAIL action. One explanation for this phenomenon is the presence of decoy receptors DcR1 and DcR2, which lack functional intracellular death domains [167]. Studies have shown that DcR2 is upregulated in some TRAIL-resistant breast and prostate cancers [168, 169]. siRNA knockdown of DcR2 in these cells sensitized them to TRAIL-induced apoptosis. When TRAIL plasmids were delivered to the same cells, their tumorigenic potential was significantly reduced. This suggests that co-delivery of siRNA to knock down decoy receptors and DNA to upregulate TRAIL expression can work synergistically to cause cancer cell apoptosis and may be a promising target for polymeric nanoparticle delivery. Similarly, other siRNA and DNA co-delivery strategies may enable breakthroughs against cancer resistance and are enabled by non-viral nanoparticles.
Treatment of genetically-based diseases such as cancer often requires a combinatorial approach, as cells can often compensate for the knockdown or overexpression of one genetic target. For the proposed treatment strategies suggested herein, co-delivery of DNA and RNA is required to occur within the same cells, not simply within the bulk of a tissue or tumor. While the materials optimal for DNA and siRNA often vary, a treatment strategy requiring co-delivery would ideally require a material optimized to deliver both. As previously demonstrated [143], a blend of nanoparticles containing different cargos is less likely to co-deliver both cargos to the same cells. Conversely, particles containing the cargo blended within each nanoparticle results in high co-delivery rates. Temporal control of DNA and RNA release [147] is also imperative for systems which would require DNA transcription and siRNA-induced knockdown to occur in a non-simultaneous fashion. Future nanoparticle designs that would have the sophistication and control to combinatorially deliver multiple types of nucleic acids against multiple targets have the potential to address the heterogeneity and mutational capabilities of genetic diseases such as cancer.
highlights.
Obstacles to intracellular nucleic acid delivery include rapid clearance from circulation, tissue and tumor targeting, cellular internalization, endosomal escape, and intracellular release.
Lipid-based and inorganic materials protect nucleic acids from degradation and condense them into nanoparticles for improved cellular uptake.
Cationic polymers self-assemble into polyplexes with nucleic acids via electrostatic interactions and possess functional groups to aid in improved cellular uptake, endosomal escape through endosomal buffering, and intracellular cargo release via biodegradable linkages.
Nanoparticle formulations optimized for the co-delivery of multiple DNA or siRNA cargos can be used to reach novel synergistic cancer therapy targets.
Therapeutic modalities such as DNA, siRNA, and CRISPR/Cas technology may benefit from non-viral nanoparticle delivery platforms for the treatment of complex genetically-based diseases.
References
- 1.Manley JL, Sharp PA, Gefter ML. RNA synthesis in isolated nuclei: in vitro initiation of adenovirus 2 major late mRNA precursor. Proceedings of the National Academy of Sciences. 1979;76:160–4. doi: 10.1073/pnas.76.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manthorpe M, Cornefert-Jensen F, Hartikka J, et al. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Human gene therapy. 1993;4:419–31. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- 3.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene therapy. 1999;6:1258–66. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 4•.Fire A, Xu SQ, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. This seminal paper describes the RNAi pathway in C. elegans and led to the development of siRNA as a gene silencing modality. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein E, Caudy AA, Hammond SM, et al. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 6.Hannon GJ. RNA interference. nature. 2002;418:244–51. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 7.Hickman MA, Malone RW, Lehmann-Bruinsma K, et al. Gene expression following direct injection of DNA into liver. Human gene therapy. 1994;5:1477–83. doi: 10.1089/hum.1994.5.12-1477. [DOI] [PubMed] [Google Scholar]
- 8.Clemens JC, Worby CA, Simonson-Leff N, et al. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6499–503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenfeld MA, Siegfried W, Yoshimura K, et al. Adenovirus-Mediated Transfer of a Recombinant α1-Antitrypsin Gene to the Lung Epithelium in Vivo. Science. 1991;252:431–4. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 10••.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nature Reviews Genetics. 2003;4:346–58. doi: 10.1038/nrg1066. This article reviews the limitations and safety concerns associated with viral vectors for gene delivery. [DOI] [PubMed] [Google Scholar]
- 11.Pack DW, Hoffman AS, Pun S, et al. Design and development of polymers for gene delivery. Nature Reviews Drug Discovery. 2005;4:581–93. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 12.Alving CR, Steck EA, Chapman WL, Jr, et al. Therapy of leishmaniasis: superior efficacies of liposome-encapsulated drugs. Proceedings of the National Academy of Sciences. 1978;75:2959–63. doi: 10.1073/pnas.75.6.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vadiei K, Lopez-Berestein G, Perez-Soler R, et al. In vitro evaluation of liposomal cyclosporine. International Journal of Pharmaceutics. 1989;57:133–8. [Google Scholar]
- 14.Scherphof GL, Dijkstra J, Spanjer HH, et al. Uptake and intracellular processing of targeted and nontargeted liposomes by rat Kupffer cells in vivo and in vitro. Ann N Y Acad Sci. 1985;446:368–84. doi: 10.1111/j.1749-6632.1985.tb18414.x. [DOI] [PubMed] [Google Scholar]
- 15.Wagner E, Plank C, Zatloukal K, et al. Influenza-Virus Hemagglutinin-Ha-2 N-Terminal Fusogenic Peptides Augment Gene-Transfer by Transferrin Polylysine DNA Complexes - toward a Synthetic Virus-Like Gene-Transfer Vehicle. Proceedings of the National Academy of Sciences. 1992;89:7934–8. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Wu GY, Wu CH. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. Journal of Biological Chemistry. 1987;262:4429–32. This paper first introduced PLL as a potential nucleic acid delivery material. [PubMed] [Google Scholar]
- 17.Giljohann DA, Seferos DS, Prigodich AE, et al. Gene regulation with polyvalent siRNA-nanoparticle conjugates. Journal of the American Chemical Society. 2009;131:2072–3. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagerman PJ. Flexibility of RNA. Annu Rev Bioph Biom. 1997;26:139–56. doi: 10.1146/annurev.biophys.26.1.139. [DOI] [PubMed] [Google Scholar]
- 19.Kebbekus P, Draper DE, Hagerman P. Persistence length of RNA. Biochemistry-Us. 1995;34:4354–7. doi: 10.1021/bi00013a026. [DOI] [PubMed] [Google Scholar]
- 20.Ogris M, Brunner S, Schuller S, et al. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Therapy. 1999;6:595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 21.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–93. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 22.Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–88. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 23.Wyman TB, Nicol F, Zelphati O, et al. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry-Us. 1997;36:3008–17. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 24.Hafez I, Maurer N, Cullis P. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Therapy. 2001;8:1188–96. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Szoka FC., Jr Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry-Us. 1996;35:5616–23. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 26.Zelphati O, Szoka FC., Jr Mechanism of oligonucleotide release from cationic liposomes. Proceedings of the National Academy of Sciences. 1996;93:11493–8. doi: 10.1073/pnas.93.21.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma A, Stellacci F. Effect of Surface Properties on Nanoparticle-Cell Interactions. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 28.Felgner JH, Kumar R, Sridhar CN, et al. Enhanced Gene Delivery and Mechanism Studies with a Novel Series of Cationic Lipid Formulations. Journal of Biological Chemistry. 1994;269:2550–61. [PubMed] [Google Scholar]
- 29.El Ouahabi A, Thiry M, Pector V, et al. The role of endosome destabilizing activity in the gene transfer process mediated by cationic lipids. Febs Lett. 1997;414:187–92. doi: 10.1016/s0014-5793(97)00973-3. [DOI] [PubMed] [Google Scholar]
- 30.Benjaminsen RV, Mattebjerg MA, Henriksen JR, et al. The Possible “Proton Sponge” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:149–57. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonawane ND, Szoka FC, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. Journal of Biological Chemistry. 2003;278:44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 32.Nel AE, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki H, Taira K. Short hairpin type of dsRNAs that are controlled by tRNAVal promoter significantly induce RNAi-mediated gene silencing in the cytoplasm of human cells. Nucleic acids research. 2003;31:700–7. doi: 10.1093/nar/gkg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynn DM, Langer R. Degradable poly (beta-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. Journal of the American Chemical Society. 2000;122:10761–8. [Google Scholar]
- 35.Woodrow KA, Cu Y, Booth CJ, et al. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–33. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radical Biology and Medicine. 1999;27:922–35. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 37.Son S, Namgung R, Kim J, et al. Bioreducible Polymers for Gene Silencing and Delivery. Accounts of Chemical Research. 2012;45 doi: 10.1021/ar200248u. [DOI] [PubMed] [Google Scholar]
- 38.Lukacs GL, Haggie P, Seksek O, et al. Size-dependent DNA mobility in cytoplasm and nucleus. Journal of Biological Chemistry. 2000;275:1625–9. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- 39.Lechardeur D, Sohn KJ, Haardt M, et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene therapy. 1999;6:482–97. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 40.Brunner S, Sauer T, Carotta S, et al. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene therapy. 2000;7:401–7. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- 41.Zanta MA, Belguise-Valladier P, Behr JP. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proceedings of the National Academy of Sciences. 1999;96:91–6. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grandinetti G, Reineke TM. Exploring the Mechanism of Plasmid DNA Nuclear Internalization with Polymer-Based Vehicles. Molecular Pharmaceutics. 2012;9:2256–67. doi: 10.1021/mp300142d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalby B, Cates S, Harris A, et al. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Ma Z, Li J, He FT, et al. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochemical and Biophysical Research Communications. 2005;330:755–9. doi: 10.1016/j.bbrc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 45.Palliser D, Chowdhury D, Wang QY, et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 46.Smisterová J, Wagenaar A, Stuart MCA, et al. Molecular shape of the cationic lipid controls the structure of cationic lipid/dioleylphosphatidylethanolamine-DNA complexes and the efficiency of gene delivery. Journal of Biological Chemistry. 2001;276:47615–22. doi: 10.1074/jbc.M106199200. [DOI] [PubMed] [Google Scholar]
- 47.Koynova R, Wang L, Tarahovsky Y, et al. Lipid phase control of DNA delivery. Bioconjugate chemistry. 2005;16:1335–9. doi: 10.1021/bc050226x. [DOI] [PubMed] [Google Scholar]
- 48.Torchilin VP, Levchenko TS, Rammohan R, et al. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome–DNA complexes. Proceedings of the National Academy of Sciences. 2003;100:1972–7. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crook K, Stevenson BJ, Dubouchet M, et al. Inclusion of cholesterol in DOTAP transfection complexes increases the delivery of DNA to cells in vitro in the presence of serum. Gene therapy. 1998;5:137–43. doi: 10.1038/sj.gt.3300554. [DOI] [PubMed] [Google Scholar]
- 50.Lu JJ, Langer R, Chen JZ. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Molecular Pharmaceutics. 2009;6:763–71. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Umeda M, Nojima S, Inoue K. Effect of lipid composition on HVJ-mediated fusion of glycophorin liposomes to erythrocytes. J Biochem-Tokyo. 1985;97:1301–10. doi: 10.1093/oxfordjournals.jbchem.a135181. [DOI] [PubMed] [Google Scholar]
- 52.Hafez IM, Cullis PR. Roles of lipid polymorphism in intracellular delivery. Adv Drug Deliver Rev. 2001;47:139–48. doi: 10.1016/s0169-409x(01)00103-x. [DOI] [PubMed] [Google Scholar]
- 53.Litzinger DC, Huang L. Phosphatidylethanolamine Liposomes - Drug Delivery, Gene-Transfer and Immunodiagnostic Applications. Biochim Biophys Acta. 1992;1113:201–27. doi: 10.1016/0304-4157(92)90039-d. [DOI] [PubMed] [Google Scholar]
- 54.Heyes J, Palmer L, Bremner K, et al. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. Journal of Controlled Release. 2005;107:276–87. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Moret I, Esteban Peris J, Guillem VM, et al. Stability of PEI–DNA and DOTAP–DNA complexes: effect of alkaline pH, heparin and serum. Journal of Controlled Release. 2001;76:169–81. doi: 10.1016/s0168-3659(01)00415-1. [DOI] [PubMed] [Google Scholar]
- 56.Chono S, Li SD, Conwell CC, et al. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. Journal of Controlled Release. 2008;131:64–9. doi: 10.1016/j.jconrel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Judge AD, Bola G, Lee ACH, et al. Design of Noninflammatory Synthetic siRNA Mediating Potent Gene Silencing in Vivo. Mol Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Semple SC, Akinc A, Chen J, et al. Rational design of cationic lipids for siRNA delivery. Nature Biotechnology. 2010;28:172–6. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 59.Ott G, Singh M, Kazzaz J, et al. A cationic sub-micron emulsion (MF59/DOTAP) is an effective delivery system for DNA vaccines. Journal of Controlled Release. 2002;79:1–5. doi: 10.1016/s0168-3659(01)00545-4. [DOI] [PubMed] [Google Scholar]
- 60.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Molecular and cellular biology. 1987;7:2745–52. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic acids research. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Tolou H. Administration of oligonucleotides to cultured cells by calcium phosphate precipitation method. Analytical biochemistry. 1993;215:156–8. doi: 10.1006/abio.1993.1568. This paper introduced the use of co-precipitation of nucleic acids with calcium phosphote crystals as a method for DNA delivery. [DOI] [PubMed] [Google Scholar]
- 63.Sokolova VV, Radtke I, Heumann R, et al. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials. 2006;27:3147–53. doi: 10.1016/j.biomaterials.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 64.Kakizawa Y, Furukawa S, Ishii A, et al. Organic-inorganic hybrid-nanocarrier of siRNA constructing through the self-assembly of calcium phosphate and PEG-based block aniomer. Journal of Controlled Release. 2006;111:368–70. doi: 10.1016/j.jconrel.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Zhang M, Ishii A, Nishiyama N, et al. PEGylated Calcium Phosphate Nanocomposites as Smart Environment-Sensitive Carriers for siRNA Delivery. Advanced Materials. 2009;21:3520–5. [Google Scholar]
- 66.Ghosh PS, Kim CK, Han G, et al. Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. 2008 doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connor EE, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–7. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 68.Sandhu KK, McIntosh CM, Simard JM, et al. Gold Nanoparticle-Mediated Transfection of Mammalian Cells. Bioconjugate Chemistry. 2002;13:3–6. doi: 10.1021/bc015545c. [DOI] [PubMed] [Google Scholar]
- 69.Rosi NL, Giljohann DA, Thaxton CS, et al. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science. 2006;312:1027–30. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 70.Cutler JI, Auyeung E, Mirkin CA. Spherical nucleic acids. Journal of the American Chemical Society. 2012;134:1376–91. doi: 10.1021/ja209351u. [DOI] [PubMed] [Google Scholar]
- 71.
- 72.Han G, You CC, Kim Bj, et al. Light-Regulated Release of DNA and Its Delivery to Nuclei by Means of Photolabile Gold Nanoparticles. Angewandte Chemie. 2006;118:3237–41. doi: 10.1002/anie.200600214. [DOI] [PubMed] [Google Scholar]
- 73.Elbakry A, Zaky A, Liebl R, et al. Layer-by-Layer Assembled Gold Nanoparticles for siRNA Delivery. Nano Letters. 2009;9:2059–64. doi: 10.1021/nl9003865. [DOI] [PubMed] [Google Scholar]
- 74•.Lee JS, Green JJ, Love KT, et al. Gold, poly (beta-amino ester) nanoparticles for small interfering RNA delivery. Nano letters. 2009;9:2402–6. doi: 10.1021/nl9009793. This paper describes a hybrid system utilizing PBAE coating of gold nanoparticles to deliver siRNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Derfus AM, Chan WCW, Bhatia SN. Intracellular delivery of quantum dots for live cell labeling and organelle tracking. Advanced Materials. 2004;16:961–6. [Google Scholar]
- 76.Gao X, Cui Y, Levenson RM, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology. 2004;22:969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 77.Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nature Biotechnology. 2002;21:41–6. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 78.Srinivasan C, Lee J, Papadimitrakopoulos F, et al. Labeling and Intracellular Tracking of Functionally Active Plasmid DNA with Semiconductor Quantum Dots. Mol Ther. 2006;14:192–201. doi: 10.1016/j.ymthe.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 79.Zhang P, Liu W. ZnO QD@PMAA-co-PDMAEMA nonviral vector for plasmid DNA delivery and bioimaging. Biomaterials. 2010;31:3087–94. doi: 10.1016/j.biomaterials.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 80.Derfus AM, Chen AA, Min DH, et al. Targeted quantum dot conjugates for siRNA delivery. Bioconjugate Chemistry. 2007;18:1391–6. doi: 10.1021/bc060367e. [DOI] [PubMed] [Google Scholar]
- 81.Chen AA, Derfus AM, Khetani SR, et al. Quantum dots to monitor RNAi delivery and improve gene silencing. Nucleic acids research. 2005;33:e190–e. doi: 10.1093/nar/gni188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slowing II, Vivero-Escoto JL, Wu CW, et al. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliver Rev. 2008;60:1278–88. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 83.Luo D, Saltzman WM. Enhancement of transfection by physical concentration of DNA at the cell surface. Nat Biotech. 2000;18:893–5. doi: 10.1038/78523. [DOI] [PubMed] [Google Scholar]
- 84.Mornet S, Lambert O, Duguet E, et al. The Formation of Supported Lipid Bilayers on Silica Nanoparticles Revealed by Cryoelectron Microscopy. Nano Letters. 2005;5:281–5. doi: 10.1021/nl048153y. [DOI] [PubMed] [Google Scholar]
- 85.Bharali DJ, Klejbor I, Stachowiak EK, et al. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11539–44. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia T, Kovochich M, Liong M, et al. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of siRNA and DNA Constructs. ACS Nano. 2009;3:3273–86. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen AM, Zhang M, Wei D, et al. Co-delivery of Doxorubicin and Bcl-2 siRNA by Mesoporous Silica Nanoparticles Enhances the Efficacy of Chemotherapy in Multidrug-Resistant Cancer Cells. Small. 2009;5:2673–7. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roy I, Ohulchanskyy TY, Bharali DJ, et al. Optical tracking of organically modified silica nanoparticles as DNA carriers: A nonviral, nanomedicine approach for gene delivery. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:279–84. doi: 10.1073/pnas.0408039101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X, Chen Y, Wang M, et al. A mesoporous silica nanoparticle – PEI – Fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials. 2013;34:1391–401. doi: 10.1016/j.biomaterials.2012.10.072. [DOI] [PubMed] [Google Scholar]
- 90•.Tinsley-Bown AM, Fretwell R, Dowsett AB, et al. Formulation of poly(d,l-lactic-co-glycolic acid) microparticles for rapid plasmid DNA delivery. Journal of Controlled Release. 2000;66:229–41. doi: 10.1016/s0168-3659(99)00275-8. This paper describes the double emulsion process commonly used to encapsulate nucleic acid cargo in PLGA particles. [DOI] [PubMed] [Google Scholar]
- 91.Zhou J, Patel TR, Fu M, et al. Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials. 2012;33:583–91. doi: 10.1016/j.biomaterials.2011.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zou W, Liu C, Chen Z, et al. Studies on bioadhesive PLGA nanoparticles: A promising gene delivery system for efficient gene therapy to lung cancer. International Journal of Pharmaceutics. 2009;370:187–95. doi: 10.1016/j.ijpharm.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 93.Woodrow KA, Cu Y, Booth CJ, et al. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–33. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ziady AG, Gedeon CR, Miller T, et al. Transfection of Airway Epithelium by Stable PEGylated Poly-L-lysine DNA Nanoparticles in Vivo. Mol Ther. 2003;8:936–47. doi: 10.1016/j.ymthe.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 95.Kim JS, Kim BI, Maruyama A, et al. A new non-viral DNA delivery vector: the terplex system. Journal of Controlled Release. 1998;53:175–82. doi: 10.1016/s0168-3659(97)00251-4. [DOI] [PubMed] [Google Scholar]
- 96.Patil ML, Zhang M, Minko T. Multifunctional Triblock Nanocarrier (PAMAM-PEG-PLL) for the Efficient Intracellular siRNA Delivery and Gene Silencing. ACS Nano. 2011;5:1877–87. doi: 10.1021/nn102711d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miyata K, Kakizawa Y, Nishiyama N, et al. Block catiomer polyplexes with regulated densities of charge and disulfide cross-linking directed to enhance gene expression. Journal of the American Chemical Society. 2004;126:2355–61. doi: 10.1021/ja0379666. [DOI] [PubMed] [Google Scholar]
- 98.Christie RJ, Matsumoto Y, Miyata K, et al. Targeted Polymeric Micelles for siRNA Treatment of Experimental Cancer by Intravenous Injection. ACS Nano. 2012;6:5174–89. doi: 10.1021/nn300942b. [DOI] [PubMed] [Google Scholar]
- 99••.Boussif O, Lezoualc’h F, Zanta MA, et al. A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in vivo: Polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7297–301. doi: 10.1073/pnas.92.16.7297. This was the first paper to demonstrate the versatility and efficacy of PEI as a gene delivery vector. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100•.Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proceedings of the National Academy of Sciences. 1999;96:5177–81. doi: 10.1073/pnas.96.9.5177. This paper provides a detailed illustration of intracellular PEI nanoparticle fate after cellular uptake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kunath K, von Harpe A, Fischer D, et al. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. Journal of Controlled Release. 2003;89:113–25. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 102.Kleemann E, Neu M, Jekel N, et al. Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG–PEI. Journal of Controlled Release. 2005;109:299–316. doi: 10.1016/j.jconrel.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 103.Breunig M, Hozsa C, Lungwitz U, et al. Mechanistic investigation of poly (ethylene imine)-based siRNA delivery: disulfide bonds boost intracellular release of the cargo. Journal of Controlled Release. 2008;130:57–63. doi: 10.1016/j.jconrel.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 104.Christensen LV, Chang CW, Kim WJ, et al. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjugate Chemistry. 2006;17:1233–40. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 105.Tang MX, Redemann CT, Szoka FC. In Vitro Gene Delivery by Degraded Polyamidoamine Dendrimers. Bioconjugate Chemistry. 1996;7:703–14. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 106.Luo D, Haverstick K, Belcheva N, et al. Poly(ethylene glycol)-Conjugated PAMAM Dendrimer for Biocompatible, High-Efficiency DNA Delivery. Macromolecules. 2002;35:3456–62. [Google Scholar]
- 107.Jeong JH, Christensen LV, Yockman JW, et al. Reducible poly(amido ethylenimine) directed to enhance RNA interference. Biomaterials. 2007;28:1912–7. doi: 10.1016/j.biomaterials.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 108.Vader P, van der Aa LJ, Engbersen JFJ, et al. Disulfide-Based Poly(amido amine)s for siRNA Delivery: Effects of Structure on siRNA Complexation, Cellular Uptake, Gene Silencing and Toxicity. Pharmaceutical Research. 2011;28:1013–22. doi: 10.1007/s11095-010-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van der Aa LJ, Vader P, Storm G, et al. Optimization of poly(amido amine)s as vectors for siRNA delivery. Journal of Controlled Release. 2011;150:177–86. doi: 10.1016/j.jconrel.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 110.Vader P, van der Aa LJ, Engbersen JFJ, et al. Physicochemical and Biological Evaluation of siRNA Polyplexes Based on PEGylated Poly (amido amine) s. Pharmaceutical Research. 2012;29:352–61. doi: 10.1007/s11095-011-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111••.Li S, Purdy WC. Cyclodextrins and their applications in analytical chemistry. Chemical Reviews. 1992;92:1457–70. This paper describes the structure and functionality of cyclodextrins as a nanocarrier for cargo such as nucleic acids. [Google Scholar]
- 112.Yang C, Li H, Goh SH, et al. Cationic star polymers consisting of α-cyclodextrin core and oligoethylenimine arms as nonviral gene delivery vectors. Biomaterials. 2007;28:3245–54. doi: 10.1016/j.biomaterials.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 113.Arima H, Kihara F, Hirayama F, et al. Enhancement of Gene Expression by Polyamidoamine Dendrimer Conjugates with α-, β-, and γ-Cyclodextrins. Bioconjugate Chemistry. 2001;12:476–84. doi: 10.1021/bc000111n. [DOI] [PubMed] [Google Scholar]
- 114.Burckbuchler V, Wintgens V, Leborgne C, et al. Development and Characterization of New Cyclodextrin Polymer-Based DNA Delivery Systems. Bioconjugate Chemistry. 2008;19:2311–20. doi: 10.1021/bc800070f. [DOI] [PubMed] [Google Scholar]
- 115.Davis ME. The First Targeted Delivery of siRNA in Humans via a Nanoparticle. From Concept to Clinic. 2009;6:659–68. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 116••.Davis ME, Zuckerman JE, Choi CHJ, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70. doi: 10.1038/nature08956. This article demonstrates evidence of siRNA efficacy in humans for the first time in a clinical trial with cyclodextrin nanocarriers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117••.Zuckerman JE, Gritli I, Tolcher A, et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proceedings of the National Academy of Sciences. 2014;111 doi: 10.1073/pnas.1411393111. This article describes the results of the first clinical trial using a synthetic vector (cyclodextrin) to systemically deliver siRNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dufès C, Uchegbu IF, Schätzlein AG. Dendrimers in gene delivery. Adv Drug Deliver Rev. 2005;57:2177–202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 119.Zinselmeyer BH, Mackay SP, Schatzlein AG, et al. The Lower-Generation Polypropylenimine Dendrimers Are Effective Gene-Transfer Agents. Pharmaceutical Research. 19:960–7. doi: 10.1023/a:1016458104359. [DOI] [PubMed] [Google Scholar]
- 120.Kim T-i, Baek J-u, Zhe Bai C, et al. Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier. Biomaterials. 2007;28:2061–7. doi: 10.1016/j.biomaterials.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 121.Taratula O, Garbuzenko OB, Kirkpatrick P, et al. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. Journal of Controlled Release. 2009;140:284–93. doi: 10.1016/j.jconrel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122••.Lynn DM, Langer R. Degradable Poly(β-amino esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. Journal of the American Chemical Society. 2000;122:10761–8. This paper introduced PBAEs as a cationic polymer capable of forming nanoparticles with nucleic acids. [Google Scholar]
- 123.Tzeng SY, Higgins LJ, Pomper MG, et al. Student award winner in the Ph.D. category for the 2013 society for biomaterials annual meeting and exposition, april 10–13, 2013, Boston, Massachusetts: biomaterial-mediated cancer-specific DNA delivery to liver cell cultures using synthetic poly(beta-amino ester)s. Journal of biomedical materials research Part A. 2013;101:1837–45. doi: 10.1002/jbm.a.34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shmueli RB, Sunshine JC, Xu Z, et al. Gene delivery nanoparticles specific for human microvasculature and macrovasculature. Nanomedicine. 2012;8:1200–7. doi: 10.1016/j.nano.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guerrero-Cázares H, Tzeng SY, Young NP, et al. Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma in Vitro and in Vivo. ACS Nano. 2014;8:5141–53. doi: 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126••.Mangraviti A, Tzeng SY, Kozielski KL, et al. Polymeric Nanoparticles for Nonviral Gene Therapy Extend Brain Tumor Survival in Vivo. ACS Nano. 2015;9:1236–49. doi: 10.1021/nn504905q. This paper demonstrates that intratumoral injection of PBAE-DNA nanoparticles leads to nanoparticle transport through tumor tissue and a significant increase in survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tzeng SY, Guerrero-Cázares H, Martinez EE, et al. Non-viral gene delivery nanoparticles based on poly(β-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32:5402–10. doi: 10.1016/j.biomaterials.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deng X, Zheng N, Song Z, et al. Trigger-responsive, fast-degradable poly(β-amino ester)s for enhanced DNA unpackaging and reduced toxicity. Biomaterials. 2014;35:5006–15. doi: 10.1016/j.biomaterials.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gu J, Wang X, Jiang X, et al. Self-assembled carboxymethyl poly (l-histidine) coated poly (β-amino ester)/DNA complexes for gene transfection. Biomaterials. 2012;33:644–58. doi: 10.1016/j.biomaterials.2011.09.076. [DOI] [PubMed] [Google Scholar]
- 130.Hong CA, Eltoukhy AA, Lee H, et al. Dendrimeric siRNA for efficient gene silencing. Angewandte Chemie. 2015;127:6844–8. doi: 10.1002/anie.201412493. [DOI] [PubMed] [Google Scholar]
- 131.Tzeng SY, Green JJ. Subtle changes to polymer structure and degradation mechanism enable highly effective nanoparticles for siRNA and DNA delivery to human brain cancer. Advanced Healthcare Materials. 2013;2:468–80. doi: 10.1002/adhm.201200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132••.Tzeng SY, Hung BP, Grayson WL, et al. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials. 2012;33:8142–51. doi: 10.1016/j.biomaterials.2012.07.036. This paper demonstrates that the addition of disulfide-containing molecules within a PBAE polymer can improve siRNA delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kozielski KL, Tzeng SY, Green JJ. A bioreducible linear poly(beta-amino ester) for siRNA delivery. Chemical Communications. 2013;49:5319–21. doi: 10.1039/c3cc40718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen J, Qiu X, Ouyang J, et al. pH and reduction dual-sensitive copolymeric micelles for intracellular doxorubicin delivery. Biomacromolecules. 2011;12:3601–11. doi: 10.1021/bm200804j. [DOI] [PubMed] [Google Scholar]
- 135.Kamat CD, Shmueli RB, Connis N, et al. Poly(β-amino ester) Nanoparticle Delivery of TP53 Has Activity against Small Cell Lung Cancer In Vitro and In Vivo. Molecular Cancer Therapeutics. 2013;12:405–15. doi: 10.1158/1535-7163.MCT-12-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu Y, Wang Y, Zhang C, et al. Core–Shell Nanoparticles Based on Pullulan and Poly(β-amino) Ester for Hepatoma-Targeted Codelivery of Gene and Chemotherapy Agent. ACS Applied Materials & Interfaces. 2014;6:18712–20. doi: 10.1021/am504203x. [DOI] [PubMed] [Google Scholar]
- 137.Segovia N, Pont M, Oliva N, et al. Hydrogel doped with nanoparticles for local sustained release of siRNA in breast cancer. Advanced healthcare materials. 2015;4:271–80. doi: 10.1002/adhm.201400235. [DOI] [PubMed] [Google Scholar]
- 138.Chaichana KL, Zadnik P, Weingart JD, et al. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2013;118:812–20. doi: 10.3171/2012.9.JNS1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. Journal of Neurosurgery. 2009;110:156–62. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 140.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New England Journal of Medicine. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 141.Cutlar L, Zhou D, Gao Y, et al. Highly Branched Poly (β-Amino Esters): Synthesis and Application in Gene Delivery. Biomacromolecules. 2015;16:2609–17. doi: 10.1021/acs.biomac.5b00966. [DOI] [PubMed] [Google Scholar]
- 142.Zhou K, Nguyen LH, Miller JB, et al. Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proceedings of the National Academy of Sciences. 2016;113:520–5. doi: 10.1073/pnas.1520756113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bhise NS, Shmueli RB, Gonzalez J, et al. A novel assay for quantifying the number of plasmids encapsulated by polymer nanoparticles. Small. 2012;8:367–73. doi: 10.1002/smll.201101718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li SD, Huang L. Targeted Delivery of Antisense Oligodeoxynucleotide and Small Interference RNA into Lung Cancer Cells. Molecular Pharmaceutics. 2006;3:579–88. doi: 10.1021/mp060039w. [DOI] [PubMed] [Google Scholar]
- 145.Chang Kang H, Bae YH. Co-delivery of small interfering RNA and plasmid DNA using a polymeric vector incorporating endosomolytic oligomeric sulfonamide. Biomaterials. 2011;32:4914–24. doi: 10.1016/j.biomaterials.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen Y, Zhu X, Zhang X, et al. Nanoparticles Modified With Tumor-targeting scFv Deliver siRNA and miRNA for Cancer Therapy. Mol Ther. 2010;18:1650–6. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bishop CJ, Tzeng SY, Green JJ. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomaterialia. 2015;11:393–403. doi: 10.1016/j.actbio.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Akinc A, Thomas M, Klibanov AM, et al. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. The journal of gene medicine. 2005;7:657–63. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 149.Schiffelers RM, Ansari A, Xu J, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic acids research. 2004;32:e149–e. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liang B, He ML, Xiao ZP, et al. Synthesis and characterization of folate-PEG-grafted-hyperbranched-PEI for tumor-targeted gene delivery. Biochemical and Biophysical Research Communications. 2008;367:874–80. doi: 10.1016/j.bbrc.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 151.Bellocq NC, Pun SH, Jensen GS, et al. Transferrin-Containing, Cyclodextrin Polymer-Based Particles for Tumor-Targeted Gene Delivery. Bioconjugate Chemistry. 2003;14:1122–32. doi: 10.1021/bc034125f. [DOI] [PubMed] [Google Scholar]
- 152.Kichler A, Chillon M, Leborgne C, et al. Intranasal gene delivery with a polyethylenimine–PEG conjugate. Journal of Controlled Release. 2002;81:379–88. doi: 10.1016/s0168-3659(02)00080-9. [DOI] [PubMed] [Google Scholar]
- 153.Mao S, Neu M, Germershaus O, et al. Influence of Polyethylene Glycol Chain Length on the Physicochemical and Biological Properties of Poly(ethylene imine)-graft-Poly(ethylene glycol) Block Copolymer/SiRNA Polyplexes. Bioconjugate Chemistry. 2006;17:1209–18. doi: 10.1021/bc060129j. [DOI] [PubMed] [Google Scholar]
- 154.Xie J, Chen K, Lee HY, et al. Ultrasmall c(RGDyK)-Coated Fe3O4 Nanoparticles and Their Specific Targeting to Integrin αvβ3-Rich Tumor Cells. Journal of the American Chemical Society. 2008;130:7542–3. doi: 10.1021/ja802003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Simberg D, Duza T, Park JH, et al. Biomimetic amplification of nanoparticle homing to tumors. Proceedings of the National Academy of Sciences. 2007;104:932–6. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Harris TJ, Green JJ, Fung PW, et al. Tissue-specific gene delivery via nanoparticle coating. Biomaterials. 2010;31:998–1006. doi: 10.1016/j.biomaterials.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Deltcheva E, Chylinski K, Sharma CM, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–7. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158••.Jinek M, Chylinski K, Fonfara I, et al. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science. 2012;337:816–21. doi: 10.1126/science.1225829. This paper reported the invention of the single guide RNA for the CRISPR/Cas system that can induce Cas9 cleavage, simplifying the system and allowing for easier application of CRISPR/Cas as a gene editing tool. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159•.Mali P, Yang L, Esvelt KM, et al. RNA-Guided Human Genome Engineering via Cas9. Science. 2013;339:823–6. doi: 10.1126/science.1232033. This paper demonstrates the versatility of the CRISPR/Cas9 system in gene editing in various cell lines using the newly-devloped single guide RNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cong L, Ran FA, Cox D, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science. 2013;339:819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jinek M, East A, Cheng A, et al. RNA-programmed genome editing in human cells. eLife. 2013;2 doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun W, Ji W, Hall JM, et al. Self-Assembled DNA Nanoclews for the Efficient Delivery of CRISPR–Cas9 for Genome Editing. Angewandte Chemie. 2015;127:12197–201. doi: 10.1002/anie.201506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 164.Pan G, O’Rourke K, Chinnaiyan AM, et al. The Receptor for the Cytotoxic Ligand TRAIL. Science. 1997;276:111–3. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 165.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. The Journal of Clinical Investigation. 104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kagawa S, He C, Gu J, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer research. 2001;61:3330–8. [PubMed] [Google Scholar]
- 167.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Current Opinion in Cell Biology. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 168.Sanlioglu AD, Dirice E, Aydin C, et al. Surface TRAIL decoy receptor-4 expression is correlated with TRAIL resistance in MCF7 breast cancer cells. BMC Cancer. 2005;5:1–17. doi: 10.1186/1471-2407-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sanlioglu AD, Karacay B, Koksal IT, et al. DcR2 (TRAIL-R4) siRNA and adenovirus delivery of TRAIL (Ad5hTRAIL) break down in vitro tumorigenic potential of prostate carcinoma cells. Cancer Gene Ther. 2007;14:976–84. doi: 10.1038/sj.cgt.7701087. [DOI] [PubMed] [Google Scholar]
- 170.Kozielski KL, Tzeng SY, Mendoza BAHd, et al. Bioreducible Cationic Polymer-Based Nanoparticles For Efficient and Environmentally Triggered Cytoplasmic siRNA Delivery to Primary Human Brain Cancer Cells. ACS Nano. 2014;8:3232–41. doi: 10.1021/nn500704t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–67. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 172.Kester M, Heakal Y, Fox T, et al. Calcium Phosphate Nanocomposite Particles for In Vitro Imaging and Encapsulated Chemotherapeutic Drug Delivery to Cancer Cells. Nano Letters. 2008;8:4116–21. doi: 10.1021/nl802098g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu T, Tang A, Zhang G, et al. Calcium phosphate nanoparticles as a novel nonviral vector for efficient transfection of DNA in cancer gene therapy. Cancer biotherapy & radiopharmaceuticals. 2005;20:141–9. doi: 10.1089/cbr.2005.20.141. [DOI] [PubMed] [Google Scholar]
- 174.Fraley R, Subramani S, Berg P, et al. Introduction of liposome-encapsulated SV40 DNA into cells. Journal of Biological Chemistry. 1980;255:10431–5. [PubMed] [Google Scholar]
- 175.Campbell RB, Fukumura D, Brown EB, et al. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer research. 2002;62:6831–6. [PubMed] [Google Scholar]
- 176.Thurston G, McLean JW, Rizen M, et al. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. Journal of clinical investigation. 1998;101:1401. doi: 10.1172/JCI965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cristiano RJ, Roth JA. Epidermal growth factor mediated DNA delivery into lung cancer cells via the epidermal growth factor receptor. Cancer gene therapy. 1995;3:4–10. [PubMed] [Google Scholar]
- 178.Tanaka M, Fraizer GC, De La Cerda J, et al. Connexin 26 enhances the bystander effect in HSVtk/GCV gene therapy for human bladder cancer by adenovirus/PLL/DNA gene delivery. Gene therapy. 2001;8:139–48. doi: 10.1038/sj.gt.3301367. [DOI] [PubMed] [Google Scholar]
- 179.Yang NS, Burkholder J, Roberts B, et al. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proceedings of the National Academy of Sciences. 1990;87:9568–72. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Visaria RK, Griffin RJ, Williams BW, et al. Enhancement of tumor thermal therapy using gold nanoparticle–assisted tumor necrosis factor-α delivery. Molecular Cancer Therapeutics. 2006;5:1014–20. doi: 10.1158/1535-7163.MCT-05-0381. [DOI] [PubMed] [Google Scholar]
- 181.Wang F, Wang YC, Dou S, et al. Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells. ACS Nano. 2011;5:3679–92. doi: 10.1021/nn200007z. [DOI] [PubMed] [Google Scholar]
- 182.Haensler J, Szoka FC. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chemistry. 1993;4:372–9. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 183.Loup C, Zanta MA, Caminade AM, et al. Preparation of Water-Soluble Cationic Phosphorus-Containing Dendrimers as DNA Transfecting Agents. Chemistry–A European Journal. 1999;5:3644–50. [Google Scholar]
- 184.Wang P, Zhao XH, Wang ZY, et al. Generation 4 polyamidoamine dendrimers is a novel candidate of nano-carrier for gene delivery agents in breast cancer treatment. Cancer Letters. 2010;298:34–49. doi: 10.1016/j.canlet.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 185.Patil ML, Zhang M, Taratula O, et al. Internally Cationic Polyamidoamine PAMAM-OH Dendrimers for siRNA Delivery: Effect of the Degree of Quaternization and Cancer Targeting. Biomacromolecules. 2009;10:258–66. doi: 10.1021/bm8009973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Koppu S, Oh YJ, Edrada-Ebel R, et al. Tumor regression after systemic administration of a novel tumor-targeted gene delivery system carrying a therapeutic plasmid DNA. Journal of Controlled Release. 2010;143:215–21. doi: 10.1016/j.jconrel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 187.Veiseh O, Kievit FM, Gunn JW, et al. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials. 2009;30:649–57. doi: 10.1016/j.biomaterials.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jones DH, Corris S, McDonald S, et al. Poly(dl-lactide-co-glycolide)-encapsulated plasmid DNA elicits systemic and mucosal antibody responses to encoded protein after oral administration. Vaccine. 1997;15:814–7. doi: 10.1016/s0264-410x(96)00266-6. [DOI] [PubMed] [Google Scholar]
- 189.Chumakova OV, Liopo AV, Andreev VG, et al. Composition of PLGA and PEI/DNA nanoparticles improves ultrasound-mediated gene delivery in solid tumors in vivo. Cancer Letters. 2008;261:215–25. doi: 10.1016/j.canlet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 190.Gonzalez H, Hwang SJ, Davis ME. New Class of Polymers for the Delivery of Macromolecular Therapeutics. Bioconjugate Chemistry. 1999;10:1068–74. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 191.Huang H, Yu H, Tang G, et al. Low molecular weight polyethylenimine cross-linked by 2-hydroxypropyl-γ-cyclodextrin coupled to peptide targeting HER2 as a gene delivery vector. Biomaterials. 2010;31:1830–8. doi: 10.1016/j.biomaterials.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 192.Meng H, Mai WX, Zhang H, et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS nano. 2013;7:994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anderson DG, Peng W, Akinc A, et al. A polymer library approach to suicide gene therapy for cancer. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16028–33. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]


