Abstract
Low birth weight is associated with cardiovascular disease and its risk factors in adulthood. However, information is limited regarding its impact on heart rate (HR), an established risk factor for cardiovascular disease. This study assessed the hypothesis that birth weight is associated with resting HR at different ages. The study sample consisted of 6,282 black and white participants enrolled in the Bogalusa Heart Study, aged 4 to 52 years with a mean age of 19.4 years. Resting HR data were available in 2,344 children (4–11 years old), 1,622 adolescents (12–19 years old) and 2,316 adults (20–52 years old). Birth certificate records, including information on birth weight and gestational age, were obtained from the Louisiana State Office of Public Health. HR showed a significant decreasing trend with increasing age, with blacks having a lower slope than whites. In multivariable linear regression analyses, adjusted for age, race, sex, BMI and gestational age, the association between lower birth weight (kg) and increased HR (beats/min) was significant in adults (regression coefficient, β= −1.21, p=0.006 in adults), but not significant in children (β= −0.31, p=0.461) and adolescents (β= −0.72, p=0.157). The association did not differ significantly between races. The birth weight-HR association did not change markedly in the models without adjustment for BMI. In conclusion, these results suggest that the association of prenatal growth retardation with increased cardiovascular disease risk in later life might be partly through its relationship with resting HR.
Keywords: birth weight, heart rate, age groups, cardiovascular disease, prenatal growth restriction
Introduction
Low birth weight at full term, an indicator of intrauterine growth restriction, has been associated with adult cardiovascular disease and its risk factors1–3. Among traditional risk factors, elevated blood pressure has been shown to be most strongly associated with low birth weight, and the effect size is amplified with increasing age4,5. Increased resting heart rate (HR), another important hemodynamic parameter and well-established independent risk factor for cardiovascular disease6–13, is associated with low birth weight in children14–16 and adults17,18. However, data on the birth weight-HR association are still limited, especially the age-related trend in the strength of the association. The present study aimed to examine the association between birth weight and resting HR in children, adolescents and adults in black and white populations.
Methods
The Bogalusa Heart Study is a series of long-term studies in a semi-rural biracial (65% white and 35% black) community in Bogalusa, Louisiana begun in 1973 by Dr. Gerald Berenson, focusing on the early natural history of cardiovascular disease since childhood. Cross-sectional surveys of children and adolescents aged 4–19 years and surveys of adults aged 20–52 years were conducted for cardiovascular risk factors in 1980–1994 and 1982–2010, respectively. Birth weight records of the participants were obtained in 2005 from the Louisiana State Public Health Office. Exclusion criteria included gestational age<37 weeks or >42 weeks of pregnancy, birth weight >4.5 kg or resting HR>150 beats/min. After exclusion, 2,344 children aged 4–11 years (59% white, and 50% male), 1,622 adolescents aged 12–19 years (57% white, and 55% male) and 2,316 adults aged 20–52 years (67% white, and 45% male) formed the study sample.
All subjects in this study gave informed consent at each examination. For those under 18 years of age, consent of a parent/guardian was obtained. Study protocols were approved by the Institutional Review Board of the Tulane University Health Science Center.
Birth weight information on the Bogalusa Heart Study participants has been obtained from the Louisiana State birth certificates maintained by the Louisiana State Office of Public Health. Information includes birth weight, gestational age, year of birth and parents’ age at birth.
Examinations of children and adults followed the same protocols. Height and weight were measured twice to ± 0.1 cm and to ± 0.1 kg, respectively. Body mass index (BMI, weight in kilograms divided by the square of the height in meters) was used as a measure of overall adiposity. Resting HR was counted at the radial pulse in a relaxed, sitting position. After at least 5 minutes resting, three counts were made by 2 trained research members, and average of the 6 values was used.
Analysis of covariance was performed using general linear models (GLM) to test differences in continuous variables in blacks versus whites and in men versus women. The differences in categorical variables were tested by a chi-square test. The rate of fetal growth was calculated as birth weight (kg)/gestational age (week) for each individual by race-sex groups, and then multiplied by the mean value of gestational age of the sample to convert into the original scale. Thus, the adjusted birth weight represented the rate of growth independent of the length of gestation period and was used in all subsequent analyses. In order to eliminate the bias due to correlations between repeated measurements, for individuals who had multiple measurements of HR in the same age period, the first measurement in childhood and the last in adolescence and adulthood were selected for analysis. The relationship between HR and birth weight was examined by multiple linear regression models, adjusting for age, sex and BMI by race groups and in the total sample (with additional adjustment for race). For categorical analyses, quartiles of gestational age-adjusted birth weight were defined using cutoff points in race-sex groups. Covariates-adjusted mean values of HR were calculated by GLM and used for trend analysis of HR by quartiles of birth weight. Statistical Analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Table 1 shows mean values and standard deviation of study variables by race, sex and age groups. Whites and women had faster HR than blacks and men, respectively, in all age groups, except that there was no race difference in adults in both men and women. Whites had greater birth weight (both adjusted and unadjusted) and gestational age than blacks; men had greater birth weight than women.
Table 1.
Mean levels (± SD) of study variables by race, sex and age groups
| Variable | White
|
Black
|
Race difference
|
|||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Childhood (4–11 years) | N=675 | N=698 | N=473 | N=498 | ||
| Age (year) | 9.0±1.7 | 8.9±1.7 | 8.8±1.9 | 8.7±1.9 | 0.020 | 0.111 |
| BMI (kg/m2) | 18.0±3.4 | 17.9±3.6 | 17.7±3.6 | 17.8±3.6 | 0.391 | 0.763 |
| HR (beats/min) | 86.4±10.3 | 89.3±10.3** | 82.0±10.1 | 85.7±10.5** | <0.001 | <0.001 |
|
|
||||||
| Adolescence (12–19 years) | N=511 | N=418 | N=383 | N=310 | ||
| Age (year) | 14.7±2.0 | 14.8±2.0 | 15.7±2.2 | 15.2±2.1** | <0.001 | 0.010 |
| BMI (kg/m2) | 22.5±5.0 | 22.2±4.8 | 22.6±5.0 | 23.1±5.4 | 0.279 | 0.052 |
| HR (beats/min) | 78.0±10.7 | 81.7±11.2** | 71.8±9.7 | 78.9±10.4** | <0.001 | 0.004 |
|
|
||||||
| Adulthood (20–52 years) | N=714 | N=831 | N=322 | N=449 | ||
| Age (year) | 33.9±9.1 | 33.3±9.0 | 32.3±9.4 | 32.4±9.3 | 0.007 | 0.079 |
| BMI (kg/m2) | 28.3±6.1 | 27.2±7.6** | 27.2±6.7 | 29.8±8.3** | 0.065 | <0.001 |
| HR (beats/min) | 69.0±9.1 | 73.5±9.8** | 68.0±9.6 | 73.0±9.6** | 0.147 | 0.253 |
|
|
||||||
| Birth registry information | N=1900 | N=1947 | N=1178 | N=1257 | ||
| Gestational age (week) | 39.80±0.82 | 39.81±0.89 | 39.66±0.88 | 39.69±0.90 | <0.001 | <0.001 |
| Birth weight (kg) | 3.46±0.48 | 3.33±0.47** | 3.19±0.51 | 3.09±0.46** | <0.001 | <0.001 |
| Birth weight (kg)a | 3.43±0.47 | 3.31±0.46** | 3.17±0.50 | 3.07±0.45** | <0.001 | <0.001 |
Adjusted for gestational age
BMI=body mass index; HR=heart rate
Sex difference within racial groups:
p<0.05,
p<0.01
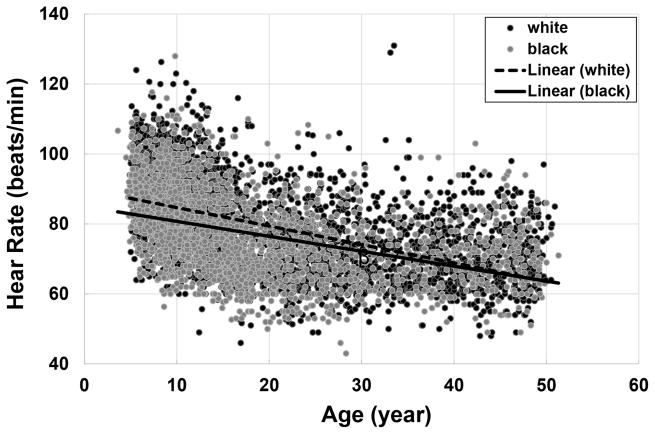
Figure 1 illustrates the relationship between age and HR by race groups. HR decreases significantly with increasing age in both blacks and whites. Despite the higher childhood HR in whites than in blacks, it decreased faster in whites as age increased, with p<0.001 for difference in slopes between race groups.
Figure 1.
Correlation between age and heart rate in whites and blacks
β=−0.528, p<0.001 in whites; β=−0.427, p<0.001 in blacks; p<0.001 for difference in slopes
Birth weight tended to be inversely associated with HR across all race-age groups except for black children, after adjusting for covariates (including BMI) in multiple regression models (Table 2). The regression coefficients did not differ significantly in blacks and whites (p=0.479 in children, p=0.604 in adolescents, and p=0.958 in adults for differences in regression coefficients between blacks and whites). In the total sample, only adults showed significant association between birth weight and HR, although children and adolesents showed similar trends (inverse associations). The regression coefficients in Table 2 had only slight changes without BMI adjustment (Table 3), which is consistent with the fact that HR and BMI were not significantly correlated in children (r=0.028, p=0.394), adolescents (r= −0.021, p=0.397) and adults (r=0.023, p=0.261) with no black-white differences.
Table 2.
Regression of heart rate on birth weight, adjusted for covariates, in black and white children, adolescents, and adults.
| White
|
Black
|
Total
|
||||
|---|---|---|---|---|---|---|
| β | P Value | β | P Value | β | P Value | |
| Childhood (n=2344) | ||||||
| Black race | −4.22 | <0.001 | ||||
| Age | −1.19 | <0.001 | −1.20 | <0.001 | −1.19 | <0.001 |
| Female sex | 2.72 | <0.001 | 3.65 | <0.001 | 3.11 | <0.001 |
| BMI | 0.06 | 0.477 | 0.06 | 0.549 | 0.06 | 0.339 |
| Birth weight a | −0.60 | 0.279 | 0.10 | 0.881 | −0.31 | 0.461 |
| Adolescence (n=1622) | ||||||
| Black race | −3.80 | <0.001 | ||||
| Age | −1.60 | <0.001 | −1.33 | <0.001 | −1.50 | 0.119 |
| Female sex | 3.64 | <0.001 | 6.34 | <0.001 | 4.76 | <0.001 |
| BMI | −0.06 | 0.378 | −0.02 | 0.831 | −0.03 | 0.509 |
| Birth weight a | −0.63 | 0.356 | −0.85 | 0.256 | −0.72 | 0.157 |
| Adulthood (n=2316) | ||||||
| Black race | −1.13 | 0.009 | ||||
| Age | −0.12 | <0.001 | −0.03 | 0.372 | −0.09 | <0.001 |
| Female sex | 4.29 | <0.001 | 4.85 | <0.001 | 4.50 | <0.001 |
| BMI | 0.03 | 0.430 | 0.05 | 0.333 | 0.04 | 0.180 |
| Birth weight a | −1.19 | 0.028 | −1.17 | 0.131 | −1.21 | 0.006 |
Gestational age-adjusted birth weight
β=regression coefficient; BMI=body mass index
Table 3.
Regression of heart rate on birth weight, adjusted for covariates, in black and white children, adolescents, and adults.
| White
|
Black
|
Total
|
||||
|---|---|---|---|---|---|---|
| β | P Value | β | P Value | β | P Value | |
| Childhood (n=2344) | ||||||
| Black race | −4.21 | <0.001 | ||||
| Age | −1.16 | <0.001 | −1.17 | <0.001 | −1.16 | <0.001 |
| Female sex | 2.73 | <0.001 | 3.67 | <0.001 | 3.11 | <0.001 |
| Birth weight a | −0.55 | 0.320 | 0.17 | 0.792 | −0.25 | 0.552 |
| Adolescence (n=1622) | ||||||
| Black race | −3.81 | <0.001 | ||||
| Age | −1.63 | <0.001 | −1.34 | <0.001 | −1.51 | <0.001 |
| Female sex | 3.65 | <0.001 | 6.32 | <0.001 | 4.75 | <0.001 |
| Birth weight a | −0.72 | 0.292 | −0.87 | 0.243 | −0.76 | 0.130 |
| Adulthood (n=2316) | ||||||
| Black race | −1.07 | 0.013 | ||||
| Age | −0.11 | <0.001 | −0.02 | 0.527 | −0.08 | <0.001 |
| Female sex | 4.27 | <0.001 | 4.97 | <0.001 | 4.51 | <0.001 |
| Birth weight a | −1.17 | 0.031 | −1.06 | 0.164 | −1.16 | 0.008 |
Gestational age-adjusted birth weight.
β=regression coefficient
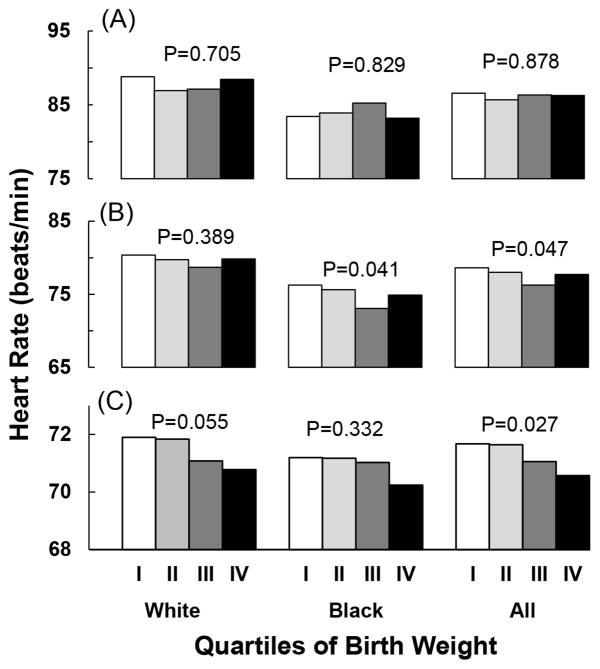
Figure 2 shows the relation between covariates-adjusted mean values of HR and quartiles of gestational age-adjusted birth weight by age group. The covariates included age, sex, BMI and race (for the combined sample). HR significantly decreased with increasing quartiles of birth weight in the combined sample of adults (p for trend =0.027). Similar but not significant trends were noted in white adults (p for trend=0.055) and black adults (p for trend=0.332). Significant decreasing trends in HR with increasing quartiles of birth weight were noted in adolescents in blacks and the total sample.
Figure 2.
Covariates-adjusted mean values of heart rate by race- and sex-specific quartiles of gestational age-adjusted birth weight in children (panel A), adolescents (panel B), and adults (panel C). Covariates included age, sex, race (for the total sample) and BMI
Discussion
Elevated resting HR is a well-known independent risk factor for cardiovascular and all-cause mortality6–13. Low birth weight, an indicator of intrauterine growth restriction, has been demonstrated to be associated with increased resting HR in children14–16 and adults17,18 in previous epidemiologic studies. Among 2,648 African school children aged 5–16 years in Congo, birth weight was significantly, inversely related to HR in both girls and boys15. The mean resting HR was significantly higher in the lower tertile of birth weight than in the higher tertile of birth weight (81.7 vs. 78.5 beats/min, p=0.028) in 573 Japanese school boys at age of 12–13 years14. Mean values of HR were significantly higher in children with preterm birth (76 beats/min) and small birth size for gestational age (79 beats/min) when compared with controls (70 beats/min) in 105 Swedish children with a mean age of 9.6 years16. In contrast, birth weight showed an inverse but nonsignificant association with HR in 228 adolescent twin pairs in the Netherlands19. In the current study of 6,282 participants enrolled in the Bogalusa Heart Study, similar negative trends in the association between birth weight and HR were noted in black and white children and adolescents; however, the p-values for the regression coefficients did not reach a significance level. Of interest, lower birth weight was found to be significantly associated with higher resting HR in adults. To the best of our knowledge, only 2 studies have, thus far, reported a significant association between low birth weight and high HR in adults in the European populations17,18. The inverse birth weight-HR association in adults of the present study is consistent with previous findings, suggesting a possible mechanism linking intrauterine growth restriction and increased cardiovascular disease risk in later life.
In our previous report from the Bogalusa Heart Study, the magnitude of birth weight-blood pressure association was found to be amplified with increasing age from childhood to adulthood5. In the current birth weight-HR association analysis, we also found increasing trends in the effect size of birth weight on HR were substantially similar to the trends in the birth weight-blood pressure association. HR and blood pressure are both important hemodynamic parameters which are highly correlated with each other in children and adults16–18,20. Detailed analyses of the age-related strength of the association of birth weight with blood pressure and HR are suggested in future studies in terms of the conjoint trait or bivariate analysis models during different growth periods.
HR has long been regarded as a symbol of sympathetic nervous system (SNS) activity21,22. Previous studies have demonstrated that low birth weight is significantly associated with elevated SNS activity in early and later life16,17,19,23. Therefore, it is generally considered that the inverse association between birth weight and HR is attributed to a large part of increased SNS activity that is initiated in utero and persists into adult life. This concept is also supported by the evidence showing that lower birth weight is significantly associated with both SNS activity markers and HR simultaneously in the same study cohorts of children and adults16,17. The findings from the current and previous studies suggest that the underlying fetal programming mechanisms including abnormalities of SNS activity and cardiovascular control and persisting changes through lifetime might be a potential link between fetal growth restriction, hypertension, HR and cardiovascular morbidity later in life.
Since the “fetal origins” hypothesis was proposed, the problem of overestimation of the association by the statistical adjustment for current body size in testing this hypothesis has been addressed, especially for the birth weight-blood pressure association5,24,25. The adjustment for current weight exaggerated the magnitude of the association when there was a genuine inverse association. This statistical artifact is known as “reversal paradox”25. Our previous report clearly showed that the strong and positive correlation between current BMI and blood pressure resulted in the overestimation of the birth weight-blood pressure association5. In this study, we did not find any marked changes in the association parameters between birth weight and HR in the models with and without adjustment for current BMI (Tables 2 and 3). The reason is that the correlation between current BMI and HR was not significant in children, adolescents and adults in the present study cohort.
This community-based epidemiologic study has certain limitations. First, SNS activity markers were not measured in this cohort. Second, covariates of socioeconomics and lifestyles were not included in the analysis models. Some variables, such as education levels, were not appropriate in children and adolescents; lifestyle variables were not available in childhood; inclusion of lifestyles in adults in the analyses would reduce about a half of the sample. In such a scenario, adjustment for these covariates would lead to a lower statistical power and incomparability of the association parameters across age groups.
Acknowledgments
This study was supported by grants 5R01ES021724 from National Institute of Environmental Health Science. Fu Wang is supported by award number 201406370184 from the State Scholarship Fund organized by the China Scholarship Council which is a non-profit institution affiliated with the Ministry of Education of China. Shengxu Li is partly supported by grant 13SDG14650068 from American Heart Association and grant 1P20GM109036-01A1 from National Institute of General Medical Sciences.
Footnotes
Disclosure(s) Statement
The authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ. Programming the baby. In: Barker DJ, editor. Mothers, babies, and disease later in life. London: BMJ Publishing Group; 1994. pp. 14–36. [Google Scholar]
- 2.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJ, Cruddas AM, Fall CH. Initiation of hypertension in utero and its amplification throughout life. BMJ. 1993;306:24–27. doi: 10.1136/bmj.306.6869.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Srinivasan SR, Berenson GS. Amplification of the association between birthweight and blood pressure with age: the Bogalusa Heart Study. J Hypertens. 2010;28:2046–2052. doi: 10.1097/HJH.0b013e32833cd31f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, Lepper M, Schoenberger JA, Lindberg HA. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112:736–749. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 8.Greenland P, Daviglus ML, Dyer AR, Liu K, Huang CF, Goldberger JJ, Stamler J. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1999;149:853–862. doi: 10.1093/oxfordjournals.aje.a009901. [DOI] [PubMed] [Google Scholar]
- 9.Kristal-Boneh E, Silber H, Harari G, Froom P. The association of resting heart rate with cardiovascular, cancer and all-cause mortality. Eight year follow-up of 3527 male Israeli employees (the CORDIS Study) Eur Heart J. 2000;21:116–124. doi: 10.1053/euhj.1999.1741. [DOI] [PubMed] [Google Scholar]
- 10.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 11.Nauman J, Janszky I, Vatten LJ, Wisloff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011;306:2579–2587. doi: 10.1001/jama.2011.1826. [DOI] [PubMed] [Google Scholar]
- 12.Palatini P, Benetos A, Grassi G, Julius S, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Pessina AC, Ruilope LM, Zanchetti A. Identification and management of the hypertensive patient with elevated heart rate: statement of a European Society of Hypertension Consensus Meeting. J Hypertens. 2006;24:603–610. doi: 10.1097/01.hjh.0000217838.49842.1e. [DOI] [PubMed] [Google Scholar]
- 13.Gillman MW, Kannel WB, Belanger A, D’Agostino RB. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J. 1993;125:1148–1154. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 14.Abe C, Minami J, Ohrui M, Ishimitsu T, Matsuoka H. Lower birth weight is associated with higher resting heart rate during boyhood. Hypertens Res. 2007;30:945–950. doi: 10.1291/hypres.30.945. [DOI] [PubMed] [Google Scholar]
- 15.Longo-Mbenza B, Ngiyulu R, Bayekula M, Vita EK, Nkiabungu FB, Seghers KV, Luila EL, Mandundu FM, Manzanza M. Low birth weight and risk of hypertension in African school children. J Cardiovasc Risk. 1999;6:311–314. doi: 10.1177/204748739900600507. [DOI] [PubMed] [Google Scholar]
- 16.Johansson S, Norman M, Legnevall L, Dalmaz Y, Lagercrantz H, Vanpee M. Increased catecholamines and heart rate in children with low birth weight: perinatal contributions to sympathoadrenal overactivity. J Intern Med. 2007;261:480–487. doi: 10.1111/j.1365-2796.2007.01776.x. [DOI] [PubMed] [Google Scholar]
- 17.Phillips DI, Barker DJ. Association between low birthweight and high resting pulse in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome? Diabet Med. 1997;14:673–677. doi: 10.1002/(SICI)1096-9136(199708)14:8<673::AID-DIA458>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Kerkhof GF, Breukhoven PE, Leunissen RW, Willemsen RH, Hokken-Koelega AC. Does preterm birth influence cardiovascular risk in early adulthood? J Pediatr. 2012;161:390–396. doi: 10.1016/j.jpeds.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 19.IJzerman RG, Stehouwer CD, de Geus EJ, van Weissenbruch MM, Delemarre-van de Waal HA, Boomsma DI. Low birth weight is associated with increased sympathetic activity: dependence on genetic factors. Circulation. 2003;108:566–571. doi: 10.1161/01.CIR.0000081778.35370.1B. [DOI] [PubMed] [Google Scholar]
- 20.Berenson GS, Patel DA, Wang H, Srinivasan SR, Chen W. Pressure-heart rate product changes from childhood to adulthood in a biracial population - a crossover phenomenon: the Bogalusa Heart Study. J Am Soc Hypertens. 2008;2:80–87. doi: 10.1016/j.jash.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–982. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- 22.Grassi G, Vailati S, Bertinieri G, Seravalle G, Stella ML, Dell’Oro R, Mancia G. Heart rate as marker of sympathetic activity. J Hypertens. 1998;16:1635–1639. doi: 10.1097/00004872-199816110-00010. [DOI] [PubMed] [Google Scholar]
- 23.Boguszewski MC, Johannsson G, Fortes LC, Sverrisdottir YB. Low birth size and final height predict high sympathetic nerve activity in adulthood. J Hypertens. 2004;22:1157–1163. doi: 10.1097/00004872-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 25.Tu YK, West R, Ellison GT, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: the “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;161:27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]