SUMMARY
The innate immune system is the frontline of host defense against microbial infections, but its rapid and uncontrolled activation elicits microbicidal mechanisms that have deleterious effects [1, 2]. Increasing evidence indicates that the metazoan nervous system, which responds to stimuli originating from both the internal and the external environment, functions as a modulatory apparatus that controls not only microbial killing pathways but also cellular homeostatic mechanisms [3–5]. Here, we report that dopamine signaling controls innate immune responses through a D1-like dopamine receptor, DOP-4, in Caenorhabditis elegans. Chlorpromazine inhibition of DOP-4 in the nervous system activates a microbicidal PMK-1/p38 mitogen-activated protein kinase signaling pathway that enhances host resistance against bacterial infections. The immune inhibitory function of dopamine originates in CEP neurons and requires active DOP-4 in downstream ASG neurons. Our findings indicate that dopamine signaling from the nervous system controls immunity in a cell non-autonomous manner and identifies the dopaminergic system as a potential therapeutic target for not only infectious diseases but also a range of conditions that arise as a consequence of malfunctioning immune responses.
RESULTS AND DISCUSSION
The ability of an organism to survive microbial infections requires tightly controlled activation of immune responses. Excessive inflammation can lead to conditions such as Crohn’s disease, rheumatoid arthritis, Alzheimer’s disease, and many other diseases that involve aberrant inflammation. Indeed, to survive an infection and return to homeostasis, animals appear to rely on reflex circuits that prevent inflammation when triggered by bacterial products or inflammatory cues [6, 7]. This rapid and precise communication between the nervous and immune systems serves as an ancestral mechanism to control immune homeostasis in metazoans.
Increasing evidence highlights the importance of the vagus nerve circuit in the mediation of anti-inflammatory effects [8]. Recent studies have demonstrated that electroacupuncture requires intact sciatic and vagus nerves to inhibit inflammation by promoting the release of dopamine in the adrenal medulla [9, 10]. Dopamine is a well-characterized catecholamine neurotransmitter that is involved in diverse functions, including movement, reward, pain perception, and drug addiction [11–13]. It has also been shown to target a number of dopamine receptors in a variety of immune cells [14, 15]. However, the precise dopaminergic neural circuits potentially involved in the control of immunity remain unknown due in part to the complexity of the nervous and immune systems of most model organisms. To dissect dopaminergic neural circuits capable of regulating immunity, we took advantage of the simple nervous system of the nematode Caenorhabditis elegans, which comprises only 302 neurons.
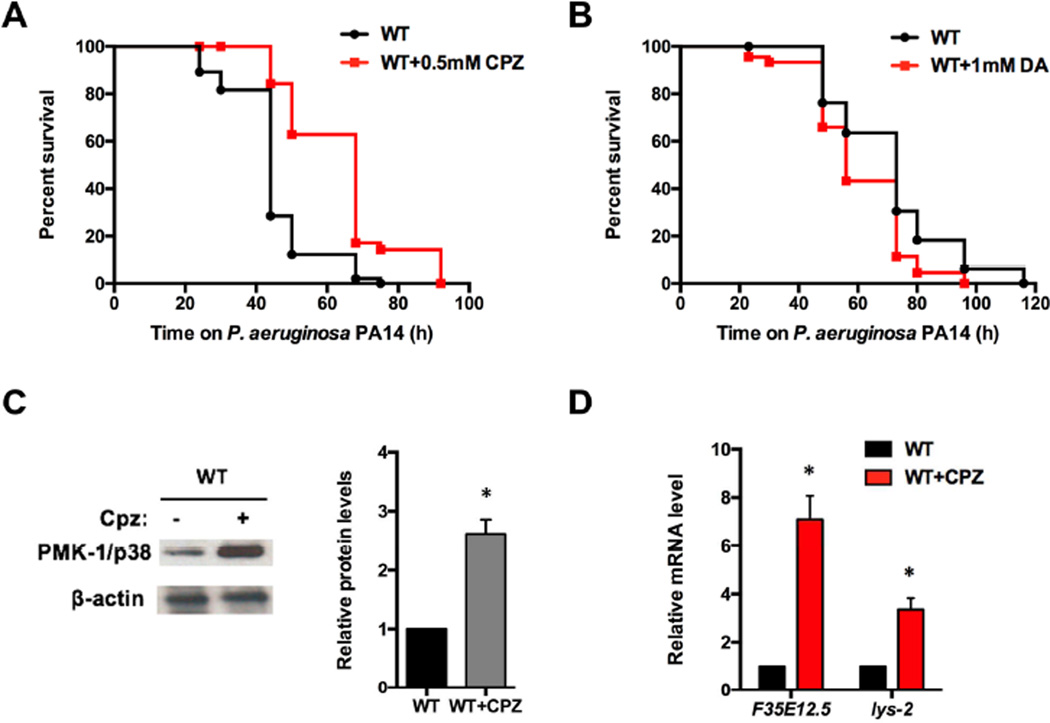
Recent findings indicate that several dopamine antagonists can activate a conserved p38/PMK-1 MAPK immune pathway in C. elegans [16]. As an initial step to determine whether drugs that target the dopaminergic nervous system may control immunity at the cell non-autonomous level, we treated wild-type animals with the dopamine antagonist chlorpromazine during exposure to the human opportunistic pathogen Pseudomonas aeruginosa. As shown in Figure 1A, animals treated with chlorpromazine exhibited enhanced resistance to P. aeruginosa-mediated killing. In contrast, wild-type animals showed a small but significantly enhanced susceptibility to the pathogen when treated with dopamine (Figure 1B). We also studied whether the enhanced resistance to P. aeruginosa-mediated killing induced by treatment with chlorpromazine correlated with higher levels of active PMK-1. Western blot analysis indicated that animals treated with chlorpromazine had higher levels of active PMK-1 (Figure 1C). Furthermore, two genes that are markers of the PMK-1 pathway, F35E12.5 and lys-2, were up-regulated in response to the chlorpromazine treatment (Figure 1D), suggesting that chemical inhibition of dopamine signaling protects C. elegans against pathogenic bacteria by inducing the expression of immune-related genes.
Figure 1. Chemical Targeting of Dopamine Signaling Affects Immune Responses.
(A) Wild-type animals were exposed to P. aeruginosa PA14 in the presence or absence of the dopamine antagonist chlorpromazine (CPZ) and scored for survival. WT vs. WT+CPZ: p < 0.001. (B) Wild-type animals were exposed to P. aeruginosa PA14 in the presence or absence of dopamine (DA) and scored for survival. WT vs. WT+DA: p < 0.01. (C) Western blot analysis of active PMK-1/p38 levels in wild-type animals treated with or without chlorpromazine. Image quantification was performed using the software program ImageJ. (D) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of F35E12.5 and lys-2 gene expression in wild-type animals treated with chlorpromazine compared with control animals. All bars represent means ± SEM. *, p < 0.05.
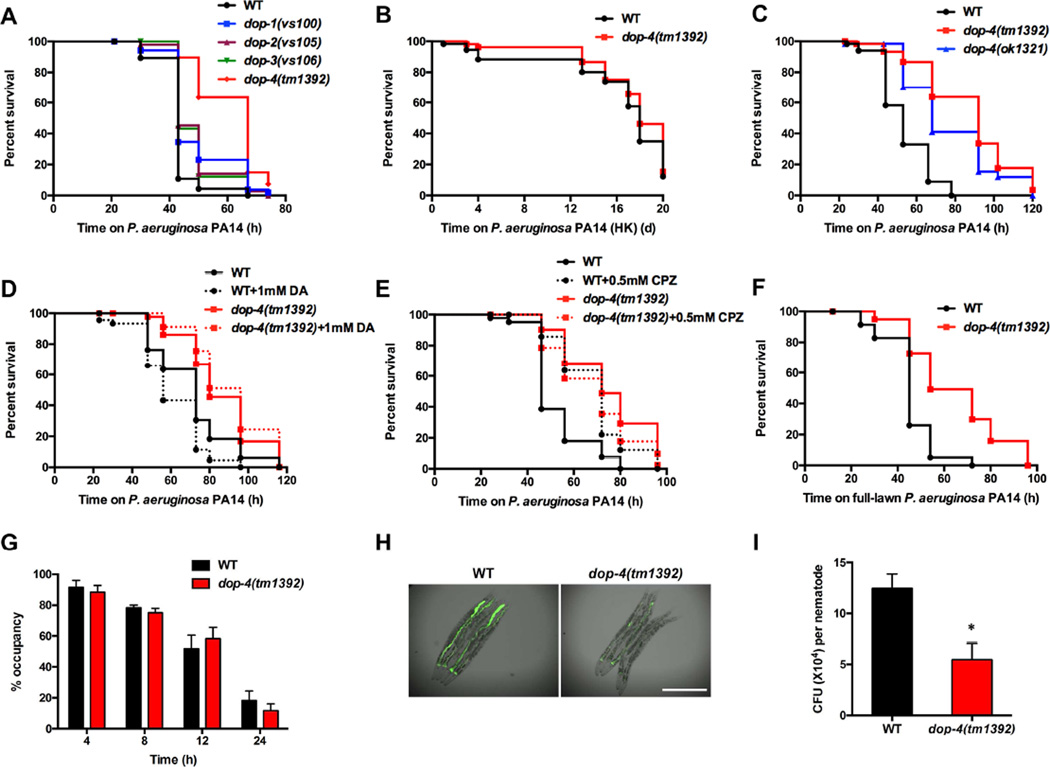
Dopamine released from pre-synaptic vesicles must engage receptors expressed in downstream neurons to perform its physiological functions in vivo. To identify the dopamine receptor/s that may be involved in the control of immune responses, we examined the susceptibility to P. aeruginosa of four strains of C. elegans lacking dopamine receptors. We found that only dop-4(tm1392) animals, which carry a 1086-bp deletion in the dop-4 gene, showed enhanced resistance to P. aeruginosa infection compared with wild-type animals (Figure 2A and Figure S1). We also found that dop-4(tm1392) animals do not show a significant lifespan extension on heat-killed bacteria compared to wild-type animals (Figure 2B), indicating that dop-4 may modulate immunity without affecting longevity. To rule out the possibility of an allelic effect caused by the dop-4(tm1392) mutation, we examined the susceptibility to P. aeruginosa-mediated killing of dop-4(ok1321) animals, which harbor a 1766-bp deletion in the dop-4 locus (Figure S1). Both dop-4(tm1392) and dop-4(ok1321) animals exhibited similar resistance to killing by P. aeruginosa (Figure 2C), suggesting that dop-4 may be involved in dopamine-mediated immune regulation. Consistent with this idea, dop-4 was required for the enhanced susceptibility to P. aeruginosa-mediated killing induced by dopamine treatment (Figure 2D). In addition, the enhanced resistance to P. aeruginosa-mediated killing of dop-4(tm1392) animals did not differ from that of dop-4(tm1392) animals treated with chlorpromazine (Figure 2E). In contrast, treatment with chlorpromazine enhanced resistance to P. aeruginosa infection in animals carrying mutations in other dopamine receptors (Figure S2), suggesting that chlorpromazine enhances immunity primarily by inhibiting the D1-like dopamine receptor DOP-4.
Figure 2. The D1-like Dopamine Receptor DOP-4 Controls Immunity against P. aeruginosa Infection.
(A) Wild-type animals and dopamine receptor mutants were exposed to P. aeruginosa PA14 and scored for survival. WT vs. dop-4(tm1392): p < 0.0001. (B) Wild-type and dop-4(tm1392) animals were exposed to heat-killed P. aeruginosa PA14 and scored for survival. WT vs. dop-4(tm1392): p > 0.1. (C) Wild-type, dop-4(tm1392), and dop-4(ok1321) animals were exposed to P. aeruginosa PA14 and scored for survival. WT vs. dop-4(tm1392): p < 0.001; WT vs. dop-4(ok1321): p < 0.001. (D) Wild-type and dop-4(tm1392) animals were exposed to P. aeruginosa PA14 in the presence or absence of dopamine and scored for survival. WT vs. WT+DA: p < 0.01; dop-4(tm1392) vs. dop-4(tm1392)+DA: p > 0.1. (E) Wild-type and dop-4(tm1392) animals were exposed to P. aeruginosa PA14 in the presence or absence of chlorpromazine and scored for survival. WT vs. WT+CPZ: p < 0.001; dop-4(tm1392) vs. dop-4(tm1392)+CPZ: p > 0.1. (F) Wild-type and dop-4(tm1392) animals were exposed to a full lawn of P. aeruginosa PA14 and scored for survival. WT vs. dop-4(tm1392): p < 0.001. (G) Wild-type and dop-4(tm1392) animals were cultured on partial pathogen lawns, and the percentage of animals on the pathogen lawn was calculated for each time point. (H) Wild-type and dop-4(tm1392) animals were exposed to P. aeruginosa PA14-GFP for 30 hours and visualized using a Leica M165 FC fluorescence stereomicroscope. Scale bar = 0.5 mm. (I) Wild-type and dop-4(tm1392) animals were exposed to P. aeruginosa PA14-GFP for 30 hours, and the colony forming units (CFU) were counted. Ten animals were used for each condition. All bars represent means ± SEM; *, p < 0.05. See also Figure S1 for allele information and Figure S2 for additional chlorpromazine treatments and feeding behaviors.
Because avoidance to P. aeruginosa is part of the C. elegans defense response against this pathogen [17, 18], we performed a so-called “full-lawn” survival assay that uses plates that are completely covered in bacteria, a condition that eliminates pathogen avoidance. We found that dop-4(tm1392) animals exhibited enhanced resistance to P. aeruginosa infection compared with wild-type animals on full-lawn plates (Figure 2F). In addition, we monitored the lawn occupancy of wild-type and dop-4(tm1392) animals on partial-lawn plates, which are normally used for survival assays. The nematode distribution on the pathogen lawn did not differ significantly between wild-type and dop-4(tm1392) animals (Figure 2G), indicating that the enhanced resistance of dop-4(tm1392) animals to P. aeruginosa infection was not due to enhanced pathogen avoidance. We further questioned whether the enhanced resistance to P. aeruginosa infection correlated with reduced bacterial accumulation in the intestine. To address this question, dop-4(tm1392) animals were infected with P. aeruginosa labelled with GFP. The dop-4(tm1392) animals showed a significant reduction of fluorescence intensity within the intestine (Figure 2H). In addition, there were fewer bacterial cells in the intestine of the dop-4 mutant compared with wild-type animals (Figure 2I). To study whether a reduced feeding behavior in dop-4(tm1392) animals may account for the reduced bacterial burden observed, we studied the pumping rate of the animals and the accumulation of fluorescent beads in the intestine. We observed that over 30 seconds or 2 minutes the pumping rate of dop-4(tm1392) is not lower than that of wild type animals (Figure S2E and S2F). In addition, the accumulation of fluorescent beads in the gut of dop-4(tm1392) and wild-type animals was comparable, while the accumulation of fluorescent beads in the feeding mutant eat-2(ad465) was lower (Figure S2G). These results indicate that dop-4(tm1392) animals do not exhibit any feeding deficiency and that the dose of infecting bacteria they received is comparable to that of wild type animals. Thus, we reasoned that the dop-4 mutation leads to the activation of microbial killing mechanisms that result in reduced bacterial burden and enhanced resistance to infection.
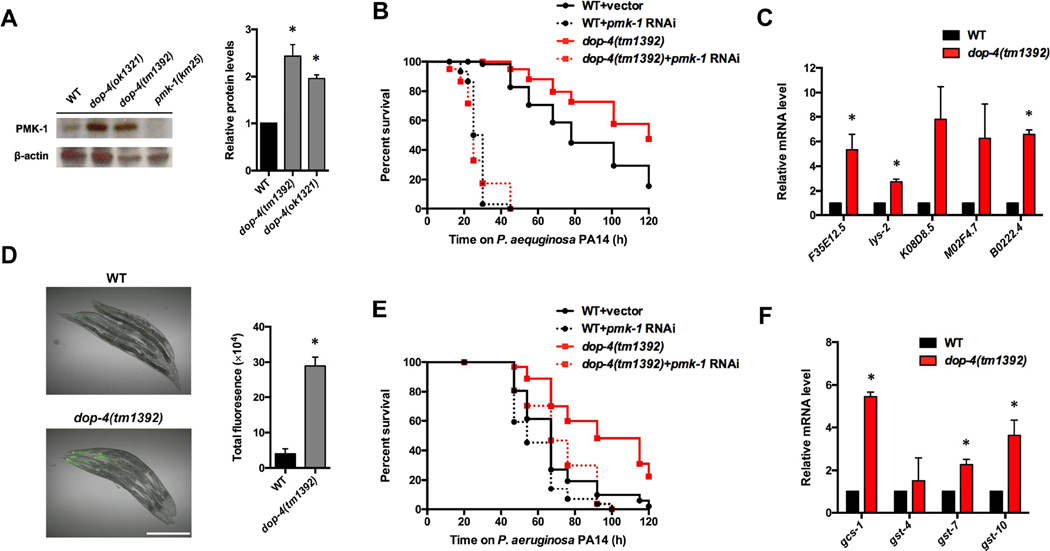
Based on these results, we hypothesized that DOP-4 acts as a negative regulator of immunity, at least in part by inhibiting the p38/PMK-1 MAPK pathway. Consistent with this idea, we observed that dop-4(tm1392) animals had higher levels of active PMK-1 compared with wild-type animals (Figure 3A). Inhibition of the pmk-1 gene by RNAi also completely suppressed the enhanced resistance to P. aeruginosa of dop-4(tm1392) animals (Figure 3B). Furthermore, PMK-1-dependent genes were up-regulated in dop-4(tm1392) animals relative to wild-type animals in the absence or presence of an infection (Figure 3C and Figure S3A). The levels of pmk-1 in dop-4(tm1392) and wild-type animals were comparable (Figure S3B), indicating that DOP-4 controls PMK-1 activation rather than expression. To study the in vivo role of DOP-4 in the regulation of PMK-1, we used a transgenic transcriptional reporter strain of PMK-1 activity that expresses GFP under the control of the promoter of F35E12.5 [19]. We observed higher levels of GFP in dop-4(tm1392);, PF35E12.5::gfp than in PF35E12.5::gfp animals (Figure 3D). We also found that animals lacking dop-4 exhibited higher levels of GFP than control animals when infected with P. aeruginosa (Figure S3C), indicating that DOP-4 functions as a negative regulator of immunity by preventing the expression of PMK-1-dependent genes in the intestine. It has been shown that the C. elegans transcription factor SKN-1 is activated by PMK-1 in response to stressful conditions [20]. SKN-1 is orthologous to mammalian Nrf transcription factors and functions to activate the oxidative stress response during pathogen infection [21]. Consistent with the notion that SKN-1 functions downstream of PMK-1, we found that RNAi of skn-1 also abolished the enhanced resistance of dop-4 animals (Figure 3E). In addition, genes controlled by SKN-1 were up-regulated in dop-4(tm1392) animals (Figure 3F).
Figure 3. A conserved p38/PMK-1 MAP kinase pathway is required for the enhanced resistance to P. aeruginosa infection of dop-4 animals.
(A) Western blot analysis of active p38 levels of wild-type, dop-4(ok1321), and dop-4(tm1392) animals. Image quantification was performed using the software program ImageJ. (B) Wild-type and dop-4(tm1392) animals fed with E. coli strain HT115 carrying a vector control or expressing double-stranded RNA (dsRNA) targeting pmk-1 were exposed to P. aeruginosa PA14 and scored for survival. WT+pmk-1 RNAi vs. dop-4(tm1392)+pmk-1 RNAi: p > 0.1. (C) Quantitative RT-PCR analysis of pmk-1–regulated genes in dop-4(tm1392) compared with wild-type animals fed on E. coli OP50. (D) Fluorescence image of wild-type and dop-4(tm1392) animals expressing F35E12.5::gfp. Young adult animals were cultured on E. coli OP50 and visualized using a Leica M165 FC fluorescence stereomicroscope. Scale bar = 0.5 mm. Fluorescence was quantified using software ImageJ. (E) Wild-type and dop-4(tm1392) animals fed with E. coli strain HT115 carrying a control vector or expressing dsRNA targeting skn-1 were exposed to P. aeruginosa PA14 and scored for survival. WT+skn-1 RNAi vs. dop-4(tm1392)+skn-1 RNAi: p > 0.1. (F) Quantitative RT-PCR analysis of skn-1–regulated genes in dop-4(tm1392) compared with wild-type animals fed on E. coli OP50. All bars represent means ± SEM; *, p < 0.05. See also Figure S3 for expression level of pmk-1–regulated genes upon P. aeruginosa infection.
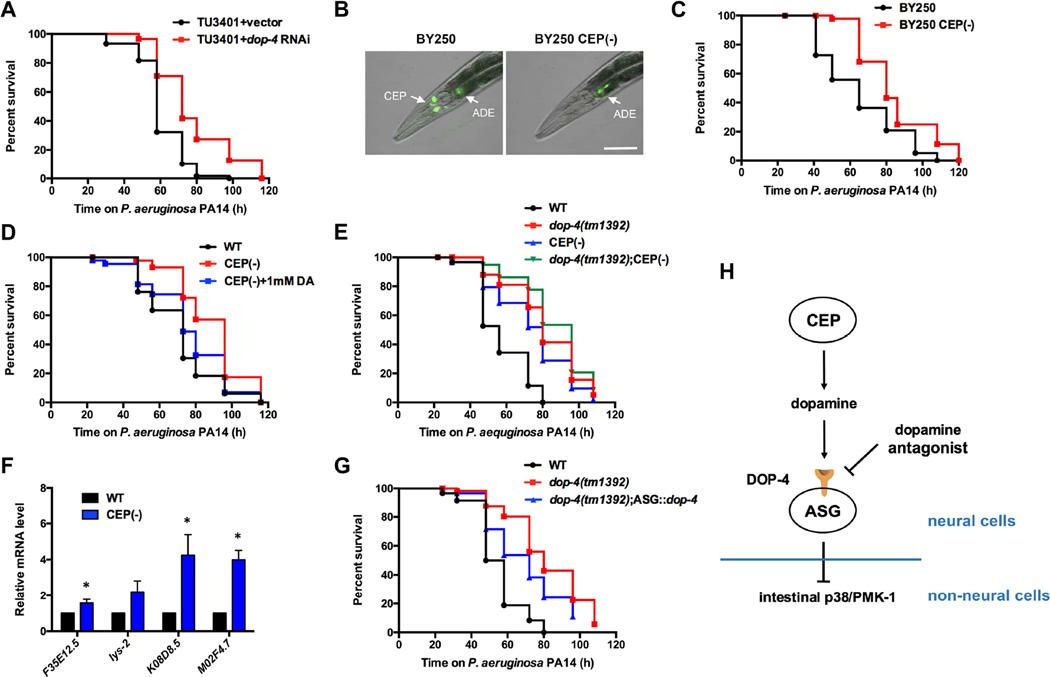
It has been reported that dop-4 is expressed in a series of neurons, including ASG, AVL, and pharyngeal neurons, as well as in intestinal cells [22]. To identify the tissue in which DOP-4 functions to regulate immune responses, we employed nematode strains that were engineered to promote RNAi only in neural or intestinal cells. We observed that inhibition of dop-4 in the nervous system but not in the intestine increased the resistance of the nematodes to P. aeruginosa infection (Figure 4A and Figure S4A), indicating that DOP-4 functions in the nervous system to control immunity. As a first step to identify the specific dopaminergic neurons responsible for the observed immune regulation, we performed targeted killing of CEP neurons. We focused on these cells because they are the only dopaminergic neurons in C. elegans hermaphrodites that are exposed to the pseudocoelomic body fluid, from where they can directly communicate through neuroendocrine signals with tissues involved in defense responses. The killing of CEP neurons was achieved by expressing the two subunits of the C. elegans caspase CED-3 (p15 and p17) under the promoters of dat-1 and ace-1, respectively. Because the expression patterns of these two genes overlap solely in the four CEP neurons, only in these neurons could the two subunits of CED-3 form a functional module to induce programmed cell death. The ablation was conducted using strain BY250, in which all dopaminergic neurons are labeled with GFP. We observed that only CEP neurons, but not other dopaminergic neurons, were ablated, confirming the efficacy of this cell-specific killing approach (Figure 4B). We found that CEP(-) animals showed enhanced resistance to P. aeruginosa-mediated killing compared with control animals (Figure 4C). In addition, the lawn occupancy of wild-type and CEP(-) animals was comparable at different time points (Figure S4B), which indicated that the pathogen resistance of CEP(-) animals is not due to enhanced pathogen avoidance. These data suggest that CEP neurons participate in immune modulation and that dopamine secreted from CEP neurons can induce downstream immune inhibition. Therefore, we explored whether treatment with exogenous dopamine could suppress the enhanced resistance to P. aeruginosa infection of CEP(-) animals. Indeed, CEP(-) animals treated with dopamine exhibited susceptibility to P. aeruginosa-mediated killing similar to that of wild-type animals (Figure 4D). No difference in the lawn occupancy of animals treated with chlorpromazine or dopamine was observed, indicating that the drugs affect immunity without altering pathogen avoidance (Figure S4C). We also observed that the ablation of CEP neurons could not further increase the resistance to P. aeruginosa of dop-4(tm1392) animals (Figure 4E). The lack of synergism between the dop-4 mutation and CEP ablation suggests that dopamine released from CEP neurons may function through the dopamine receptor DOP-4 to inhibit downstream immune pathways. In support of this idea, we found that PMK-1-dependent genes were up-regulated in CEP(-) animals (Figure 4F). Therefore, we predicted that expression of dop-4 in neurons downstream CEP would suppress the enhanced resistance to P. aeruginosa of dop-4(tm1392) animals. Because ASG neurons are the only dop-4-expressing cells reported to have direct synaptic connectivity with CEP neurons, we tested whether dop-4 is required in these cells. We found that single-neuron rescue of dop-4 in ASG neurons could at least partially suppress the enhanced resistance to P. aeruginosa-mediated killing of dop-4 animals (Figure 4G).
Figure 4. Dopaminergic CEP neurons control immunity through DOP-4 expressed in ASG neurons.
(A) The neural-specific RNAi strain TU3401 fed with vector control or dop-4 RNAi bacteria was exposed to P. aeruginosa PA14 and scored for survival. TU3401+vector vs. TU3401+dop-4 RNAi: p < 0.01. (B) Fluorescence image showing the targeted ablation of CEP neurons. Scale bar = 20 μm. (C) BY250 and BY250 CEP(-) animals were exposed to P. aeruginosa PA14 and scored for survival. BY250 vs. BY250 CEP(-): p < 0.01. (D) Wild-type, CEP(-) and CEP(-) animals treated with dopamine were exposed to P. aeruginosa PA14 and scored for survival. WT vs. CEP(-): p < 0.01; WT vs. CEP(-)+DA: p > 0.1. (E) Wild-type, dop-4(tm1392), CEP(-), and dop-4(tm1392);CEP(-) animals were exposed to P. aeruginosa PA14 and scored for survival. WT vs. dop-4(tm1392);CEP(-): p < 0.001; dop-4(tm1392) vs. dop-4(tm1392);CEP(-): p > 0.1. (F) Quantitative RT-PCR analysis of pmk-1–regulated genes in CEP(-) compared with wild-type animals. Bars represent means ± SEM. *, p < 0.05. (G) Wild-type, dop-4(tm1392), and dop-4(tm1392);Pgcy-15::dop-4(tm1392) animals were exposed to P. aeruginosa PA14 and scored for survival. dop-4(tm1392) vs. dop-4(tm1392);Pgcy-15::dop-4(tm1392): p < 0.05. (H) Proposed model of dopamine regulation of innate immunity. See also Figure S4.
In the present study, we have shown that dopamine signaling negatively regulates the innate immune response through a D1-like dopamine receptor, DOP-4, by down-regulating the p38/PMK-1 MAPK pathway. Furthermore, a putative neural circuit involving dopaminergic neurons CEP and DOP-4-expressing neurons ASG has been shown to control the immune response against pathogen infections (Figure 4H). The ablation of CEP neurons conferred a similar enhanced resistance to infection, as observed in the dop-4 mutant animals. In addition, exogenous dopamine suppressed the enhanced resistance to pathogen infection caused by CEP ablation. The identification of this neural circuit and the demonstration that chemical inhibition of dopamine signaling in the nervous system can control immune pathways at the cell non-autonomous level provides proof of concept for the use of neural interventions to control infections and conditions that involve aberrant immune functions.
Supplementary Material
Highlights.
Inhibition of dopamine signaling protects against bacterial infections
Chlorpromazine enhances immunity by inhibiting a D1-like dopamine receptor
Dopamine signaling regulates p38 MAP kinase activity
Dopaminergic neurons control immunity in the C. elegans intestine
In Brief.
Cao and Aballay show that chemical inhibition of the neurotransmitter dopamine in the nervous system enhances a signaling pathway that functions in the intestine to fight bacterial infections. The identification of this neural circuit provides proof of concept for the use of neural interventions to control infections and the immune system.
Acknowledgments
This work was supported by National Institutes of Health grants GM0709077 and AI117911 (to A.A.). We thank Joel Meyer (Duke University, Durham, NC) for providing strain BY250, Dong Yan (Duke University, Durham, NC) for providing the Punc-122::gfp co-injection marker, Gary Ruvkun (Massachusetts General Hospital, Harvard Medical School, Boston, MA) for providing the intestine-specific RNAi strain MGH171, and Ryan Baugh (Duke University, Durham, NC) for providing eat-2(ad465) mutants. Some strains used in this study were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
X.C. and A.A. conceived and designed experiments. X.C. performed experiments. X.C. and A.A. analyzed the data and wrote the paper.
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen Recognition and Innate Immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Cho JH, Feldman M. Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies. Nat Med. 2015;21:730–738. doi: 10.1038/nm.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aballay A. Role of the Nervous System in the Control of Proteostasis during Innate Immune Activation: Insights from C. elegans. PLoS Pathog. 2013;9:e1003433. doi: 10.1371/journal.ppat.1003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veiga-Fernandes H, Mucida D. Neuro-Immune Interactions at Barrier Surfaces. Cell. 2016;165:801–811. doi: 10.1016/j.cell.2016.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. The Journal of Experimental Medicine. 2012;209:1057–1068. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex[mdash]linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavan SS, Tracey KJ. Regulating innate immunity with dopamine and electroacupuncture. Nat Med. 2014;20:239–241. doi: 10.1038/nm.3501. [DOI] [PubMed] [Google Scholar]
- 10.Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotharius J, Brundin P. Pathogenesis of parkinson's disease: dopamine, vesicles and [alpha]-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 12.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 13.Beaulieu J-M, Gainetdinov RR. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacological Reviews. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 14.Levite M. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiologica. 2016;216:42–89. doi: 10.1111/apha.12476. [DOI] [PubMed] [Google Scholar]
- 15.Pacheco R, Prado CE, Barrientos MJ, Bernales S. Role of dopamine in the physiology of T-cells and dendritic cells. Journal of Neuroimmunology. 2009;216:8–19. doi: 10.1016/j.jneuroim.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y, Cao X, Aballay A. Whole-Animal Chemical Screen Identifies Colistin as a New Immunomodulator That Targets Conserved Pathways. mBio. 2014;5 doi: 10.1128/mBio.01235-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A. Innate Immunity in Caenorhabditis elegans Is Regulated by Neurons Expressing NPR-1/GPCR. Science. 2008;322:460–464. doi: 10.1126/science.1163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meisel JD, Kim DH. Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends in Immunology. 2014;35:465–470. doi: 10.1016/j.it.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Bolz DD, Tenor JL, Aballay A. A conserved PMK-1/p38 MAPK is required in caenorhabditis elegans tissue-specific immune response to Yersinia pestis infection. J Biol Chem. 2010;285:10832–10840. doi: 10.1074/jbc.M109.091629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes & Development. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Hoeven R, McCallum KC, Cruz MR, Garsin DA. Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in C. elegans. PLoS Pathog. 2011;7:e1002453. doi: 10.1371/journal.ppat.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugiura M, Fuke S, Suo S, Sasagawa N, Van Tol HHM, Ishiura S. Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans. Journal of Neurochemistry. 2005;94:1146–1157. doi: 10.1111/j.1471-4159.2005.03268.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.