Abstract
Given the rising costs of imaging, there is increasing pressure to provide evidence for direct additive impact on clinical care. Appropriate use criteria (AUC) were developed to optimize test-patient selection, and are increasingly used by payers to assess reimbursement. However, these criteria were created by expert consensus with limited systematic validation. The aims of this study were therefore to determine: 1) rates of active clinical change resulting from stress cardiovascular magnetic resonance (CMR) imaging; and 2) whether the AUC can predict these changes. We prospectively enrolled 350 consecutive outpatients referred for stress CMR. Categories of “active changes in clinical care” due to stress CMR were pre-defined. Appropriateness was classified according to the 2013 AUC. Multivariable logistic regression analysis was used to identify factors independently associated with active change. Overall, stress CMR led to an active change in clinical care in about 70% of patients. Rates of change in clinical care did not vary significantly across AUC categories (p=0.767). In a multivariable model adjusting for clinical variables and AUC, only ischemia (OR 6.896, 95% CI 2.637–18.032, p<0.001), known CAD (OR 0.300, 95% CI 0.161–0.559, p<0.001), and age (OR 0.977, 95% CI 0.954–1.000, p=0.050) independently predicted significant clinical change. In conclusion, stress CMR made a significant impact on clinical management, resulting in active change in clinical care in about 70% of patients. AUC categories were not an independent predictor of clinical change. Clinical change was independently associated with presence of ischemia, absence of known CAD, and younger age.
Keywords: Cardiovascular magnetic resonance imaging, stress testing, appropriate use criteria
INTRODUCTION
The appropriate use criteria (AUC) for stress cardiac-magnetic-resonance (CMR) have recently been published as part of a multimodality approach to detection and risk assessment of stable ischemic heart disease1. These criteria were created by expert consensus with limited systematic validation2–10 – particularly for CMR. We recently reported downstream utilization rates of angiography and revascularization procedures after stress-CMR, based on the most recent AUC11. However, stress CMR routinely provides significant information beyond ischemia assessment12, 13. We therefore hypothesized that the overall clinical impact of stress-CMR may extend beyond just angiography and revascularization procedures to other aspects of patient management and care14. Moreover, given that the purpose of the AUC is to optimize test-patient selection, one might expect it to predict active change in clinical management resulting from the test15. The aims of this study were therefore: 1) to determine overall rates of active clinical change resulting from stress CMR in the outpatient setting; and 2) to determine whether the AUC can predict these rates of active change.
METHODS
350 consecutive outpatients referred for CMR stress testing were prospectively enrolled in a single academic medical center. Patients were excluded if they had metallic implants incompatible with CMR, glomerular filtration rate < 30 ml/min, high degree atrio-ventricular block, severe active wheezing from asthma or severe claustrophobia. Subjects were asked to abstain from caffeine-containing products for at least 12 h prior to the test. Information on baseline demographic variables and prior laboratory testing was obtained from patient interviews and the electronic medical record. Patients gave informed written consent for the protocol, which was approved by the local institutional review board.
Images were acquired on a 3 T scanner (Philips Achieva, Philips Medical Systems, Best, the Netherlands) using a six-element phased-array receiver coil as previously described16. Steady-state free-precession cine images were acquired in multiple short-axis and three long-axis views (repetition time, 3.0 ms; echo time, 1.5 ms; flip angle, 40°; slice thickness 6 mm).
The patient table was then partially pulled outside the scanner bore to allow direct observation of the patient and full access. A 0.4 mg bolus of regadenoson (Lexiscan, Astellas Pharma Inc) was infused under continuous electrocardiography and blood pressure monitoring. Approximately 1 min after regadenoson administration, the perfusion sequence was applied and Gadolinium contrast (0.075 mmol/kg gadoteridol, Bracco Diagnostics) followed by a saline flush (30 ml) was infused (4.5 ml/s) via an antecubital vein. On the console, the perfusion images were observed as they were acquired, with breath-holding starting from the appearance of contrast in the right ventricular cavity. Imaging was completed 10 to 15 s after the gadolinium bolus had transited the left ventricular myocardium. Perfusion images consisted of three to four short-axis slices obtained every heartbeat with a saturation-recovery, gradient-echo sequence (repetition time 2.8 ms; echo time 1.1 ms; flip angle 20°; voxel size, 2.5 × 2.5 × 8 mm). Aminophylline (100 mg IV) was administered immediately after stress perfusion imaging16. Rest perfusion images were acquired 15 min after stress imaging with an additional contrast bolus (0.075 mmol/kg gadoteridol) using identical sequence parameters. Five minutes after rest perfusion, late gadolinium enhancement (LGE) imaging was performed with a 2D segmented gradient echo phase-sensitive inversion-recovery sequence in the identical views as cine-CMR. Inversion delay times were typically 280 to 360 ms. Perfusion and LGE images were visually interpreted by standard methods12.
Two general cardiologists reviewed all clinical information dated before the CMR stress test. These reviewers were blinded to the results of the CMR and to the clinical course subsequent to the test. The CMR stress tests were classified as “appropriate’, “maybe appropriate” or “rarely appropriate” as defined by the 2013 AUC1. A third blinded independent physician adjudicated any discrepancy between the interpreters.
Two general cardiologists blinded to AUC classification independently assessed the clinical impact of each stress CMR by review of the electronic medical records through to the next outpatient visit with the ordering provider. If referral for coronary angiography was made then occurrence of revascularization was noted. Clinical impact was defined a priori in one of the following two mutually exclusive categories: 1) active change in care and 2) no change in care. Categories of active change included referral to coronary angiography, revascularization, pre-operative clearance, medication change, subspecialty referral, ordering of additional diagnostic testing, and discharge from cardiology clinic. Categorization strictly required the presence of a statement by the referring physician in the follow-up clinical note - stating that the change (e.g. discharge from clinic or medication change) was initiated as a result of the stress CMR results. Patients could be included in more than one category of the active change group.
Normally distributed data were expressed as mean ± SD. Continuous variables were compared by the Student’s t-test or Wilcoxon rank-sum (depending on data normality). Comparisons of discrete variables were made using the chi-square test; Fisher’s exact test was used when the assumptions of the chi-square test were not met. To identify which clinical indices were associated with active clinical change, we performed univariable (unadjusted) logistic regression analysis to estimate the unadjusted odds ratios (OR) and the 95% confidence intervals (CIs) for baseline clinical variables and AUC categorization. For the multivariable model, covariates were chosen on the basis of established clinical risk factors as well as significant univariate predictors (at p<0.10) from the list of baseline characteristics. A p value of <0.05 was considered statistically significant.
RESULTS
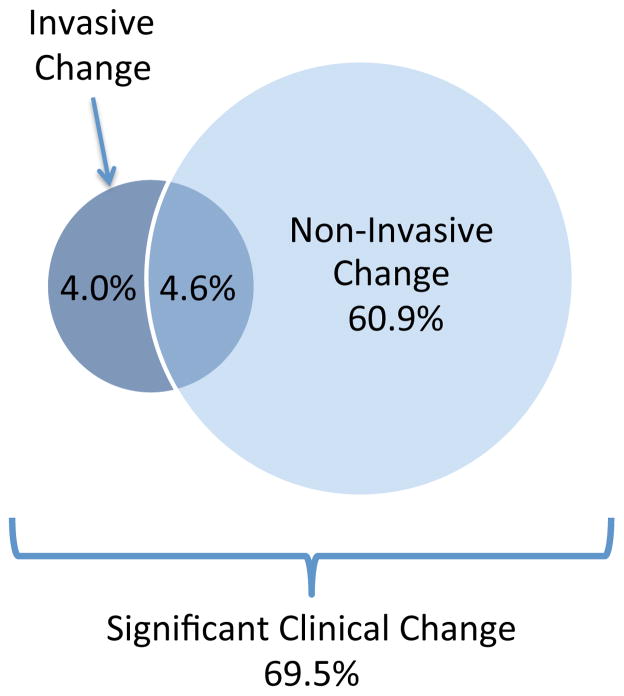
Table 1 summarizes baseline patient characteristics. In the overall cohort, 243 (69.5%) of stress CMRs resulted in an active change in care, and 107 (30.5%) led to no change (Table 2 and Figure 1). The most common active changes were discharge from cardiology clinic (21.1%) or medication change (18.3%) (Table 2 and 3). The majority of active changes were non-invasive (65.5%) as opposed to invasive (8.6%) in nature (Figure 1). A significant minority (4.6%) underwent both a non-invasive and invasive change in management.
Table 1.
Clinical Baseline Characteristics of Enrolled Patients. Values are expressed as mean (±SD) or number (percentage).
| CHARACTERISTICS | Total (N=350) |
|---|---|
| Age (years) | 59 (±13.7) |
| Body Mass Index (kg/m2) | 30.6 (±5.9) |
| Male | 46.3% |
| Diabetes Mellitus | 34.9% |
| Hyperlipidemia | 53.4% |
| Smoker | 18.9% |
| Hypertension | 74.9% |
| Known Coronary Artery Disease | 31.4% |
| Left Ventricular Ejection Fraction | 59 (±11.5) |
| Late Gadolinium Enhancement present | 20.3% |
| Ischemia present | 13.7% |
Table 2. Breakdown of categories of clinical change.
Please note that patients could have more than one category of clinical change.
| CATEGORY | DEFINITION | N=350 (%) |
|---|---|---|
| Active Change in Clinical Care | Angiography with Revascularization | 19 (5.4%) |
| Angiography without Revascularization | 27 (7.7%) | |
| Preoperative Clearance | 39 (11.1%) | |
| Medication Changes | 64 (18.3%) | |
| Subspecialty Consultation | 33 (9.4%) | |
| Additional Diagnostic Test Ordered | 32 (9.1%) | |
| Discharge from Cardiology Clinic | 74 (21.1%) | |
| No Active Change in Clinical Care | Continuation of pre-CMR Care | 107 (30.5%) |
Figure 1. Clinical Impact of CMR.
On the basis of CMR findings, 65.5% of patients had a non-invasive change in management and 8.6% of patients had an invasive change. In 4.6% of patients CMR resulted in both a non-invasive and invasive change in management. In total, CMR had a significant clinical impact on 69.5% of patients.
Table 3.
Medication changes occurring as a result of stress CMR results.
| DRUG CLASS | INITIATION or INCREASE | DISCONTINUATION or DECREASE |
|---|---|---|
| Beta-Blocker | 17 | 3 |
| ACE inhibitor/Angiotensin Receptor Blocker | 14 | 3 |
| Cholesterol Lowering | 13 | 0 |
| Antiplatelet | 12 | 5 |
| Diuretic | 8 | 2 |
| Nitrate | 6 | 1 |
| Calcium Channel Blocker | 3 | 1 |
| Hydralazine | 2 | 0 |
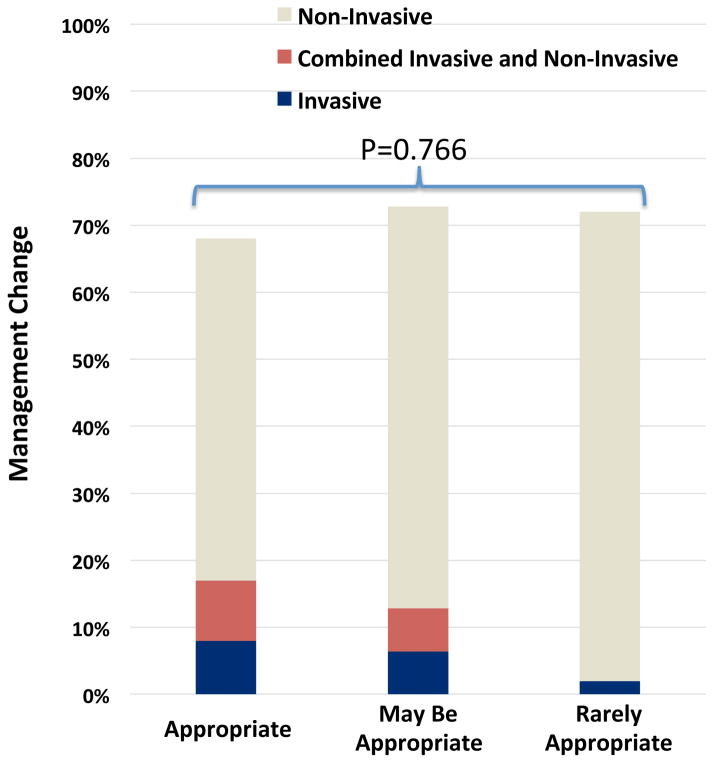
Based on the 2013 AUC, 52% of stress CMRs were classified as appropriate, 36% as maybe appropriate, and 12% as rarely appropriate. The most common rarely appropriate categories in this study are shown in table 4. The overall rates of active clinical change were similar between studies classified as appropriate (68%), may be appropriate (72%), and rarely appropriate (72%)(p=0.766) (Figure 2). However, there were significantly more invasive changes in the appropriate group compared with the rarely appropriate group (16.5% vs 2.3%, p=0.015%).
Table 4.
Most common rarely appropriate classifications.
| AUC DESCRIPTION | N |
|---|---|
| Symptomatic with low pre-test probability of CAD and interpretable ECG and able to exercise | 23 |
| Follow up testing (> 90 Days) with asymptomatic or stable symptoms with an abnormal prior stress imaging study < 2 years ago | 3 |
| Follow up testing (> 90 Days) with asymptomatic or stable symptoms with a normal prior stress imaging study or non-obstructive CAD on angiogram and low global CAD risk | 3 |
Figure 2. Clinical change categorized by AUC.
The overall rates of active clinical change were similar between studies classified as appropriate (68%), may be appropriate (72%), and rarely appropriate (72%)(p=0.766). However, there were significantly more invasive changes in the appropriate group compared with the rarely appropriate group (16.5% vs 2.3%, p=0.015%).
Presence of ischemia (OR 3.517, 95% CI 1.447–8.550, p=0.006), known coronary artery disease (OR 0.374, 95% CI 0.232–0.603, p<0.001), hyperlipidemia (OR 0.520, 95% CI 0.325–0.831, p=0.006), and age (OR 0.976, 95% CI 0.959–0.995, p=0.010) were significant univariable predictors of significant clinical impact (Table 5). In a multivariable model adjusting for clinical variables and AUC, only ischemia (OR 6.896, 95% CI 2.637–18.032, p<0.001), known coronary artery disease (OR 0.300, 95% CI 0.161–0.559, p<0.001), and age (OR 0.977, 95% CI 0.954–1.000, p=0.050) independently predicted significant clinical change (Table 5). AUC categories were not an independent predictor of clinical change (OR 0.936, 95% CI 0.637–1.376, p=0.736). AUC were still not an independent predictor of clinical change even if surgical clearance was excluded as a category of change (OR 0.891, 95% CI 0.689–1.153, p=0.380).
Table 5.
Univariable and multivariable predictors of clinical change.
| VARIABLES | Univariable | Multivariable | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Age | 0.976 (0.959–0.994) | 0.010 | 0.977 (0.954–1.000) | 0.050 |
| Body Mass Index | 1.019 (0.980–1.060) | 0.348 | 1.004 (0.959–1.051) | 0.858 |
| Male | 1.016 (0.932–1.109) | 0.713 | 1.004 (0.863–1.170) | 0.950 |
| Diabetes Mellitus | 1.147 (0.709–1.857) | 0.576 | 1.667 (0.923–3.011) | 0.090 |
| Hyperlipidemia | 0.520 (0.325–0.831) | 0.006 | 0.600 (0.338–1.067) | 0.082 |
| Smoking | 0.854 (0.482–1.513) | 0.589 | 0.901 (0.482–1.681) | 0.742 |
| Hypertension | 0.937 (0.553–1.588) | 0.809 | 1.562 (0.794–3.070) | 0.196 |
| Known Coronary Artery Disease | 0.374 (0.232–0.603) | <0.001 | 0.300 (0.161–0.559) | <0.001 |
| Appropriate Use Criteria | 1.168 (0.838–1.629) | 0.360 | 0.936 (0.637–1.376) | 0.736 |
| Left Ventricular Ejection Fraction | 0.993 (0.973–1.013) | 0.512 | 0.996 (0.973–1.019) | 0.742 |
| Late Gadolinium Enhancement | 1.661 (0.965–2.860) | 0.067 | 1.268 (0.653–2.465) | 0.483 |
| Ischemia | 3.517 (1.447–8.550) | 0.006 | 6.896 (2.637–18.032) | <0.001 |
DISCUSSION
In this study we have shown that stress CMR made a significant impact on clinical management, resulting in active change in clinical care in about 70% of patients. However, overall rates of change were similar across AUC categories (p=0.766), highlighting the current limitations of the AUC. To the best of our knowledge this is the first study to systematically and prospectively assess the overall clinical impact of stress CMR based on the AUC.
Studies classified as appropriate resulted in significantly greater invasive changes than studies classified as rarely appropriate. Despite this, the overall rates of active change in clinical care were similar across AUC categories. This finding does not appear to be driven by surgical clearance of rarely appropriate cases. Since there are 43 rarely appropriate studies and surgical clearance accounted for just 4. Our study suggests that outpatient stress CMR testing can be useful in patient management even when it does not lead to angiography or revascularization and that the current AUC may be too narrowly focused on invasive outcomes 17.
In this study, AUC categories were not an independent predictor of clinical change following stress-CMR. In contrast, clinical change was independently associated with presence of ischemia, absence of known CAD, and younger age. Ischemia as a predictor of clinical change is not surprising. However, it is not clear why absence of known CAD and younger age should be independently associated with clinical change. It is interesting to speculate that perhaps a normal study in these individuals tended to result in exclusion of CAD as a cause for their symptoms and consequent discharge from clinic. However, overall 302 studies were negative for ischemia, and 76.2% of these negative studies for ischemia continued to be followed up in clinic. Thus not all negative studies automatically resulted in clinic discharge.
Although LGE and ejection fraction are well known predictors of adverse cardiovascular outcomes18, 19, our endpoint of “clinical change” is significantly different and not associated with these variables. For example, a patient with known left ventricular dysfunction will likely not have a clinical change on the basis of a depressed CMR derived ejection fraction. Similarly LGE was present most commonly in the setting of patients with known CAD, thus finding LGE typically did not lead the physician to change management.
Future validation of the AUC requires prospective randomized outcome trials, ideally integrating multiple different imaging modalities and strategies. However, such studies are challenging to fund, and are unlikely to be performed. One approach is to compare the prognostic ability of stress CMR across AUC categories. This was undertaken by Doukky et al in a large nuclear study, which suggested that inappropriate use of SPECT was associated with reduced prognostic value20. In those patients whose scans were appropriate or uncertain, abnormal scans were of significant value in predicting major adverse cardiac events. However, in those with inappropriate scans, abnormal studies did not achieve significance in predicting adverse cardiac events.
In this study, the great majority (88%) of tests ordered were classified as appropriate or maybe appropriate. It is possible that growing pre-certification demands by third-party payers may have affected physicians test orderings patterns. The upcoming changes in Medicare reimbursement which have been legislated by the US Congress are likely to significantly increase pressure on physicians in this regard9, 10. Interestingly, a recent meta-analysis systematically reviewed published evidence to identify whether the promulgation of AUC over the last 10 years has led to improvements in the proportion of appropriate cardiac imaging requests21. They found that rates of reported appropriate use in imaging showed improvements for transthoracic echocardiography and coronary CT but not for stress echocardiography, SPECT imaging or transesophageal echocardiography. The authors provided no data for CMR.
The performance of this study at a single US academic medical center limits its generalizability to other practice settings. Differences may exist in adherence to AUC by region, practice type, practice size, clinician experience, and payer mix that cannot be captured by this single-center study. In this study, active change was assigned if an action was taken because of the stress CMR, with no consideration of whether the change was clinically indicated. Therefore, active change in care in our study may not necessarily indicate better care. This study was limited by not providing data regarding clinical outcomes such as hospitalization, heart failure, myocardial infarction or mortality. The inclusion of surgical clearance as a category of change may be criticized, but these patients constitute an important portion of cases referred to most general cardiology clinics. Even if surgical clearance is excluded, 60% (n=209) of patients still had an active clinical change. Similarly discharge from clinic is a major change in patient management and needs to be accounted for. Of note, the AUC were not independent predictors of clinical change even if these two categories were excluded from the analysis[OR=0.814 (0.617–1.072), p=0.143]. Cost-effectiveness was not assessed in this study and needs to be the subject of future studies. Finally, assessment of active change relied on review of the electronic medical records, which may have led to misclassification of impact owing to incomplete documentation.
Acknowledgments
FUNDING SOURCES
Dr Chung was supported by an NIH grant (T32HL072742).
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, Min JK, Patel MR, Rosenbaum L, Shaw LJ, Stainback RF, Allen JM American College of Cardiology Foundation Appropriate Use Criteria Task F. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ, Kramer CM, Lesser JR, Martin ET, Messer JV, Redberg RF, Rubin GD, Rumsfeld JS, Taylor AJ, Weigold WG, Woodard PK, Brindis RG, Hendel RC, Douglas PS, Peterson ED, Wolk MJ, Allen JM, Patel MR American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working G, American College of R, Society of Cardiovascular Computed T, Society for Cardiovascular Magnetic R, American Society of Nuclear C, North American Society for Cardiac I, Society for Cardiovascular A, Interventions and Society of Interventional R. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA American College of Cardiology Foundation Appropriate Use Criteria Task F, American Society of Nuclear C, American College of R, American Heart A, American Society of E, Society of Cardiovascular Computed T, Society for Cardiovascular Magnetic R and Society of Nuclear M. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53:2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 4.American College of Cardiology Foundation Appropriate Use Criteria Task F, American Society of E, American Heart A, American Society of Nuclear C, Heart Failure Society of A, Heart Rhythm S, Society for Cardiovascular A, Interventions, Society of Critical Care M, Society of Cardiovascular Computed T, Society for Cardiovascular Magnetic R. Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Ward RP, Weiner RB. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57:1126–1166. doi: 10.1016/j.jacc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, Rubin GD, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC, Jr American College of Cardiology Foundation Appropriate Use Criteria Task F, Society of Cardiovascular Computed T, American College of R, American Heart A, American Society of E, American Society of Nuclear C, North American Society for Cardiovascular, I, Society for Cardiovascular A Interventions, Society for Cardiovascular Magnetic R. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–1894. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Hendel RC. Utilization management of cardiovascular imaging pre-certification and appropriateness. JACC Cardiovasc Imaging. 2008;1:241–248. doi: 10.1016/j.jcmg.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Iglehart JK. Health insurers and medical-imaging policy--a work in progress. N Engl J Med. 2009;360:1030–1037. doi: 10.1056/NEJMhpr0808703. [DOI] [PubMed] [Google Scholar]
- 8.Shor R. Using Appropriate Use Criteria to Address Pre-Authorization. J Am Coll Cardiol. 2015;66:1300–1302. doi: 10.1016/j.jacc.2015.07.049. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari VA, Whitman B, Blankenship JC, Budoff MJ, Costa M, Weissman NJ, Cerqueira MD, Council ACCI. Cardiovascular imaging payment and reimbursement systems: understanding the past and present in order to guide the future. JACC Cardiovasc Imaging. 2014;7:324–332. doi: 10.1016/j.jcmg.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Soman P, Kelly R. Imaging at the 2014 ACC Legislative Conference: a debrief. JACC Cardiovasc Imaging. 2015;8:118–120. doi: 10.1016/j.jcmg.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 11.McGraw S, Mirza O, Bauml MA, Rangarajan VS, Farzaneh-Far A. Downstream clinical consequences of stress cardiovascular magnetic resonance based on appropriate use criteria. J Cardiovasc Magn Reson. 2015;17:35. doi: 10.1186/s12968-015-0137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klem I, Heitner JF, Shah DJ, Sketch MH, Jr, Behar V, Weinsaft J, Cawley P, Parker M, Elliott M, Judd RM, Kim RJ. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47:1630–1638. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbasi SA, Ertel A, Shah RV, Dandekar V, Chung J, Bhat G, Desai AA, Kwong RY, Farzaneh-Far A. Impact of cardiovascular magnetic resonance on management and clinical decision-making in heart failure patients. J Cardiovasc Magn Reson. 2013;15:89. doi: 10.1186/1532-429X-15-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matulevicius SA, Rohatgi A, Das SR, Price AL, DeLuna A, Reimold SC. Appropriate use and clinical impact of transthoracic echocardiography. JAMA Intern Med. 2013;173:1600–1607. doi: 10.1001/jamainternmed.2013.8972. [DOI] [PubMed] [Google Scholar]
- 16.Dandekar VK, Bauml MA, Ertel AW, Dickens C, Gonzalez RC, Farzaneh-Far A. Assessment of global myocardial perfusion reserve using cardiovascular magnetic resonance of coronary sinus flow at 3 Tesla. J Cardiovasc Magn Reson. 2014;16:24. doi: 10.1186/1532-429X-16-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong W, Eagle KA. Appropriate use criteria in echocardiography: is no change the same as no benefit? JAMA Intern Med. 2013;173:1609–1610. doi: 10.1001/jamainternmed.2013.7273. [DOI] [PubMed] [Google Scholar]
- 18.Kwon DH, Halley CM, Carrigan TP, Zysek V, Popovic ZB, Setser R, Schoenhagen P, Starling RC, Flamm SD, Desai MY. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc Imaging. 2009;2:34–44. doi: 10.1016/j.jcmg.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Klem I, Shah DJ, White RD, Pennell DJ, van Rossum AC, Regenfus M, Sechtem U, Schvartzman PR, Hunold P, Croisille P, Parker M, Judd RM, Kim RJ. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging. 2011;4:610–619. doi: 10.1161/CIRCIMAGING.111.964965. [DOI] [PubMed] [Google Scholar]
- 20.Doukky R, Hayes K, Frogge N, Balakrishnan G, Dontaraju VS, Rangel MO, Golzar Y, Garcia-Sayan E, Hendel RC. Impact of appropriate use on the prognostic value of single-photon emission computed tomography myocardial perfusion imaging. Circulation. 2013;128:1634–1643. doi: 10.1161/CIRCULATIONAHA.113.002744. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca R, Negishi K, Otahal P, Marwick TH. Temporal Changes in Appropriateness of Cardiac Imaging. J Am Coll Cardiol. 2015;65:763–773. doi: 10.1016/j.jacc.2014.11.057. [DOI] [PubMed] [Google Scholar]