Abstract
Background
Lymphocytic Esophagitis (LyE) is a recently described clinicopathological condition, but little is known about its features and clinical associations.
Aim
To characterize patients with LyE, compare them to non-LyE controls, and identify risk factors.
Methods
We conducted a retrospective study of all patients ≥18 years old who underwent upper endoscopy with esophageal biopsy between January 1, 2000 and June 1, 2012. Archived pathology slides were re-reviewed and LyE was diagnosed if there was lymphocyte-predominant esophageal inflammation with no eosinophils or granulocytes. Three non-LyE controls groups were also defined: reflux, eosinophilic esophagitis (EoE), and normal. Clinical data were extracted from electronic medical records, and LyE cases were compared to non-LyE controls.
Results
27 adults were diagnosed with LyE, and the majority were female (63%). The most common symptom was dysphagia (70%). 52% had a prior or current diagnosis of reflux. Endoscopic findings included strictures (37%), erosive esophagitis (33%), rings (26%), and hiatal hernia (26%); 33% of patients required dilation. After histology re-review, 78% of LyE patients were found to have more than 20 lymphs/hpf. In comparison to the normal, reflux and EoE controls, patients with LyE tended to be non-white (p<0.01), were more commonly tobacco users (p=0.02), and less likely to have seasonal allergies (p=0.02).
Conclusion
LyE commonly presents with dysphagia due to esophageal strictures which require dilation. Smoking was associated with LyE whereas atopy was not. LyE should be considered as a diagnostic possibility in patients with these characteristics undergoing upper endoscopy.
Keywords: Lymphocyte, dysphagia, heartburn, chest pain, endoscopy
Introduction
Lymphocytic Esophagitis (LyE) is a recently described histopathological condition first defined by Rubio et al. in 2006 as a histologic subset of chronic esophagitis characterized by >20 intraepithelial lymphocytes (IELs) per high-power field (HPF) with no more than rare granulocytes [1]. Subsequently, as recognition of this histopathologic condition has become more widespread, there has also been some controversy related to LyE as studies have both questioned prior findings and attempted to better characterize this new entity [2–8].
No definite clinical associations have been detected in adults, though both gastroesophageal reflux disease (GERD) and Crohn’s disease have been linked to LyE [9,10]. Because of the relative rarity of LyE, consensus regarding the defining features and clinical associations still does not exist and great variation has been present in sample sizes and control groups. We have clinically encountered patients with LyE and found diagnosis and management to be a challenge due to lack of data.
Therefore, the aim of our study was to characterize adult patients with LyE. We sought to compare patients with LyE to non-LyE controls (GERD, eosinophilic esophagitis (EoE), and normal on esophageal biopsy) and to identify risk factors for the condition and endoscopic findings.
Methods
We performed a retrospective study of all patients ≥18 years old who had undergone upper endoscopy (EGD) with esophageal biopsy at the University of North Carolina at Chapel Hill between January 1, 2000, and June 1, 2012. In order to identify a cohort of patients who could have LyE, all pathology reports from this time frame were searched for terms referencing increased lymphocytes or a lymphocyte-predominant infiltrate as these were broad terms that could capture LyE diagnoses, even if the LyE itself was not recognized at the time of the prior endoscopy. The archived pathology slides were acquired for these cases and were independently re-reviewed by a study pathologist who was blinded to the original diagnoses. As there are no published diagnostic guidelines and we wanted to be broad in identifying potential cases, LyE was diagnosed if there were ≥10 lymphocytes/hpf (hpf=0.24mm2) in the esophageal epithelium and no eosinophils or granulocytes.
Three non-LyE control groups were also defined by reviewing the above pathology database and selecting the first 20 patients with biopsy findings consistent with the following diagnoses: 1) patients with GERD (defined by clinical symptoms and a mixed inflammatory pattern on biopsy); 2) patients with eosinophilic esophagitis (EoE) (defined by consensus guidelines); [11] and 3) patients with a normal esophageal biopsy. Patients with “normal” esophageal biopsy are believed to be reflective of our general population at UNC undergoing endoscopic evaluation. Patients with vasculitis, lichen planus, leukemia, infectious esophagitis (cytomegalovirus, herpes, candida), graft vs host disease, or multiple myeloma were excluded [11,12].
Data including patient demographics, co-morbidities, tobacco or alcohol use, medications, endoscopic findings, treatment, and outcomes were independently extracted for all four study groups from electronic medical records, pathology reports, and endoscopic databases at UNC, by two separate reviewers. The reviewers then compared findings and re-reviewed data jointly to reach consensus on any points of discrepancy. If there was still disagreement, the data were adjudicated by the senior author.
Descriptive statistics was performed to summarize characteristics of patients with LyE. One way analysis of variance and chi squared testing were done to compare patients with LyE to controls, GERD patients, and EoE patients using Stata 13. IRB approval was obtained at the University of North Carolina prior to initiation of the study.
Results
A total of 27 patients with LyE were identified, with the first diagnosis made in 2004. The average age was 56 years, most patients were female (63%) and white (59%) (Table 1). The most common symptoms at presentation were dysphagia (70%), heartburn (26%), chest pain (19%), nausea/vomiting (19%), and abdominal pain (15%). About half of patients had a prior or current history of alcohol and tobacco use, and the most commonly used medications were proton-pump inhibitors (59%) and non-steroidal anti-inflammatory drugs (64%).
Table 1.
Baseline Characteristics of LyE Patients (n = 27)
| Age, yrs (mean ± SD) | 56 ± 16 | |
| Male, n (%) | 10 (37) | |
| Caucasian, n (%) | 16 (59) | |
| BMI, kg/m2 (mean ± SD) | 27 ± 9 | |
| Year of initial diagnosis, n (%) | ||
| 2004 | 1 (4) | |
| 2005 | 2 (7) | |
| 2007 | 1 (4) | |
| 2008 | 3 (11) | |
| 2009 | 5 (18) | |
| 2010 | 5 (18) | |
| 2011 | 8 (30) | |
| 2012 | 2 (7) | |
| Symptoms, n (%) | ||
| Dysphagia | 19 (70) | |
| Asymptomatic | 3 (11) | |
| Heartburn | 7 (26) | |
| Abdominal Pain | 4 (15) | |
| Chest Pain | 5 (19) | |
| Nausea/vomiting | 5 (19) | |
| Odynophagia | 1 (4) | |
| Pertinent Medical History, n (%) | ||
| Hx of Crohn’s disease | 1 (4) | |
| Hx of Ulcerative colitis | 0 | |
| Hx of or active GERD | 14 (52) | |
| Hx of BE | 0 | |
| Hx of EoE | 1 (4) | |
| Hx of Achalasia | 1 (4) | |
| Hx of Drug medication allergies | 10 (37) | |
| Hx of Food allergies | 1 (4) | |
| Hx of Seasonal allergies | 1 (4) | |
| Hx of Asthma | 5 (19) | |
| Hx of Eczema | 1 (4) | |
| Hx of IBS | 3 (11) | |
| Hx of cancer | 4 (14) | |
| Habits, n (%) | ||
| Alcohol Use (prior or current) | 10 (37) | |
| Tobacco Use (prior or current) | 13 (48) | |
| Pertinent Medication History, n (%) | ||
| PPI use, n (%) | 15 (59) | |
| NSAID use (n=11), n (%) | 7 (64) | |
For concomitant conditions, one patient (4%) had been previously diagnosed with inflammatory bowel disease and 14 (52%) had a current or prior diagnosis of GERD (Table 1). Atopic conditions were not common in this group with 1 patient (4%) with a history of food allergy, 1 (4%) with seasonal allergies, 5 (19%) with asthma, and 1 (4%) with eczema.
The vast majority of patients had an abnormal upper endoscopy (82%) at diagnosis (Table 2). Endoscopic findings included a narrow caliber esophagus (44%), esophageal stricture (37%), erosive esophagitis as defined endoscopically (33%), esophageal rings (26%), erythema (26%), and hiatal hernia (26%) (Figure 1). Esophageal dilation was performed in 9 patients (33%). Of the patients who had strictures, 30% were pan-esophageal (representing diffuse esophageal narrowing), 30% were proximal, and 40% were distal.
Table 2.
Endoscopic findings, Lymphocytic count and Treatment
| n (%) | ||
|---|---|---|
| Endoscopic Findings | ||
| Abnormal | 23 (82) | |
| Narrow Caliber | 12 (44) | |
| Esophageal Stricture | 10 (37) | |
| Esophageal Rings | 7 (26) | |
| Erythema | 7 (26) | |
| Hiatal Hernia | 7 (26) | |
| Erosive Esophagitis | 9 (33) | |
| Mucosal Pallor | 3 (11) | |
| Abnormal Vascular Pattern | 2 (7) | |
| Desquamation | 2 (7) | |
| Ulcer | 1 (4) | |
| Candidiasis | 4 (15) | |
| Lymphocyte Count (n =25) | ||
| 10–20 lymph/hpf | 4 (15) | |
| 21–40 lymph/hpf | 14 (52) | |
| 41–80 lymph/hpf | 1 (4) | |
| >80 lymph/hpf | 6 (22) | |
| Medication Added or Changed After Endoscopy | ||
| PPI daily | 6 (22) | |
| GI cocktail (Maalox, viscous lidocaine, and donnatal) | 1 (4) | |
| Fluticasone | 1(4) | |
| Prednisone Taper | 1(4) | |
Figure 1.
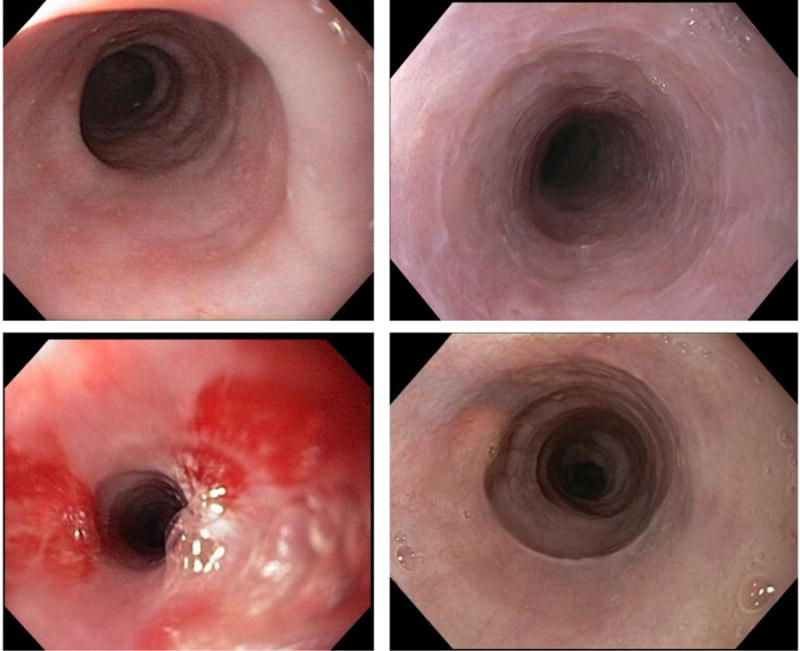
Endoscopic findings of patients with lymphocytic esophagitis illustrating narrow caliber esophagus, esophageal strictures, fine esophageal rings/webs, and erythema.
On histologic re-examination of the original biopsy slides, 52% of patients had between 21–40 lymphocytes/hpf, 4% had between 41–80 lymphocytes/hpf and 22% had greater than 80 lymphocytes/hpf (Figure 2) (Table 2).
Figure 2.
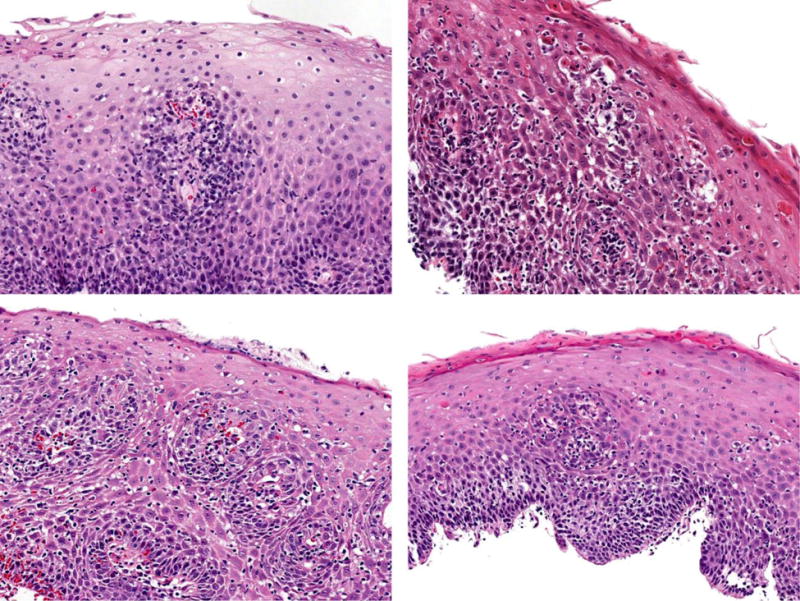
Histologic findings of patients with lymphocytic esophagitis showing esophageal squamous mucosa with variable spongiosis and increased numbers of intraepithelial lymphocytes in a diffuse distribution, ranging in numbers from mild to striking, occasionally forming small lymphocytic clusters, particularly in the peripapillary areas.
Based on the clinical and histologic findings, 9 patients had a medication change. These included: initiation or increase in proton-pump inhibitor dose in 6 patients, addition of a GI cocktail in 1, initiation of swallowed fluticasone in 1, and initiation of oral prednisone taper in 1 (Table 2). Of the patients who had a medication change, only 1 patient had follow up EGD, 2 patients who were started on a PPI and 1 patient who was treated with swallowed fluticasone had symptomatic improvement.
When patients with LyE were compared to patients with a normal esophageal biopsy, patients with GERD, and patients with eosinophilic esophagitis (Table 3), patients with LyE were more likely to be non-white (41% vs. 15% normal vs. 0% GERD vs. 5% EoE; p < 0.01) and use tobacco (64% vs. 30% normal vs. 55% GERD vs. 55% EoE; p = 0.02). LyE patients had comparable rates of drug and food allergies, but were less likely to have allergic rhinitis (4% vs. 25% normal vs. 25% GERD vs. 40% EoE; p =0.02). Other clinical features were similar.
Table 3.
LyE patient characteristics compared to controls
| LyE (n= 27) |
Normal (n= 20) |
GERD (n=20) |
EoE (n= 20) |
P value | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Age in years (Mean ± SD) | 56 ± 16 | 57 ± 12 | 61 ± 15 | 36 ± 12 | < 0.001 | |
| Female (%) | 63 | 80 | 60 | 50 | 0.26 | |
| Race (%) | ||||||
| Caucasian | 59 | 85 | 100 | 95 | ||
| Black | 37 | 15 | 0 | 5 | <0.01 | |
| Hispanic | 4 | 0 | 0 | 0 | ||
| Atopic Conditions (%) | ||||||
| Drug | 37 | 60 | 50 | 32 | 0.49 | |
| Food allergies | 4 | 10 | 5 | 15 | 0.65 | |
| Allergic Rhinitis | 4 | 25 | 25 | 40 | 0.02 | |
| Asthma | 19 | 10 | 10 | 40 | 0.14 | |
| Eczema | 4 | 0 | 0 | 0 | 0.67 | |
| IBD (%) | 4 | 0 | 10 | 0 | 0.45 | |
| Alcohol use (%) | 42 | 45 | 55 | 61 | 0.34 | |
| Tobacco use prior and current (%) | 64 | 30 | 55 | 55 | 0.02 | |
| Endoscopic Findings (%) | ||||||
| Hiatal Hernia | 26 | 40 | 50 | 10 | 0.11 | |
| Erosive Esophagitis | 33 | 25 | 40 | 0 | 0.01 | |
| Stricture | 37 | 15 | 25 | 35 | 0.35 | |
| Rings | 26 | 20 | 10 | 55 | 0.01 | |
| Dilation during endoscopy (%) | 33 | 20 | 10 | 35 | 0.20 | |
Discussion
LyE is a recently described rare condition of the esophagus. It has not yet been well characterized, and correlation between the histologic findings and clinical features are not always clear [3,8,9,10,13,14]. Because of this, we aimed to characterize patients with LyE at our center, compare them to non-LyE controls, and identify risk factors in order to provide more data to guide care. In sum, we found 27 adults diagnosed with LyE starting as early as 2004, and the vast majority of patients had more than 20 lymphs/hpf on histology. Diagnosis tended to be in the 6th decade, and in comparison to our GERD, EoE and “normal” controls, patients with LyE tended to be non-white, were more commonly tobacco users, and less likely to have atopy. In contrast to EoE, there was also a female preponderance in LyE. However, in comparison to patients with “normal” findings, there were fewer females diagnosed with LyE, though this group still had a majority of females. In addition, we did not see a clear relation between LyE and Crohn’s disease, though previously diagnosed GERD was common. Of note, 85% of the patients in our “normal” cohort were Caucasian which is consistent with prior racial demographic data from the University of North Carolina [15].
From a symptom standpoint, our data is consistent with several other studies that have also noted dysphagia to be the most common symptom at presentation of LyE [2,16]. Relatively few of our patients complained of other upper GI symptoms such as heartburn, abdominal pain, chest pain, nausea/vomiting, or odynophagia, which is similar to other studies [2,3,5,16,17]. However, the possible association between smoking and LyE has not been previously observed. The pathogenesis of LyE is yet unknown, but it has been hypothesized that possible causes of LyE may include a hypersensitivity reaction to an ingestant or an autoimmune phenomenon [1,3,4,14]. Additionally, it has been proposed that LyE may be an early sign of GERD in patients with no other endoscopic findings [10,18]. Given our finding of a significant association with smoking, it is intriguing to speculate whether an element of cigarette smoke may act as a topical trigger of the condition. However, further studies investigating the association with smoking are needed to confirm our findings and determine any etiologic mechanisms.
Several studies have reported an association between LyE and inflammatory bowel disease, but this has not been consistent. For example, Rubio et. al compared 20 patients with LyE to 61 patients with other types of esophagitis and found an association of LyE with Crohn’s disease (CD), particularly amongst pediatric patients [1]. Similarly, in a pediatric population with known Crohn’s disease, 28% of those patients were found to have increased lymphocytes [9]. A large pediatric cohort study by Sutton et. al found a significant association of CD in children with LyE (19% of children with LyE had CD and 12% of children with CD had LyE) [3,19]. This was not replicated in the study conducted by Purdy et. al [3]. A less frequent association was found in a study among adults by Basseri et. al [6]. Additionally, in a study of a very large esophageal biopsy database, Haque and Genta found that in adults, LyE affects predominantly older women and is not associated with CD [5]. In our study, we identified only one patient with concomitant inflammatory bowel disease (Crohn’s disease).
It is important to differentiate between esophageal lichen planus and LyE. The most characteristic histologic finding in esophageal lichen planus is a bandlike or lichenoid lymphocytic infiltrate obscuring the interface between lamina propria and basal layer epithelium, with or without Civatte bodies. The histologic lesions illustrated in our report lack a lichenoid infiltrate. Lymphocytes are numerous, but they are generally distributed uniformly within a spongiotic epithelium. Lymphocyte-mediated epithelial cell injury is present in most lesions, but the injury is higher in the epithelial layer. Therefore, we believe that LyE is a different histologic entity than lichen planus, and the two do not overlap in our series.
Limitations of our study include the fact that this was a retrospective case series at a single center. Therefore, we did not have standardized follow up data and there were few patients who had repeat endoscopic assessments. Because of the relatively small number of cases, we were unable to control for potential confounders or perform detailed sub-analyses. However, there were also several strengths. We conducted an exhaustive review of pathologic records to capture all patients with possible LyE, even if the diagnosis had not been made clinically, and then confirmed the diagnosis of our cases after re-review of pathology slides by an expert pathologist. This strategy yielded a cohort size that is comparable to other studies. Additionally, we compared our findings in patients with LyE to patients with normal esophagus, GERD, and EoE controls to help contextualize the findings and identify risk factors.
In conclusion, though it is rare, LyE should be considered as a diagnostic possibility in patients with clinical symptoms of dysphagia undergoing upper endoscopy. Our data would suggest it is more likely to be seen in older female patients who smoke and who do not have atopy. With wider recognition of LyE it is also important to ensure training of pathologists to recognize this condition and ensure semi-quantitative reporting of lymphocyte numbers noted when clinically appropriate. Larger studies are needed to better characterize LyE and gain information on the natural history of this condition.
Acknowledgments
Funding: This research was funded by T32 DK07634 (SP; CCR) from the National Institutes of Health
Footnotes
Relevant Financial Disclosures: None of the authors have conflicts related to this study.
Author Contributions (all approved the final draft): Sarina Pasricha: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis; Amit Gupta: acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content; Craig Reed: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content; Olga Speck: acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content; John Woosley: study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content; Evan Dellon: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content.
References
- 1.Rubio CA, Sjodahl K, Lagergren J. Lymphocytic esophagitis: a histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125:432–437. [PubMed] [Google Scholar]
- 2.Cohen S, Saxena A, Waljee AK, et al. Lymphocytic esophagitis: a diagnosis of increasing frequency. J Clin Gastroenterol. 2012;46:828–832. doi: 10.1097/MCG.0b013e3182500de8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purdy JK, Appelman HD, Golembeski CP, McKenna BJ. Lymphocytic esophagitis: a chronic or recurring pattern of esophagitis resembling allergic contact dermatitis. American journal of clinical pathology. 2008;130:508–513. doi: 10.1309/D3PCF6D6YYMQRX9A. [DOI] [PubMed] [Google Scholar]
- 4.Kasirye Y, John A, Rall C, Resnick J. Lymphocytic esophagitis presenting as chronic dysphagia. Clin Med Res. 2012;10:83–84. doi: 10.3121/cmr.2011.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut. 2012;61:1108–1114. doi: 10.1136/gutjnl-2011-301014. [DOI] [PubMed] [Google Scholar]
- 6.Basseri B, Vasiliauskas EA, Chan O, et al. Evaluation of peripapillary lymphocytosis and lymphocytic esophagitis in adult inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2013;9:505–511. [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka K, Rubio CA, Dlugosz A, et al. Narrow-band imaging magnifying endoscopy in adult patients with eosinophilic esophagitis/esophageal eosinophilia and lymphocytic esophagitis. Gastrointest Endosc. 2013;78:659–664. doi: 10.1016/j.gie.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Lebwohl B, Green PH, Genta RM. Letter: lymphocytic gastritis and coeliac disease - authors’ reply. Alimentary pharmacology & therapeutics. 2015;42:938. doi: 10.1111/apt.13355. [DOI] [PubMed] [Google Scholar]; Maejima R, Uno K, Iijima K, et al. A Japanese case of lymphocytic esophagitis. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2015 doi: 10.1111/den.12578. [DOI] [PubMed] [Google Scholar]
- 9.Ebach DR, Vanderheyden AD, Ellison JM, Jensen CS. Lymphocytic esophagitis: a possible manifestation of pediatric upper gastrointestinal Crohn’s disease. Inflammatory bowel diseases. 2011;17:45–49. doi: 10.1002/ibd.21347. [DOI] [PubMed] [Google Scholar]
- 10.Ronkainen J, Walker MM, Aro P, et al. Lymphocytic oesophagitis, a condition in search of a disease? Gut. 2012;61:1776. doi: 10.1136/gutjnl-2012-302329. [DOI] [PubMed] [Google Scholar]
- 11.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108:679–692. doi: 10.1038/ajg.2013.71. quiz 693. [DOI] [PubMed] [Google Scholar]
- 12.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.e26. doi: 10.1016/j.jaci.2011.02.040. quiz 21–22. [DOI] [PubMed] [Google Scholar]
- 13.Hendy PJ, Wong DS, Florin TH. Spontaneous oesophageal perforation: an unreported complication of lymphocytic oesophagitis. Gut. 2013;62:1668–1669. doi: 10.1136/gutjnl-2013-305455. [DOI] [PubMed] [Google Scholar]
- 14.Vangimalla S, Gordon I, Thota PN. Lymphocytic Esophagitis in Common Variable Immune Deficiency. The American journal of gastroenterology. 2016;111:170. doi: 10.1038/ajg.2015.178. [DOI] [PubMed] [Google Scholar]
- 15.Jensen ET, Kappelman MD, Kim HP, Ringel-Kulka T, Dellon ES. Early life exposures as risk factors for pediatric eosinophilic esophagitis. Journal of pediatric gastroenterology and nutrition. 2013;57:67–71. doi: 10.1097/MPG.0b013e318290d15a. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar KB, Ayyar BKS, S J, Genta RM, Melton SD. Clinical, endoscopic and histological features of patients with lymphocytic esophagitis compared to patients with GERD. Gastroenterology. 2014:5085. Mo 1846. Abstract. [Google Scholar]
- 17.Basseri B, Levy M, Wang HL, et al. Redefining the role of lymphocytes in gastroesophageal reflux disease and eosinophilic esophagitis. Dis Esophagus. 2010;23:368–376. doi: 10.1111/j.1442-2050.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 18.Ronkainen J, Aro P, Storskrubb T, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scandinavian journal of gastroenterology. 2005;40:275–285. doi: 10.1080/00365520510011579. [DOI] [PubMed] [Google Scholar]
- 19.Sutton LM, Heintz DD, Patel AS, Weinberg AG. Lymphocytic esophagitis in children. Inflammatory bowel diseases. 2014;20:1324–1328. doi: 10.1097/MIB.0000000000000100. [DOI] [PubMed] [Google Scholar]


