SUMMARY
Neuromodulators influence the activities of collections of neurons, and have profound impacts on animal behavior. Male courtship drive is complex, and is subject to neuromodulatory control. Using the fruit fly, Drosophila melanogaster, we identified neurons in the brain (inferior posterior slope; IPS) that impact courtship drive, and were controlled by tyramine—a biogenic amine related to dopamine, whose roles in most animals are enigmatic. We knocked out a tyramine-specific receptor, TyrR, which was expressed in IPS neurons. Loss of TyrR led to a striking elevation in courtship activity between males. This effect occurred only in the absence of females, as the TyrRGal4 mutant males exhibited a wild-type preference for females. Artificial hyperactivation of IPS neurons caused a large increase in male-male courtship, while suppression of IPS activity decreased male-female courtship. We conclude that TyrR is a receptor for tyramine, and suggest that it serves to curb high levels of courtship activity through functioning as an inhibitory neuromodulator.
INTRODUCTION
Neurotransmitters and neuromodulators that are derived from tyrosine are evolutionarily conserved, and are critical mediators of animal behavior. Dopamine and the related catecholamine, norepinephrine, are synthesized through a simple pathway that begins with conversion of tyrosine into DOPA (Figure 1A). Tyrosine is also a substrate for production of octopamine, which is structurally similar to norepinephrine. Octopamine is produced in both mammals and invertebrates [1], although its role as a neuromodulator and neurotransmitter is best characterized in insects, where it promotes an array of behaviors. These range from male aggression [2, 3] to learning and memory in flies [4], female post-mating behaviors [5, 6], sleep [7], foraging [8] and others.
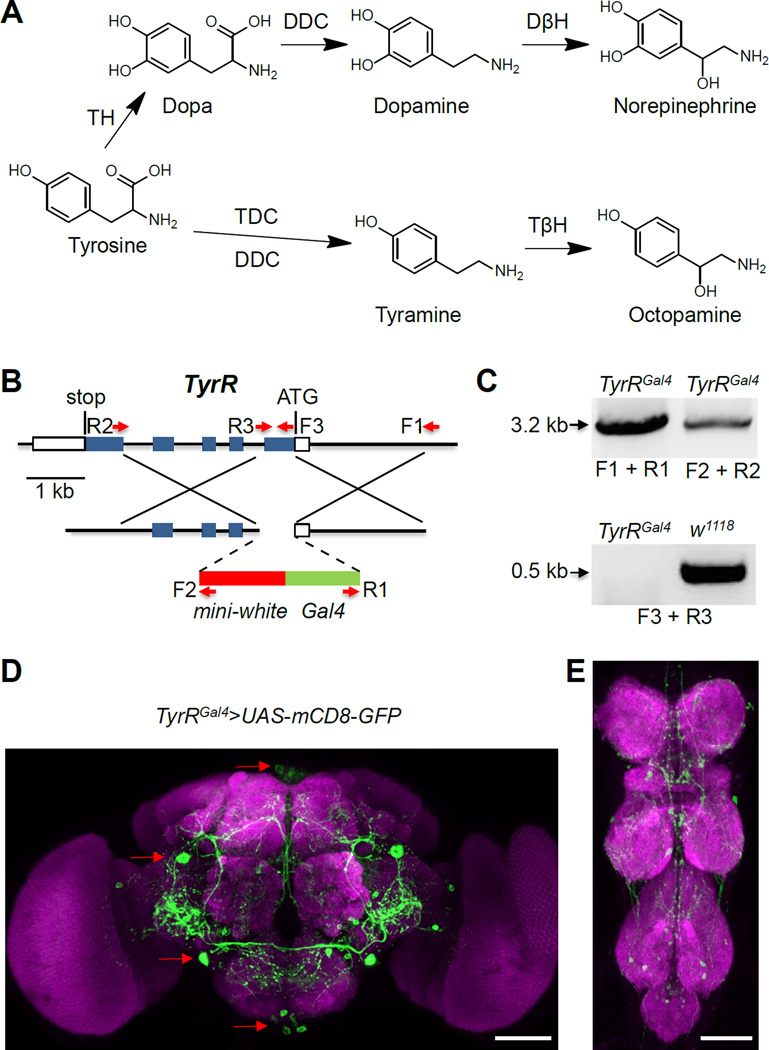
Figure 1. Synthesis of tyramine and knockout and expression of TyrR.
(A) Biosynthetic pathway of biogenic amines derived from tyrosine. DβH, dopamine β-hydroxylase; DDC, Dopa decarboxylase; TDC, tyrosine decarboxylase; TβH, tyramine β-hydroxylase; TH, tyrosine hydroxylase. Drosophila DDC is also capable of converting tyrosine to tyramine in vitro [49].
(B) Schematic of the TyrR locus (90C2-90C3) and the targeting construct used to generate the TyrRGal4 allele. The boxes represent exons and the coding regions are shown in blue. Indicated are the translational start codon (ATG), stop codon and the forward (F) and reverse (R) PCR primers.
(C) PCR confirmation of the deletion in TyrRGal4 and the replacement of the mini-white and Gal4 in TyrRGal4. We prepared genomic DNA and performed PCR using the primer pairs indicated in (B).
(D) UAS-mCD8-GFP expression driven under the control of the TyrRGal4/+ in the brain. The red arrows indicate the location of some of the neurons expressing the TyrR reporter. SMP, superior medial protocerebrum; PLP, posteriorlateral protocerebrum; IPS, inferior posterior slope; GNG, gnathal ganglia. MB, mushroom body; AL, antennal lobe; OL, optical lobe.
(E) TyrR-reporter expression in the ventral nerve cord. TG, thoracic ganglion; AG, abdominal ganglion.
The scale bars represent 50 µM.
The biosynthesis of octopamine is initiated by decarboxylation of tyrosine to produce tyramine, which is present at low levels in many mammalian tissues, including the brain [9]. Due to its concentration in trace amounts, it has long been thought to serve primarily as a biosynthetic precursor of octopamine, and not as a neuroactive chemical in its own right. Nevertheless, the discovery of a specific family of G-protein coupled receptors (GPCRs), some members of which are activated primarily by tyramine, raises the possibility that tyramine may function independently as a neuromodulator [10]. Indeed, the concentration of tyramine is altered in a variety of human neurological disorders, including schizophrenia, Parkinson’s disease, attention deficit hyperactivity disorder (ADHD), Tourette syndrome, and phenylketonuria [11]. Nevertheless, the functions of tyramine are enigmatic, especially in mammals.
The brains of the fruit fly harbor populations of neurons that produce tyramine, and not octopamine [12, 13], arguing against a trivial role for tyramine exclusively as a metabolic intermediate. A few experiments in insects address this possibility. For example, a Drosophila mutation affecting a receptor for both octopamine and tyramine (Oct-TyrR) results in reduced odor avoidance [14]. However, it is unclear whether the phenotype reflects a role for octopamine or tyramine since Oct-TyrR is activated by tyramine and octopamine with similar potency. Application of tyramine to Drosophila tissue, or injections of tyramine into the blowfly or moth produce a variety of physiological responses [15, 16]. However, the tyramine might be metabolically converted to other biogenic amines that elicit function. At this time, there is no clear genetic evidence indicating a role for tyramine as an independent neuromodulator in Drosophila. Despite the presence of tyramine in the brains of animals that include mammals and insects, C. elegans is the only organism for which genetic evidence supports a role of tyramine as a neuromodulator [17–20].
The Drosophila genome encodes multiple GPCRs that are activated by biogenic amines, one of which (TyrR) is activated specifically by tyramine but not by other biogenic amines tested, including octopamine, dopamine, serotonin and histamine [21]. Here, we generated a null mutation in TyrR and found that the mutant males displayed a profound increase in male-male courtship, but no change in gender preference. We found that TyrR was expressed and functioned in a set of tyramine-responsive neurons in the Drosophila brain called the inferior posterior slope (IPS). Genetic hyperactivation of IPS neurons induced a significant elevation in male-male courtship, similar to the mutant males. Conversely, inactivation of these neurons decreased male-female courtship. We conclude that basal IPS activity is required to permit sufficient levels of sexual drive for male-female courtship. In addition, through activation of TyrR, we suggest that tyramine serves as an inhibitory neuromodulator to reduce sexual drive.
RESULTS
Expression of the TyrR-reporter in a small subset of neurons in the brain
Seven Drosophila GPCRs are receptors for octopamine and/or tyramine. However, TyrR (CG7431) is the only receptor that is potently and specifically activated by tyramine, and not other biogenic amines [>1000-fold; 21, 22]. To dissect the physiological role of TyrR, we generated a TyrR knock-out allele by ends-out homologous recombination (TyrRGal4). We deleted ~0.7 kb encoding the N-terminal region and the first two transmembrane domains of TyrR. In addition, we introduced a Gal4 gene reporter at the site of the normal TyrR translation initiation codon (Figure 1B). We confirmed the TyrR knockout and Gal4 knock-in by PCR (Figures 1B and 1C).
We analyzed the expression pattern of the TyrR reporter (TyrRGal4/+) in the brain and ventral nerve cord (VNC) using UAS-mCD8-GFP and detected GFP staining in 20–25 cells per brain hemisphere (Figure 1D). These neurons were mainly in four regions of the brain [23]: the superior medial protocerebrum (SMP), the posteriorlateral protocerebrum (PLP), the inferior posterior slope (IPS) and the gnathal ganglia (GNG). There were also several GFP-positive neurons in each ganglia of the VNC (Figure 1E). However, we did not detect TyrR reporter expression in the peripheral nervous system.
Loss of TyrR increased male-male courtship, but did not alter gender preference
We found that the TyrRGal4 mutant flies were healthy and fertile. However, TyrRGal4 males displayed a large increase in male-male courtship. We quantified this behavior by introducing 8–10 TyrRGal4 males into a small Petri dish, and found that they chased each other and formed chains of courting males, resulting in a strikingly high chaining index (Figures 2A and 2B; Movie S1). This behavioral phenotype was due to loss of TyrR since we phenocopied the increased male-male courtship by knocking down TyrR by RNAi (UAS-TyrRRNAi and TyrRGal4/+) or by placing the TyrRGal4 mutation in trans with a deficiency (Df) that uncovered the gene (Figure 2A). Single TyrRGal4 males also showed strong courtship toward single w1118 target males (Figure 2C). We suppressed the increased male-male courtship by introducing either a wild-type genomic transgene or a UAS-TyrR transgene under the control of the TyrR reporter (TyrRGal4; Figures 2A and 2C). Thus, TyrR was necessary to inhibit high levels of male-male courtship.
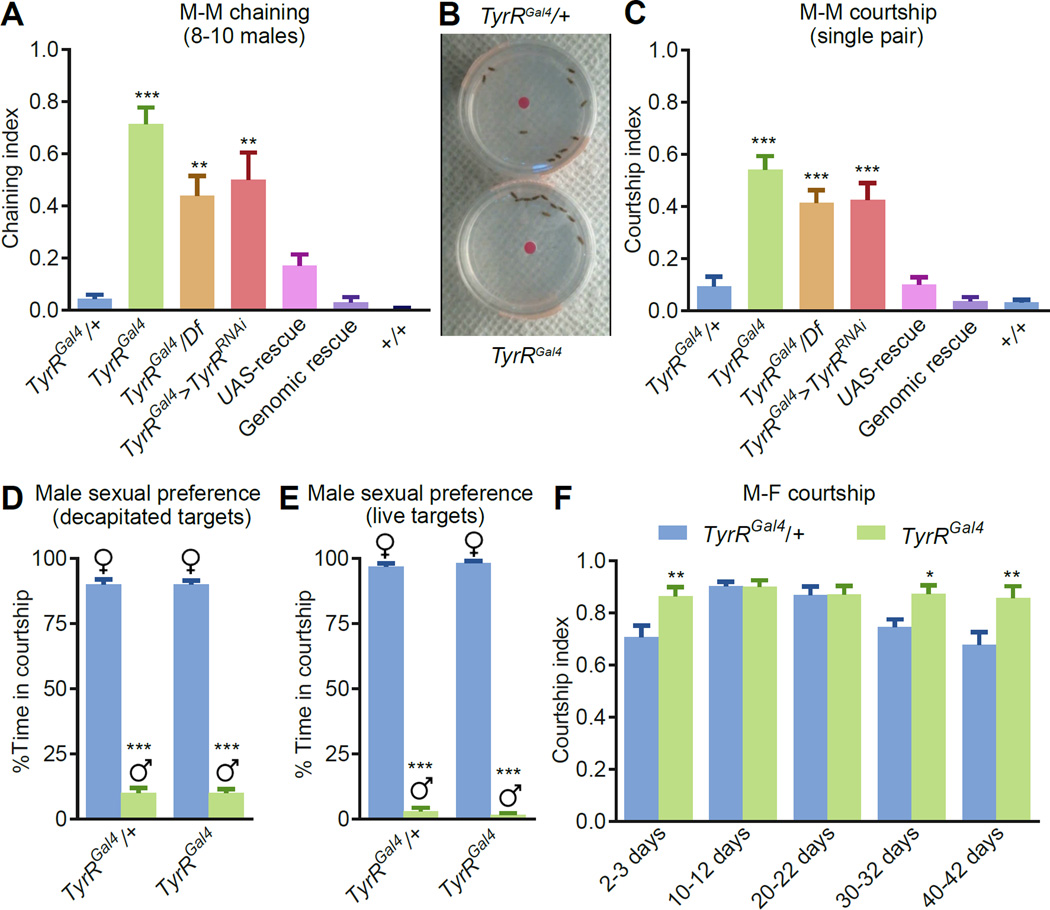
Figure 2. TyrRGal4 mutant showed enhanced courtship behavior.
(A) Chaining indices for groups of males of the indicated genotypes. The “wild type” +/+ flies used here and in other panels are w+ flies that have been outcrossed to w1118 for five generations. p<0.01; p<0.001 compared to control. n=7–10.
(B) A snapshot from Movie S1 showing male-male chaining exhibited by TyrRGal4 (bottom) but not the heterozygous control (top).
(C) Courtship indices using one target (w1118) male and one tester male of the indicated genotypes. p<0.001 compared to the TyrRGal4/+ control. n=24–29 trials/genotype.
(D) Testing sexual preference. Single TyrRGal4/+ and TyrRGal4 males were tested for courtship behavior towards a single decapitated female and a single decapitated male. p<0.001 compared to the other gender. n=24.
(E) Single TyrRGal4/+ and TyrRGal4 males were tested for courtship behavior towards a single w1118 female and a single fruM null (fruLexA/fru4–40) male. p<0.001 compared to the other gender. n=24.
(F) Courtship indices using different age groups of w1118 females and heterozygous or TyrRGal4 homozygous males. The ages of the target females are indicated. n=13–15 trials/genotype.
The error bars depict the means ±SEMs. All analyses of two samples employed Mann-Whitney tests. To analyze multiple samples we used the Kruskal-Wallis and Dunn’s post hoc test. **p<0.01. ***p<0.001.
See also Figure S1.
To address whether TyrRGal4 flies exhibited a change in gender preference, we performed choice assays. We placed a decapitated wild-type female and a decapitated wild-type male into a chamber that contained a TyrRGal4 mutant or a control male. We found that gender preference was unchanged in the mutant flies (Figure 2D). We repeated the gender preference experiments using live targets. Because fruM null (fruLexA/fru4–40) males do not court females, we allowed the TyrRGal4 males to choose between one female and one fruM male. We found that the TyrRGal4 males showed the same strong bias for female flies as did the controls (Figure 2E). However, when presented with fruM males alone, the TyrRGal4 males elicited strong courtship to these males as well (Figure S1A). We then paired sexually naive mutant males with isogenic wild-type control (w1118) virgin females, and compared their behavior to heterozygous control males. Both mutant and control males displayed similar initiation latencies and copulation durations (Figure S1B and S1C). In addition, the enhanced male-male courtship behavior was not likely due to a general increase in arousal since the TyrRGal4 males did not show elevated aggression or locomotion. Rather, these behaviors were decreased relative to the controls (Figure S1D and S1E).
TyrR mutant males exhibited increased courtship towards females
To determine whether TyrRGal4 males exhibited increased courtship towards females, we initially tested 4–10 day old males and 10–12 day old females. However, since the vast majority of control (TyrRGal4/+) males courted these females, there was insufficient sensitivity to discern whether or not the TyrRGal4 mutant males increased courtship towards females (Figure 2F). Therefore, we performed additional male-female courtship assays using females that ranged in age. The control males displayed lower courtship behavior towards young (2–3 day old) and older females (30–32 and 40–42 days old) relative to the mid-aged (10–12 and 20–22 day old) females (Figure 2F). However, TyrRGal4 males retained nearly maximal courtship indices towards females regardless of their age (Figure 2F). In contrast to the increased courtship activity of the mutant males, TyrRGal4 female flies exhibited normal post-mating behaviors, such as decreased receptivity and remating and increased egg laying (Figure S1F-S1H).
Hyperactivating TyrR-expressing neurons recapitulates the TyrR mutant phenotype
Because the TyrRGal4 mutant showed strong male-male chaining, we tested whether artificial inactivation of TyrRGal4 neurons would induce similarly robust behavior. To silence the TyrRGal4 neurons, we expressed the tetanus toxin light chain (UAS-TNT) or the inwardly rectifying K+ channel (UAS-Kir2.1) under control of the TyrR-Gal4 (TyrRGal4/+). Surprisingly, introduction of these transgenes did not induce male chaining behavior (Figure 3A). Rather, inactivation of TyrRGal4-expressing neurons with UAS-TNT suppressed the dramatic elevation in chaining behavior exhibited by the TyrRGal4 mutant males (Figure 3A). Moreover, silencing these neurons with UAS-TNT also significantly decreased the courtship indices toward females (Figure 3B). These phenotypes were not due to any obvious effects on neurite architecture resulting from expressing TNT (Figure S2A and S2B), although we cannot formally exclude any developmental phenotypes.
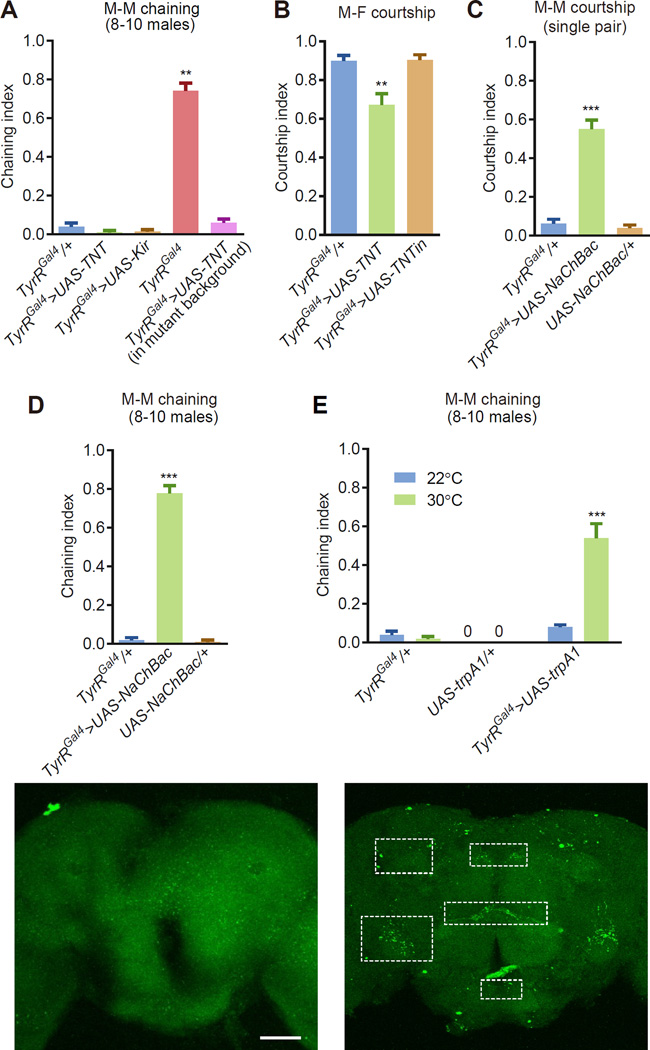
Figure 3. Activating or silencing TyrR neurons affected courtship behavior.
(A) Chaining indices using groups of 8–10 males. To silence the TyrR neurons, we expressed UAS-TNT or UAS-Kir2.1 under control of the TyrR-Gal4 (TyrRGal4/+). TyrRGal4>UAS-TNT flies contain two copies of the TyrRGal4 insertion, and are therefore mutants. Statistical significance was determined by comparing the data to the TyrRGal4/+ control. n=5–8.
(B) Single pair male-female courtship after silencing TyrR-expressing neurons by expressing UAS-TNT in the males. We used males of the indicated genotypes and control (w1118) virgin females. Changes were compared to the TyrRGal4/+ control. n=24 trials/genotype.
(C) Male-male courtship indices in males with hyperactivated TyrR-expressing neurons. We combined one male expressing UAS-NachBac in TyrR neurons (TyrRGal4/+) in combination with one control (w1118) male. Controls expressing just the Gal4 or UAS transgene are included. n=18–20 trials/genotype.
(D) Chaining indices exhibited by groups of males with TyrR neurons constitutively activated by UAS-NachBac. n=5–13
(E) Chaining among groups of males in which we thermally hyperactivated TyrR neurons with UAS-trpA1. n=5–7.
(F and G) Putative synaptic connections between TyrRGal4 and fruM neurons in the brain using the GRASP technique. All GRASP signals represent endogenous GFP fluorescence (no GFP antibodies were used). (F) Representative negative control showing the lack of GFP signal when using one rather than both GRASP components.
(F) GRASP-mediated GFP reconstitution observed in the SCL, AVLP, CRE, AL and PENP regions (dotted rectangles) due to TyrRGal4 neurons expressing CD4::spGFP1–10 and fruLexA neurons expressing CD4::spGFP11. SCL, superior clamp; AVLP, anterior ventrolateral protocerebrum; CRE, crepine; AL, antennal lobe; PENP, periesophageal neuropils. The scale bars represent 50 µm.
The error bars represent the means ±SEMs. We used the Kruskal-Wallis and Dunn’s post hoc test. **p<0.01. ***p<0.001.
See also Figure S2.
To determine the effects on courtship behavior resulting from hyperactivation of the TyrR-expressing neurons, we used a transgene expressing NaChBac to chronically raise the resting potential and thereby increase both the spontaneous and induced firing rates. We also used a trpA1 transgene to acutely hyper-stimulate these neurons by thermally activating TRPA1. We found that expression of NaChBac in TyrRGal4 neurons greatly increased male courtship toward individual target males, and generated strong male-male chaining behavior (Figures 3C and 3D). We obtained similar results by expressing TRPA1 in TyrR-neurons, and shifting the animals to a 30°C enviro nment to conditionally hyperactive these neurons (Figure 3E). However, activation of trpA1 in TyrRGal4 mutant males did not cause a further increase in courtship behavior (Figure S2C and S2D).
Fruitless (Fru) and Doublesex (Dsx) are two transcriptional factors that impact male-specific behaviors. Activation of FruM or Dsx neurons in solitary males induces courtship behaviors [24]. However, we did not detect any overlap between the TyrR reporter and either anti-FruM or anti-DsxM staining (Figure S2E and S2F), indicating that FruM and DsxM are expressed in different subsets of neurons in the brain from TyrR. We also tested the possibility that the TyrRGal4 neurons transiently expressed fru during development using a lineage tracing approach (fru-FLP and UAS>stop>mCD8-GFP), but did not detect GFP signals.
We then used the GRASP technique (GFP reconstitution across synaptic partners) to test whether TyrR-positive neurons might contact fruM neurons to affect courtship behaviors, since fruM function is critical for building the potential for nearly all aspects of male sexual behavior [25, 26]. The GRASP method employs two complementary fragments of GFP (spGFP11 and spGFP1–10) tethered to the membranes of different cells [27, 28]. If the cells are in contact, GFP is functionally reconstituted. Since GRASP requires dual expression systems (e.g. LexA/LexAop and GAL4/UAS), we used the fruLexA and the TyrRGal4 [29] to drive LexAop-mCD4-spGFP11 and UAS-mCD4-spGFP1–10, respectively. Brains expressing just one or the other GFP fragment did not exhibit fluorescent signals (Figure 3F). In contrast, flies expressing both GFP fragments displayed GFP fluorescence in several regions of the brain including the SCL (superior clamp), AVLP (anterior ventrolateral protocerebrum), CRE (crepine), AL (antennal lobe) and PENP (periesophageal neuropils) (Figure 3G). The positive GRASP signals suggest that there are synaptic contacts between TyrR and fruM neurons, and that they may function within a common neural circuit to regulate courtship.
Hyperactivation of cholinergic IPS neurons stimulated male chaining behavior
The TyrRGal4 was expressed in the brain and the VNC. To address whether hyperactivation of TyrR-positive neurons in the brain contributed to inducing high levels of male-male courtship, we eliminated most TyrR expression in the VNC by introducing a Gal4 repressor (Gal80) in the VNC (Tsh-Gal80) [30]. This Gal4 suppressor removed TyrR reporter expression in the VNC, except for a pair of neurons between the first and second ganglion (Figures 4A and 4B). We found that thermal activation of TRPA1 in this remaining subset of TyrR-expressing cells caused a similarly high level of male-male chaining as did TRPA1 activation of the full set of TyrR-expressing cells (Figure 4C). We then used the VGluT-Gal80 [31] to suppress the TyrR-Gal4 in the brain, except for the IPS neurons and very weak expression in the GNG (Figure 4D). Thermal activation of TRPA1 in these remaining TyrR-positive brain neurons induced male-male chaining (Figure 4C), indicating the important contribution of IPS or GNG neurons in controlling male-male courtship.
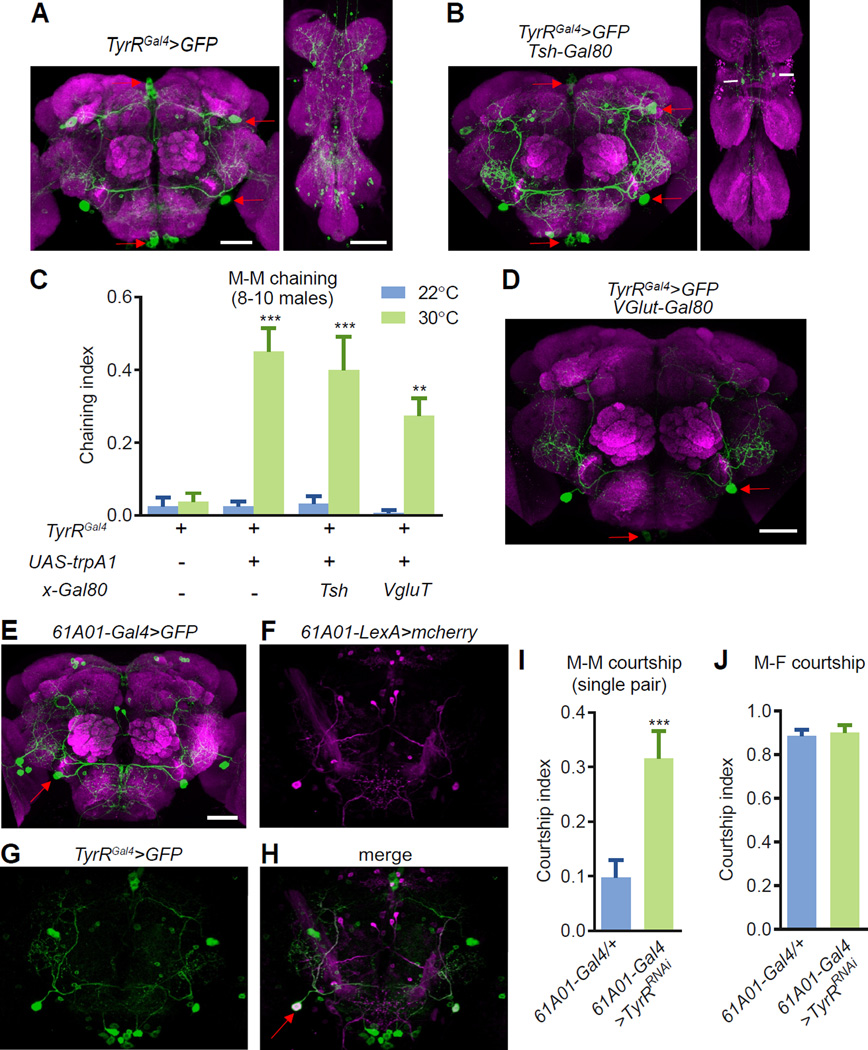
Figure 4. TyrR controls courtship behavior through IPS neurons.
(A) Staining of an adult male brain (left) and VNC (right) using the TyrR-Gal4 reporter (TyrRGal4/+) in combination with UAS-mCD8-GFP. Anti-GFP (green) and anti-nc82 (panneuropil marker, magenta).
(B) Elimination of TyrR-reporter expression in most cells in the VNC using a Gal4 repressor (Gal80): Tsh::Gal80. Anti-GFP (green) and anti-nc82 (pan-neuropil marker, magenta).
(C) Male chaining resulting from thermal hyperactivation of defined subsets of TyrR-expressing neurons using two Gal80 transgenes. To determine statistical significance, we compared the chaining indices at 22°C (no TRPA1 activation) and 30°C (TRPA1 activated). n=4–7 trials/genotype.
(D) VGlut::Gal80 restricted TyrRGal4 expression to the IPS and GNG regions of the male brain. The image was obtained from an animal maintained at room temperature.
(E) UAS-mCD8-GFP expressed under the control of the 61A01-Gal4. The red arrow indicates the IPS neurons in the brain. Anti-GFP, green; anti-nc82, magenta.
(F-H) IPS neurons co-expressed the TyrRGal4 and the 61A01-LexA. The brains contained the following transgenes: 61A01-LexA/UAS-mCD8-GFP;TyrRGal4/LexAop2-mCherry. Anti-GFP, green; anti-DeRed, magenta.
(I) Male-male courtship indices after RNAi knockdown of TyrR in 61A01-Gal4 neurons. n=20–22.
(J) Male-female courtship indices after RNAi knockdown of TyrR in 61A01-Gal4 neurons. n=15–19.
The immunohistochemistry experiments were performed at room temperature. The scale bars represent 50 µm. The error bars indicate the means ±SEMs. Mann-Whitney test were performed. **p<0.01. ***p<0.001.
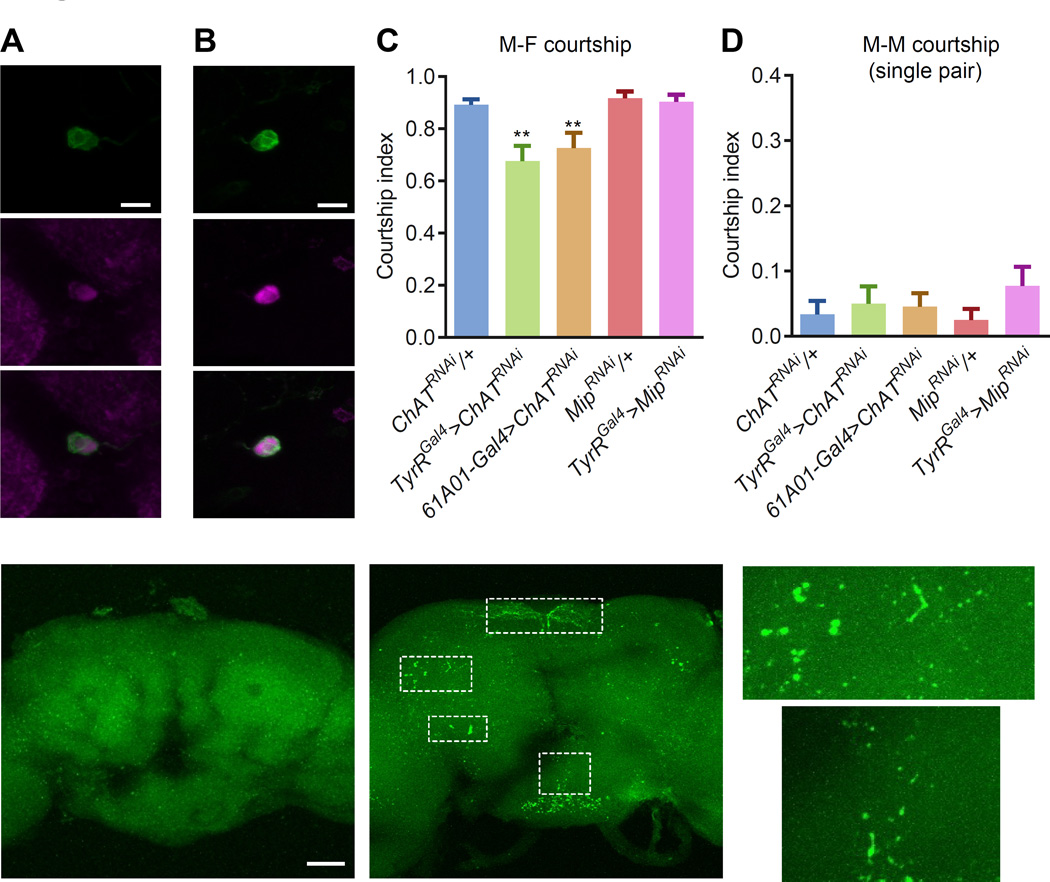
Since the IPS neurons (2–3 cells per hemisphere) project extensive processes to SCL, AVLP and PENP, while the GNG neurons project outside the brain (Figure 4D), we propose that the IPS neurons are key neurons in regulating male courtship. We found that a Janelia Gal4 (61A01-Gal4) also labeled IPS but not other TyrR neurons (Figures 4E-4H). To determine the effects of loss of TyrR in IPS neurons, we used the 61A01-Gal4 to drive UAS-TyrR-RNAi and found that these animals also showed strong male-male courtship behaviors (Figure 4I), while leaving male-female courtship intact (Figure 4J). Taken together, our data indicate that activation of TyrR-expressing IPS (TyrRIPS) neurons is sufficient to stimulate male-male courtship, and that stimulation of TyrR inhibited the activity of these neurons.
To identify candidate neurotransmitters or neuromodulators that may be expressed and function in TyrRIPS neurons to promote courtship we performed whole-mount immunostaining using a panel of antibodies against acetylcholine transferase (ChAT), serotonin (5-HT), tyrosine hydroxylase (TH) and tyrosine decarboxylase 2 (TDC2), which is the only tdc gene expressed in neurons [32]. We found that the TyrRIPS neurons stained with anti-ChAT (Figure 5A), but not with the other antibodies tested (Figure S3A-3C). The 61A01-Gal4 used a promoter region for the gene encoding the neuropeptide myoinhibiting peptide (Mip). TyrRIPS neurons also expressing MIP (Figure 5B and Figure S3D). We knocked down ChAT and Mip in TyrR-expressing neurons using RNAi and found that suppressing ChAT but not Mip decreased the male-female courtship index (Figure 5C), even though MipRNAi was effective in knocking down expression of MIP (Figure S3E and S3F). We obtained similar results after knocked down ChAT with the 61A01-Gal4 (Figure 5C). However, RNAi-mediated knockdown of ChAT or Mip had no significant effect on increasing male-male courtship (Figure 5D).
Figure 5. Functional and anatomical analyses of IPS neurons.
(A) Double immunostaining using anti-GFP (green; TyrRGal4>UAS-mCD8-GFP) and anti-choline acetyltransferase (ChAT, magenta) in IPS neurons.
(B) Double immunostaining using anti-GFP (green) and anti-MIP (magenta) in IPS neurons.
(C) Single-pair male-female courtship indices after RNAi knockdown of Mip or ChAT in male TyrR neurons. The females were w1118 virgins. n=23–25.
(D) Single-pair male-male courtship indices after RNAi knockdown of Mip or ChAT in male TyrR neurons. n=18–21
(E-H) Images of brains showing putative synaptic connections between IPS and FruM neurons using the GRASP technique. All GRASP signals represent endogenous GFP fluorescence (no GFP antibodies were used). (E) Representative image showing a lack of GPF signal in a brain expression only one of the two GRASP components. (F) GRASP-mediated GFP reconstitution in the SMP, SCL, AVLP and PENP regions (dotted rectangles). The 61A01-LexA neurons expressed CD4::spGFP11 and the fruNP21-Gal4 neurons expressed CD4::spGFP1–10. (G-H) Close-up images of the boxed regions (SCL and PENP) in F.
The scale bars represent 10 µm in A and B and 50 µm in E and F.
See also Figure S3.
To test whether TyrRIPS neurons form contacts with FruM neurons in the male brain, we used GRASP. We drove the two halves of a split-GFP on the external cell membranes using the fruNP21-Gal4 [33] and the 61A01-LexA. The 61A01-LexA driver labeled the IPS neurons and neural projections in the SCL, AVLP and PENP, which are derived mainly from IPS neurons (Figures 4F and 4H). Expression of one half of the split-GFP under the control of either driver did not yield GFP-positive signals (e.g. Figure 5E). In contrast, upon expression of both halves of the GFP, we detected reconstituted GFP signals in SMP, SCL, AVLP and PENP (Figures 5F-5H). This was not due to expression of fru in 61A01 neurons since we did not detect a GFP signal in an intersectional experiment using UAS>stop>mCD8-GFP/Cyo;fruFLP/TM6b and 61A01-GAL4, the latter of which labels the same neurons as the 61A01-LexA, in additional to several others (Figure 4E and 4F). Thus, we suggest that a portion of GRASP signal observed in these regions reflects synaptic contact between IPS and fruM neurons.
TyrR neurons respond to tyramine specifically
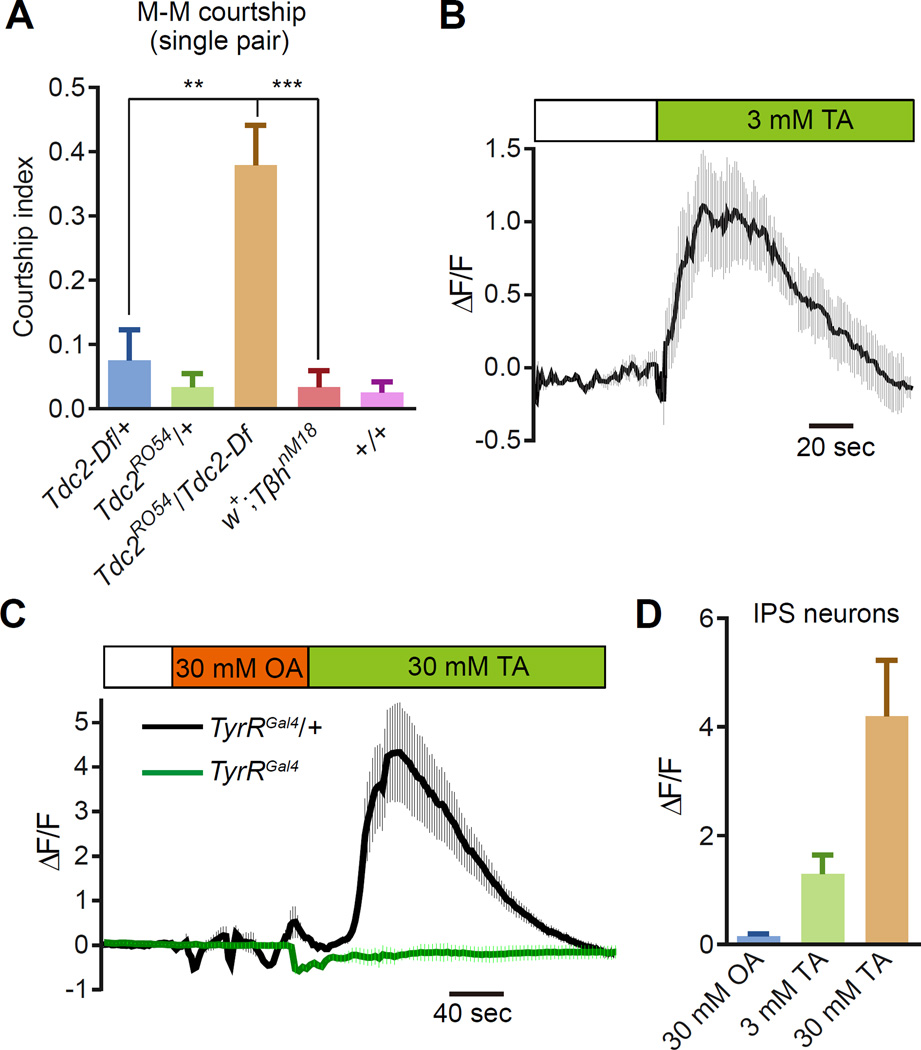
TyrR is activated by tyramine and not octopamine in vitro [21], suggesting that in vivo TyrR is receptor for tyramine but not octopamine. To address this proposal, we performed genetic and Ca2+ imaging analyses. Tyrosine is biosynthetically converted to tyramine, which in turn is the precursor for octopamine. These biogenic amines are produced through the sequential action of tyrosine decarboxylase (TDC) and tyramine β-hydroxylase (TbH), respectively (Figure 1A). We found that the Tdc2RO54 mutant flies, which are missing the enzyme needed for generating tyramine, showed elevated male-male courtship (Figure 6A). However, a null mutation that eliminates the tyramine β-hydroxylase (TbhnM18) that is essential for octopamine biosynthesis [34] did not cause an increase in male-male courtship (Figure 6A).
Figure 6. TyrR neurons respond to tyramine specifically.
(A) Male-male courtship indices using one target (w1118) male and one tester male of the indicated genotypes. n=12 trials/genotype
(B) Ca2+ imaging of TyrR neurons (TyrRGal4>UAS-GCaMP6f) in a brain subjected to 3 mM tyramine (TA). The ΔF/F represents the evoked fluorescence change from the baseline. The traces were averaged from 8 samples. The solid line represents the mean and the shaded areas indicate SEMs.
(C) Ca2+ response displayed by IPS neurons upon application of either 30 mM octopamine (OA) or 30 mM TA. Shown are the responses of the homozygous TyrR mutant (green trace) and the TyrRGal4 heterozygous control (black trace). (D) Ca2+ responses resulting from application of either 3 or 30 mM TA to IPS neurons. The 30 mM OA serves as a negative control. n=9 samples.
See also Figure S4.
To test whether tyramine activates TyrR in the brain, we performed Ca2+ imaging using the Ca2+ indicator GCaMP6f [35]. We isolated fly brains and found that application of tyramine drove a significant increase in Ca2+ signals in TyrR-expressing neurons (Figure 6B). The effect using the whole mount preparation embedded in gel required much higher concentrations of tyramine than used in vitro, in which the receptors are overexpressed and directly exposed to chemicals [21]. In some experiments, we observed Ca2+ oscillations (Figure S4A, Movie S2), a frequent consequence of activation of Gq-coupled GPCRs, which is attributable to repeated Ca2+ release and uptake from intracellular stores. Since IPS neurons were critical for regulating male courtship behaviors, we focused on these cells. Application of 30 mM octopamine did not stimulate a significant Ca2+ response in IPS neurons (Figures 6C and 6D). By contrast, application of either 3 or 30 mM tyramine induced a rise in Ca2+ (Figures 6C and 6D). This tyramine-induced Ca2+ response depended on TyrR, since it was eliminated in the TyrRGal4 mutant (Figure 6C). Thus, it appears that TyrR is activated in vivo by tyramine but not octopamine, consistent with in vitro studies [21].
DISCUSSION
Nearly all wild-type Drosophila males court and mate with females. However, among wild-type flies the frequency of male-male courtship is low. Nevertheless, there are multiple mutations that increase male-male courtship [reviewed in 36]. The changes in behavior are typically due to deficits in identifying males, such as occurs upon elimination of male pheromones or the corresponding receptors [36]. We found that TyrRGal4 mutant males exhibit a dramatic increase in male-male sexual activity. In contrast to previous mutations that increase male-male courtship [37–39], the TyrRGal4 flies discriminate between male and females. When provided a choice between the two genders, the TyrRGal4 mutants select females at the same high proportion as wild-type males. These results suggest that the strong male-male courtship activity was not due a deficit in sensing repulsive male pheromones. Furthermore, TyrRGal4 males also exhibited increased courtship towards young and aged females. These phenotypes were due to loss of TyrR and not potential effects of the mini-white transgene since we rescued the TyrR phenotype with a wild-type TyrR transgene. Moreover, heterozygous control males harboring the mini-white gene (TyrR/+) display wild-type levels of courtship behavior. Furthermore, we recapitulated the increased male-male courtship by RNAi knockdown of TyrR. Consistent with the conclusion that tyramine modulates male courtship activity, Tdc2 but not Tβh mutant males show elevated levels of male-male courtship.
We propose that the TyrR-expressing neurons control overall male sexual drive. In support of this concept, suppressing the normal activity of TyrR-expressing neurons in wild-type males significantly reduced male-female courtship behavior. This manipulation slightly reduced male-male courtship behavior in wild-type. However, the effect was not statistically significant since basal male-male courtship activity was very low. Nevertheless, silencing TyrR+ neurons in TyrRGal4 mutant males eliminated the high male-male courtship activity. Conversely, when we artificially activated the TyrR+ neurons in wild-type males, the animals displayed a strong elevation in male-male courtship behavior. Thus, the dramatic increase in male-male courtship reflected an increase in overall sexual activity, rather than an increase in same-sex preference. Based on GRASP studies, we suggest that the TyrR+ neurons function through the FruM neural circuits. Thus, the normal low activity of TyrR-positive neurons is permissive for male-female courtship. Higher activity stimulates greater courtship drive such that the animals will also court males, but only if females are not present since even at artificially elevated levels of activity, the males still prefer females if both gender targets are available. This role for the TyrR-expressing neurons differs from P1 neurons, which promote distinct behaviors, aggression and courtship, at low and high activity levels, respectively [40].
The TyrRGal4 phenotype was due to a requirement for tyramine for controlling courtship behavior since we found that TyrR-expressing neurons were activated by tyramine but not octopamine, consistent with in vitro data indicating that the TyrR is activated specifically by tyramine [21]. Tyramine is most likely acting as a neuromodulator, rather than as a neurotransmitter, since TyrR is a GPCR rather than an ionotropic receptor. In further support of this model, we did not detect any GRASP signals using the Tdc2-LexA and TyrRGal4, suggesting that the tyramine-producing neurons are not in direct contact with the TyrR-expressing neurons. However, a caveat is that the Tdc2-LexA recapitulates only a subset of the Tdc2 neurons (Figure S4B).
A neuromodulator can either be excitatory or inhibitory, depending on the receptor that is activated. Our data suggest that as a consequence of activating TyrR, tyramine serves an inhibitory rather than excitatory neuromodulator, which curbs sexual activity. In favor of this proposal are the genetic activation and inactivation experiments. Artificial stimulation of TyrR-expressing neurons increased male-male courtship, while inhibition of these neurons reduced male-female courtship.
The model that TyrR is an inhibitory neuromodulator receptor in vivo is consistent with in vitro studies showing that tyramine reduces the amplitude of EJP in neuromuscular junctions. [41, 42]. Our in vivo Ca2+ imaging results, as well as an in vitro analysis [21] indicate that TyrR is coupled to Gq, which typically leads to neuronal activation [43]. However, loss of Gq/G11 signaling can increase neuronal activity as well [44]. This could potentially occur through inhibiting glutamate release, gating of a Ca2+- activated K+ channel or through promiscuous coupling of a Gq/G11-coupled receptors to Gi/o G-proteins [44, 45]. In support of this latter possibility, two related Drosophila catecholamine receptors are coupled to both Gq and Gi proteins [46, 47].
We found that TyrR activity was required in a small group of neurons (TyrRIPS) in the brain for controlling courtship drive. Tyramine-induced inhibition of TyrRIPS neurons was strictly dependent on TyrR since the response was eliminated in TyrRGal4 mutant brains. Based on our findings using the GRASP technique, we propose that TyrRIPS neurons may form synaptic connections with FruM neurons, which regulate courtship. We propose that courtship behavior is enhanced by release of acetylcholine from basal or highly activated TyrRIPS neurons. In support of this proposal, TyrRIPS cells expressed acetylcholine transferase (ChAT), and knockdown of ChAT in these cells reduced courtship behavior.
In conclusion, our findings show that in Drosophila tyramine is not simply a biosynthetic intermediate for octopamine. Rather, it has an important function in the neuromodulation of male courtship drive through its specific receptor, TyrR. However, it does not affect gender preference. Given that the presence of tyramine as a trace monogenic amine in the mammalian brain [48], the question arises as to whether tyramine also functions in mammals as a neuromodulator of behavior.
EXPERIMENTAL PROCEDURES
Animals
Flies were maintained on conventional cornmeal-agar-molasses medium under a 12 h light:12 h dark cycle at 25°C and ambient humidit y. Specific details regarding the strains used in the experiments can be found in the Supplemental Experimental Procedures.
Generation of the TyrRGal4 mutant and transgenes
We generated the TyrRGal4 allele by ends-out homologous recombination. See Extended Experimental Procedures for details.
Behavioral assays
Unless indicated otherwise, the behavioral assays were carried out 3 h before the onset of darkness. Tester males were raised in isolation for 4–10 days post-eclosion. Target w1118 males and virgin females were segregated by sex, and group-housed for 4–10 days old. For age-dependent courtship assays, w1118 virgin females were aged in groups for intended days. A detailed protocol is included with the Supplemental Experimental Procedures.
Immunohistochemistry
For details on procedures and antibodies used see the Extended Experimental Procedures.
Statistical analyses
Statistical analyses were performed using Prism6 (GraphPad Software). We used nonparametric tests to analyze all data, except for Figure S1E and S1F (Fisher’s exact test). We performed the Mann-Whitney test for two groups of data. For comparison of three or more sets of data, we performed Kruskal-Wallis test and Dunn’s post hoc test.
Supplementary Material
Acknowledgments
This work was supported by a grant to C.M. from the National Institute on Deafness and Other Communication Disorders (DC007864) and a NIH Director’s Pioneer Award (1DP1AI124453). J.H. received support from the 973 Program (2013CB127600). We thank Dr. Gong-yin Ye for partial support of J.H. (funded by the China National Science Fund for Distinguished Young Scholars (31025021), and Drs. Barry Dickson, Gerald Rubin, Leslie Vosshall , Kristin Scott, Yi Rao, Toshihiro Kitamoto, Reinhard Predel, Mariana Wolfner, Rachel Wilson, Vivian Budnik and Sarah Certel for sharing valuable fly stocks and antibodies. We also thank the Physics Machine Shop at UCSB for fabricating behavioral chambers and Jinfei Ni for testing locomotor activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.H. and C.M. designed the experiments, interpreted the data and wrote the manuscript. J.H. and W.L. performed most of the experiments. Y.Q. and J.L. helped perform some immunohistochemistry and molecular biology experiments.
REFERENCES
- 1.Axelrod J, Saavedra JM. Octopamine. Nature. 1977;265:501–504. doi: 10.1038/265501a0. [DOI] [PubMed] [Google Scholar]
- 2.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 3.Certel SJ, Savella MG, Schlegel DC, Kravitz EA. Modulation of Drosophila male behavioral choice. Proc. Natl. Acad. Sci. USA. 2007;104:4706–4711. doi: 10.1073/pnas.0700328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila . Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezaval C, Nojima T, Neville MC, Lin AC, Goodwin SF. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 2014;24:725–730. doi: 10.1016/j.cub.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heifetz Y, Lindner M, Garini Y, Wolfner MF. Mating regulates neuromodulator ensembles at nerve termini innervating the Drosophila reproductive tract. Curr. Biol. 2014;24:731–737. doi: 10.1016/j.cub.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65:670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koon AC, Ashley J, Barria R, DasGupta S, Brain R, Waddell S, Alkema MJ, Budnik V. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat. Neurosci. 2011;14:190–199. doi: 10.1038/nn.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulton AA. Identification, distribution, metabolism, and function of meta and para tyramine, phenylethylamine and tryptamine in brain. Adv. Biochem. Psychopharmacol. 1976;15:57–67. [PubMed] [Google Scholar]
- 10.Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branchek TA, Blackburn TP. Trace amine receptors as targets for novel therapeutics: legend, myth and fact. Curr. Opin. Pharmacol. 2003;3:90–97. doi: 10.1016/s1471-4892(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 12.Selcho M, Pauls D, Huser A, Stocker RF, Thum AS. Characterization of the octopaminergic and tyraminergic neurons in the central brain of Drosophila larvae. J. Comp. Neurol. 2014;522:3485–3500. doi: 10.1002/cne.23616. [DOI] [PubMed] [Google Scholar]
- 13.Busch S, Selcho M, Ito K, Tanimoto H. A map of octopaminergic neurons in the Drosophila brain. J. Comp. Neurol. 2009;513:643–667. doi: 10.1002/cne.21966. [DOI] [PubMed] [Google Scholar]
- 14.Kutsukake M, Komatsu A, Yamamoto D, Ishiwa-Chigusa S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster . Gene. 2000;245:31–42. doi: 10.1016/s0378-1119(99)00569-7. [DOI] [PubMed] [Google Scholar]
- 15.Nisimura T, Seto A, Nakamura K, Miyama M, Nagao T, Tamotsu S, Yamaoka R, Ozaki M. Experiential effects of appetitive and nonappetitive odors on feeding behavior in the blowfly, Phormia regina: a putative role for tyramine in appetite regulation. J. Neurosci. 2005;25:7507–7516. doi: 10.1523/JNEUROSCI.1862-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirashima A, Yamaji H, Yoshizawa T, Kuwano E, Eto M. Effect of tyramine and stress on sex-pheromone production in the pre- and post-mating silkworm moth, Bombyx mori . J. Insect Physiol. 2007;53:1242–1249. doi: 10.1016/j.jinsphys.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Pirri JK, McPherson AD, Donnelly JL, Francis MM, Alkema MJ. A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron. 2009;62:526–538. doi: 10.1016/j.neuron.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ringstad N, Abe N, Horvitz HR. Ligand-gated chloride channels are receptors for biogenic amines in C. elegans . Science. 2009;325:96–100. doi: 10.1126/science.1169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendesky A, Tsunozaki M, Rockman MV, Kruglyak L, Bargmann CI. Catecholamine receptor polymorphisms affect decision-making in C. elegans . Nature. 2011;472:313–318. doi: 10.1038/nature09821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnelly JL, Clark CM, Leifer AM, Pirri JK, Haburcak M, Francis MM, Samuel AD, Alkema MJ. Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 2013;11:e1001529. doi: 10.1371/journal.pbio.1001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazzamali G, Klaerke DA, Grimmelikhuijzen CJ. A new family of insect tyramine receptors. Biochem. Biophys. Res. Commun. 2005;338:1189–1196. doi: 10.1016/j.bbrc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Ohta H, Inoue N, Takao H, Kita T, Ozoe F, Ozoe Y. Molecular cloning and pharmacological characterization of a Bombyx mori tyramine receptor selectively coupled to intracellular calcium mobilization. Insect. Biochem. Mol. Biol. 2009;39:842–849. doi: 10.1016/j.ibmb.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, et al. A systematic nomenclature for the insect brain. Neuron. 2014;81:755–765. doi: 10.1016/j.neuron.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Pan Y, Robinett CC, Baker BS. Turning males on: activation of male courtship behavior in Drosophila melanogaster . PLoS One. 2011;6:e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 26.Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila . Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellert DJ, Knapp JM, Manoli DS, Meissner GW, Baker BS. Midline crossing by gustatory receptor neuron axons is regulated by fruitless doublesex and the Roundabout receptors. Development. 2010;137:323–332. doi: 10.1242/dev.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clyne JD, Miesenbock G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila . Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 31.Bussell JJ, Yapici N, Zhang SX, Dickson BJ, Vosshall LB. Abdominal-B neurons control Drosophila virgin female receptivity. Curr. Biol. 2014;24:1584–1595. doi: 10.1016/j.cub.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 33.Kimura K, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- 34.Monastirioti M, Linn CE, Jr, White K. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto D, Sato K, Koganezawa M. Neuroethology of male courtship in Drosophila: from the gene to behavior. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2014;200:251–264. doi: 10.1007/s00359-014-0891-5. [DOI] [PubMed] [Google Scholar]
- 37.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 2008;11:874–876. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoopfer ED, Jung Y, Inagaki HK, Rubin GM, Anderson DJ. P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila . eLife. 2015:4. doi: 10.7554/eLife.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ormerod KG, Hadden JK, Deady LD, Mercier AJ, Krans JL. Action of octopamine and tyramine on muscles of Drosophila melanogaster larvae. J. Neurophysiol. 2013;110:1984–1996. doi: 10.1152/jn.00431.2013. [DOI] [PubMed] [Google Scholar]
- 42.Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster . Neurosci. Lett. 2002;329:324–328. doi: 10.1016/s0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- 43.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Wettschureck N, van der Stelt M, Tsubokawa H, Krestel H, Moers A, Petrosino S, Schütz G, Di Marzo V, Offermanns S. Forebrain-specific inactivation of Gq/G11 family G proteins results in age-dependent epilepsy and impaired endocannabinoid formation. Mol. Cell. Biol. 2006;26:5888–5894. doi: 10.1128/MCB.00397-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endoh T. Characterization of modulatory effects of postsynaptic metabotropic glutamate receptors on calcium currents in rat nucleus tractus solitarius. Brain Res. 2004;1024:212–224. doi: 10.1016/j.brainres.2004.07.074. [DOI] [PubMed] [Google Scholar]
- 46.Bayliss A, Roselli G, Evans PD. A comparison of the signalling properties of two tyramine receptors from Drosophila. J. Neurochem. 2013;125:37–48. doi: 10.1111/jnc.12158. [DOI] [PubMed] [Google Scholar]
- 47.Robb S, Cheek TR, Hannan FL, Hall LM, Midgley JM, Evans PD. Agonist-specific coupling of a cloned Drosophila octopamine/tyramine receptor to multiple second messenger systems. EMBO J. 1994;13:1325–1330. doi: 10.1002/j.1460-2075.1994.tb06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shariatgorji M, Nilsson A, Goodwin RJ, Kallback P, Schintu N, Zhang X, Crossman AR, Bezard E, Svenningsson P, Andren PE. Direct targeted quantitative molecular imaging of neurotransmitters in brain tissue sections. Neuron. 2014;84:697–707. doi: 10.1016/j.neuron.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Livingstone MS, Tempel BL. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila . Nature. 1983;303:67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.