Abstract
Background
Most sudden cardiac death (SCD) events occur in the general population among persons who do not have any prior history of clinical heart disease. We sought to develop a predictive model of SCD among US adults.
Methods
We evaluated a series of demographic, clinical, laboratory, electrocardiographic, and echocardiographic measures in participants in the Atherosclerosis Risk in Communities (ARIC) Study (n=13,677) and the Cardiovascular Health Study (CHS) (n=4,207) who were free of baseline cardiovascular disease. Our initial objective was to derive a SCD prediction model using the ARIC cohort and validate it in CHS. Independent risk factors for SCD were first identified in the ARIC cohort to derive a 10-year risk model of SCD. We compared the prediction of SCD to non-SCD and all-cause mortality in both the derivation and validation cohorts. Further, we evaluated whether the SCD prediction equation was better at predicting SCD than the 2013 ACC/AHA CVD Pooled Cohort risk equation.
Results
There were a total of 345 adjudicated SCD events in our analyses, and the 12 independent risk factors in the ARIC study included age, male sex, African American race, current smoking, systolic blood pressure, use of antihypertensive medication, diabetes, serum potassium, serum albumin, HDL, estimated GFR, and QTc interval. Over a 10-year follow-up period, a model combining these risk factors showed good to excellent discrimination for SCD risk (C statistic 0.820 in ARIC and 0.745 in CHS). The SCD prediction model was slightly better in predicting SCD than the 2013 ACC/AHA Pooled Cohort risk equations (C statistic 0.808 in ARIC and 0.743 in CHS). Only the SCD prediction model, however, demonstrated similar and accurate prediction for SCD using both the original, uncalibrated score and the recalibrated equation. Finally, in the echocardiographic subcohort, a left ventricular ejection fraction <50% was present in only 1.1% of participants and did not enhance SCD prediction.
Conclusions
Our study is the first to derive and validate a generalizable risk score that provides well-calibrated, absolute risk estimates across different risk strata in an adult population of white and African American individuals without a clinical diagnosis of cardiovascular disease.
Keywords: sudden cardiac death, arrhythmia, sudden cardiac death, population, risk prediction
Introduction
Sudden cardiac death (SCD) is defined as an unexpected, pulseless condition attributable to a cardiac arrhythmia.1 Most cardiac arrests present without warning symptoms and are nearly always fatal despite resuscitation attempts.2, 3 As a result, preventive strategies have focused on using implantable cardioverter-defibrillators (ICD) in the highest risk subgroups of the population, such as those with an advanced cardiomyopathy and depressed left ventricular ejection fraction (LVEF).4 Most SCD cases, however, occur in the general population,5–7 and the majority have no clinically recognized heart disease prior to the event.8–10 Therefore, when SCD occurs in the general population, it is typically the initial manifestation of cardiovascular disease.
The Institute of Medicine recently published “Strategies to Improve Cardiac Arrest Survival: A Time To Act” and emphasized the need for more effective prevention and resuscitation programs.11 Expert panels have highlighted the lack of a baseline model that estimates an individual’s risk for SCD as a major obstacle to primary prevention efforts that target SCD risk stratification among the general population.12, 13 Multiple population-based studies have demonstrated independent associations between specific risk factors and biomarkers of inflammation, myocyte injury, and neurohormonal activation with risk for SCD.2, 14–16 However, there is no widely accepted model of individual risk estimation for SCD.12, 13, 17 We sought to develop and evaluate a SCD prediction model among US adults without a history of cardiovascular disease (CVD). As part of this approach, we evaluated a comprehensive panel of traditional and novel cardiovascular risk factors in two large, bi-racial, population-based cohorts. We also compared SCD prediction using the current, 2013 American College of Cardiology / American Heart Association (ACC/AHA) CVD Pooled Cohort risk equations, which were developed to calculate the 10-year risk of a first cardiovascular event including nonfatal MI, coronary heart disease death, or fatal or nonfatal stroke.18
Methods
The Atherosclerosis Risk in Communities (ARIC) Study and the Cardiovascular Health Study (CHS) are population-based, prospective cohorts designed to improve understanding of cardiovascular disease risk. The ARIC Study included 15,792 persons 45 to 64 years old at baseline (1987–1989). These individuals were chosen from four communities in Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis suburbs, Minnesota; and Washington County, Maryland.19 The CHS included 5,888 participants >65 years of age identified from Forsyth County, NC; Sacramento County, CA; Washington County, MD, and Pittsburgh, PA by use of Medicare eligibility lists. The original CHS cohort included 5,201 participants recruited in 1989 and 1990. An additional 687 African Americans were recruited in 1992 to 1993.20 The ARIC and CHS protocols were approved by the institutional review board at each participating center, and informed consent was obtained from each study participant.
This analysis excluded participants who had prevalent cardiovascular disease defined by a previous history of coronary heart disease, heart failure, or stroke. Participants with missing covariates were also excluded. We performed an extensive review of the baseline data definitions and study sources so that only comparable clinical variables across the two cohorts were included. The final study sample consisted of 17,884 participants (13,677 from ARIC and 4,207 from CHS) (Figure 1).
Figure 1.
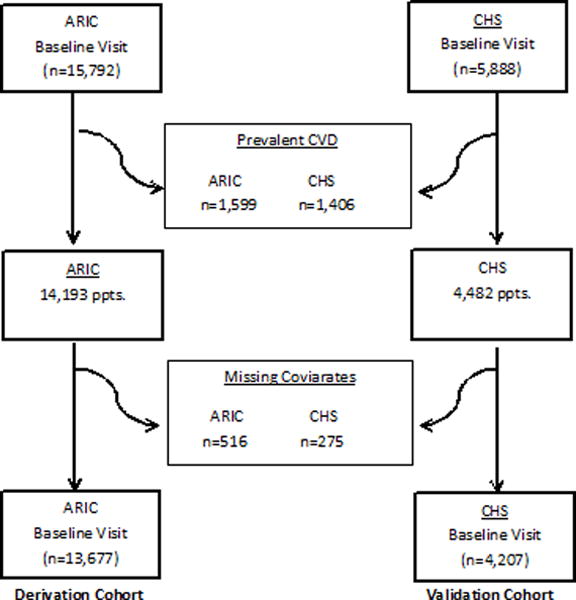
Study Overview
Baseline variables from ARIC and CHS participants were assessed for these analyses. The baseline visit in each study consisted of a comprehensive examination including a thorough medical history, physical examination, laboratory testing, and a twelve-lead electrocardiogram (ECG). In order to harmonize the baseline data between the two cohorts, each variable was defined similarly. Age, sex, race, education level and income were determined by self-report. Education was dichotomized by whether or not participants had graduated from high school. Income groups were categorized into annual household earnings of <$8,000, $8,000–34,999, and ≥$35,000. Height and weight were measured by standard protocol; and body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. Blood pressure (systolic and diastolic) was measured in seated participants after a 5-minute rest. Questionnaires assessed alcohol intake and self-reported smoking status (current, former, never). Diabetes was defined as a fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or the use of hypoglycemic medications. A family history of coronary heart disease was defined as disease in a sibling among CHS participants or any immediate family member in the ARIC study. Physical activity was evaluated in the ARIC study and CHS using a questionnaire that assessed each participant’s self-reported activity (see Supplemental Methods). Estimated glomerular filtration rate was calculated from creatinine using the Chronic Kidney Disease Epidemiology Collaboration Equation.21 Total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, serum albumin (per 0.3 g/dL decrease), potassium (centered at 4.4, per 0.5 mmol/L increase), and hemoglobin (per 1g/dL) were all modeled continuously. Twelve-lead ECGs were assessed in both cohorts for the following parameters: atrial fibrillation (ECG-defined), Cornell voltage, which evaluates left ventricular thickness and is calculated from the sum of the S-wave in V3 and R wave in aVL,22, 23 corrected QT interval,24 QRS duration,25 heart rate, and left bundle branch block status.
Echocardiography was performed on a subgroup of participants in both ARIC and CHS. Of the 13,677 ARIC participants included in our analysis, echocardiographic studies were available in 1568 individuals. In CHS, of the 4,207 participants in our study, echocardiograms were completed on 3,731 participants. In ARIC, echocardiography was performed using an Acuson XP 128/10c machine (Siemens Medical, Iselin, NJ) with both M-mode and pulsed Doppler examination following a standard protocol.26 Left ventricular ejection fraction (LVEF) was determined semi-quantitatively using visual assessment and a modified Quinones formula.27 Left ventricular systolic dysfunction was defined as an ejection fraction <50%.28, 29 In CHS, echocardiographic examinations included 2-dimensional and Doppler methods performed with the Toshiba SSH-160A sonographic units.30 The CHS Echocardiography Reading Center classified the ejection fraction in qualitative terms as normal, borderline or abnormal.31
Outcome Ascertainment
In both ARIC and CHS, comprehensive data were gathered on cardiovascular events and deaths from hospital records; interviews with physicians, next of kin, and/or witnesses; death certificates; and autopsy reports when available. The causes of death were adjudicated by respective ARIC and CHS events’ committees (see Supplemental Methods section). The primary outcome, SCD, was defined similarly in both ARIC and CHS: a sudden pulseless condition from a cardiac origin in a previously stable individual occurring out of the hospital or in the emergency department.32–35 For unwitnessed deaths, the participant must have been seen within 24 hours of the arrest in a stable condition and without evidence of a non-cardiac cause of cardiac arrest. These definitions concur with those proposed by the National Heart, Lung, and Blood Institute working group on SCD.1 For all cases, participants could not have life threatening non-cardiac comorbidities or be under hospice or nursing home care. Each event was independently adjudicated by two physicians. If there was a disagreement, a third investigator reviewed the event to provide final classification.32, 33 A blinded second physician review of a random sample of 70 of these death records showed an 88% interviewer agreement and k = 0.74 for SCD.
Statistical Analysis
Our initial objective was to derive a SCD prediction model using the ARIC cohort and validate it in CHS. We first harmonized 26 variables across both cohorts and calculated the mean and median values of relevant sociodemographic characteristics, cardiovascular risk factors, serum measures and ECG variables across ARIC and CHS. When possible, each characteristic was modeled as a linear variable.
Model Building: Derivation Cohort
We evaluated the univariate association between each baseline variable in the ARIC study and SCD risk. Those variables associated with SCD at p<0.05 were entered into a multivariable model. Backwards stepwise regression was then performed, and a retention criteria of p<0.1 was used to identify candidate variables for our prediction model. Age and sex interactions were evaluated. Based on 10-years of follow-up, the beta coefficients for each variable retained in the final multivariable model were evaluated.
Prediction modeling
Model-based 10 year risks of SCD, non-sudden cardiac death, and all-cause mortality were calculated in both ARIC and CHS. The SCD prediction equations used the score calculated from the risk factors and corresponding regression coefficients obtained from the derivation cohort (ARIC). To determine if our SCD prediction equation was a better prediction tool than previously published risk scores, we calculated risk for each outcome using the 2013 ACC/AHA CVD Pooled Cohort risk equation. As part of this assessment, we calculated the discrimination (c-statistics) and quantified each prediction model’s ability to separate those who experience a specific outcome from those who do not.36 We also assessed calibration, which evaluates how closely the predicted outcome corresponds with the observed event. We utilized Nam and D’Agostino’s modified Hosmer-Lemeshow chi-square statistic.37 The SCD prediction score and ACC/AHA CVD Pooled Cohort risk equation were used to divide subjects into deciles of risk for experiencing each outcome within 10 years. A chi-square statistic was calculated to compare the differences between predicted and actual event rates. In addition, we recalibrated both the SCD prediction score and the ACC/AHA CVD Pooled Cohort risk equation for each outcome in each cohort. In this scenario, the average incidence rate is replaced by the mean risk in the cohort and the 10-year Kaplan Meier event rate for each outcome. To do this, we multiplied the risk for each person by the ratio of the 10-year Kaplan Meier over the mean of the risks to obtain the correct calibration. Recalibration does not affect the discrimination derived beta coefficients or discrimination. Given that CHS is comprised of elderly individuals, all prediction statistics in the validation cohort were assessed utilizing a competing risk approach.38, 39 Finally, in the subgroup of participants that had an echocardiogram, we evaluated whether the addition of LVEF to the baseline model enhanced SCD prediction. The proportional hazards assumption was not violated in any of these analyses. SAS software version 9.2 (SAS Institute Inc., Cary, NC), R (version 3.0.2) and Stata (College Station, TX, version 13.1) were used for all analyses.
Secondary Analysis
As part of a secondary analysis, we evaluated in CHS the association of the following biomarkers with SCD risk: CRP, NT-pro BNP, and troponin T measured with a high sensitivity assay. Biomarkers were evaluated both continuously (log-transformed variable per doubling) and categorically in the multivariable model for SCD in CHS. This step was repeated with each biomarker categorized into quintiles.
Results
For these analyses, there were a total of 345 adjudicated SCD events. Over a median [interquartile range (IQR)] follow-up of 13.1 [IQR 2.3] years in the ARIC study, there were 171 SCD events; in CHS, over a median follow-up of 14.2 [IQR 8.2] years, there were 174 SCD cases. Baseline characteristics of eligible individuals are presented in Table 1. The average age was 54±6 years in ARIC and 72±5 years in CHS. The majority of participants for these analyses were women, 56% of ARIC and 61% of CHS. African Americans comprised 26% of ARIC and 15% of CHS.
Table 1.
Baseline Characteristics of Atherosclerosis Risk in Communities Study and Cardiovascular Health Study participants without prevalent cardiovascular disease
| Variable, mean (SD) or N (%) |
ARIC (n=13677) |
CHS (4207) |
|---|---|---|
| Demographics | ||
| Age | 54.0 (6) | 72 (5) |
| Female | 7606 (56%) | 2566 (61%) |
| Black | 3485 (26%) | 610 (15%) |
| High School Degree | 10647 (78%) | 3457 (82%) |
| Income | ||
| <$8,000 | 1862 (14%) | 543 (13%) |
| $8,000–34,999 | 5886 (43%) | 2451 (58%) |
| ≥ $35,000 | 5929 (43%) | 949 (23%) |
| CVD Risk Factors | ||
| Current Smoker | 3521 (26%) | 522 (12%) |
| Alcohol ≥3 drinks/wk | 3477 (25%) | 771 (18%) |
| BMI, kg/m2 (Body mass index) | 27.5 (5.2) | 26.6 (4.7) |
| Physical Activity, scale 0–5 | 2.4 (0.8) | |
| Physical Activity (kcal/wk) | 1100 [405,2374] | |
| Systolic Blood Pressure, mmHg | 121 (19) | 136(21) |
| Diastolic Blood Pressure, mmHg | 74 (11) | 71 (11) |
| Diabetes | 1410 (10%) | 578 (14%) |
| Family History of CHD/CVD | 1121 (8%) | 1416 (34%) |
| Serum Measures | ||
| Potassium, mmol/L | 4.4 (0.5) | 4.2 (0.4) |
| Albumin, g/dL | 3.9 (0.3) | 4.0 (0.3) |
| Hemoglobin, g/dL | 13.9 (1.4) | 14.0 (1.4) |
| Total cholesterol, md/dl | 214 (42) | 212 (39) |
| HDL, mg/dl | 52 (17) | 56 (16) |
| LDL, mg/dl | 137 (39) | 130 (35) |
| eGFR, ml/min/1.73m2 | 96 (15) | 80 (19) |
| ECG | ||
| Atrial fibrillation | 19 (0.5%) | 77(2%) |
| Cornell voltage, mm | 12.1 (5.3) | 15.4 (4.2) |
| QTc Interval, msec | 416 (18) | 428 (24) |
| QRS Interval, msec | 97 (12) | 91 (16) |
| Heart Rate, beats per minute | 67 (10) | 65(11) |
| Left bundle branch block | 6 (0.05%) | 49 (1%) |
| Biomarkers, median [IQR] | ||
| CRP, mg/L | 2.38 [1.20, 4.25] | |
| NT-proBNP, ng/dL | 103 [54, 197] | |
| High sensitivity troponin T, ng/L | 4.72 [2.99, 9.07] |
Using backwards selection to derive the prediction model, the following variables were retained as independent risk factors for SCD in the ARIC study: age, male sex, African American race, current smoker, systolic blood pressure, use of antihypertensive medication, diabetes, serum potassium, serum albumin, HDL, eGFR, and the corrected QT interval (Table 2). Serum potassium fit the model as a quadratic term, and eGFR fit the model best when it was divided into categories of <60, 60–90, and >90 ml/min/1.73m2. Age and sex-based interactions were not significant, and we did not derive separate age and sex-based equations.
Table 2.
Estimated Beta Coefficients in the Derived SCD Prediction Model.
| Esimated Beta | Standard Error | |
|---|---|---|
| Demographics | ||
| Age (centered at 54, per 1 year increase) | 0.043 | 0.014 |
| Male | 0.858 | 0.175 |
| African American | 0.597 | 0.179 |
| CVD Risk Factors | ||
| Current smoker | 0.881 | 0.159 |
| Systolic blood pressure (per 1SD increase) | 0.347 | 0.062 |
| Antihypertensive medication use | 0.322 | 0.171 |
| Diabetes | 0.792 | 0.181 |
| Serum Measures | ||
| Potassium (centered at 4.4, per 0.5 mmol/L increase) | −0.004 | 0.007 |
| Quadratic potassium term | 0.0009 | 0.0003 |
| Albumin (per 0.3 g/dL decrease) | 0.253 | 0.081 |
| HDL (per SD decrease) | 0.202 | 0.097 |
| eGFR 60–90 ml/min/1.73m2 | 0.315 | 0.175 |
| eGFR <60 ml/min/1.73m2 | 0.849 | 0.348 |
| QTc Interval (per SD increase) | 0.158 | 0.052 |
Based on 10 years of follow-up, the SCD prediction equation derived from the ARIC study is 1–0.99522exp(Σβx – 3.0702734) where β is the regression coefficient and x is the level for each risk factor.
SCD Prediction
Based on 10 years of follow-up, the SCD prediction equation derived from the ARIC study is 1–0.99522(Σβx–3.0702734) where β is the regression coefficient and x is the level for each risk factor. The SCD prediction model resulted in moderate prediction of SCD (Table 3) and was slightly better than the ACC/AHA CVD Pooled Cohort risk equations in discriminating 10-year risk of SCD in ARIC and CHS. The SCD prediction model revealed a large gradient of risk in both ARIC and CHS with the highest decile having a 10-year SCD risk of approximately 5% in ARIC and over 10% in CHS (Figures 2A and 2B). Further, the overall prediction for SCD was similar and accurate using both the original, uncalibrated SCD prediction score (Table 3) and the recalibrated equation (supplemental Table 1). In addition, the ACC/AHA CVD Pooled Cohort risk equations were not well calibrated in CHS as the model’s predicted risk of SCD was consistently higher than the observed one using both the original and recalibrated equation. Further, the SCD prediction model results in slightly enhanced discrimination of SCD risk compared with non-SCD in both cohorts (Table 3). In comparison the ACC/AHA CVD Pooled Cohort risk equations are better at predicting non-SCD than SCD in both cohorts. Neither one of the two prediction models was well calibrated for the non-SCD outcome even after recalibrating using the baseline ten year event rates of non-SCD in each cohort. Finally, the prediction of all-cause mortality using either one of these prediction equations was relatively weak.
Table 3.
Model Discrimination and Calibration for SCD, Non-Sudden Cardiac Death, and All-cause Mortality using the 10 Year Risk Prediction Equation
| ARIC | CHS | |
|---|---|---|
|
| ||
| Sudden Cardiac Death | ||
| SCD Prediction Score | ||
| C-statistic (95% CI) | 0.820 (0.785, 0.854) | 0.745 (0.701, 0.789) |
| Calibration chi-square (p-value) | 11.46 (0.25) | 13.74 (0.088) |
| ACC/AHA CVD risk equation | ||
| C-statistic (95% CI) | 0.808 (0.772, 0.844) | 0.743 (0.700, 0.768) |
| Calibration chi-square (p-value) | 851.0 (<0.001) | 24.71 (0.003) |
| Non-Sudden Cardiac Death | ||
| SCD Prediction Score | ||
| C-statistic (95% CI) | 0.818 (0.784, 0.853) | 0.720 (0.693, 0.747) |
| Calibration chi-square (p-value) | 20.90 (0.01) | 45.17 (<0.001) |
| ACC/AHA CVD risk equation | ||
| C-statistic (95% CI) | 0.823 (0.791, 0.856) | 0.753 (0.728, 0.779) |
| Calibration chi-square (p-value) | 800.6 (<0.001) | 34.72 (<0.001) |
| All-Cause Mortality | ||
| SCD Prediction Score | ||
| C-statistic (95% CI) | 0.731 (0.715, 0.747) | 0.681 (0.666, 0.696) |
| Calibration chi-square (p-value) | 6058 (<0.001) | 91.40 (<0.001) |
| ACC/AHA CVD risk equation | ||
| C-statistic (95% CI) | 0.728 (0.712, 0.743) | 0.692 (0.678, 0.707) |
| Calibration chi-square (p-value) | 34.59 (<0.001) | 68.32 (<0.001) |
Figure 2A. ARIC 10 year calibration plot using the SCD prediction model.
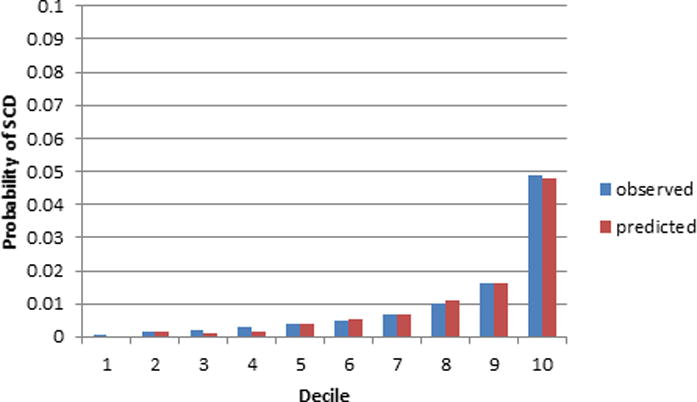
The x-axis refers to deciles of predicted SCD risk. Each bar in the graph represents the average observed and predicted SCD risk.
Figure 2B. CHS 10 year calibration plot using the SCD prediction model.
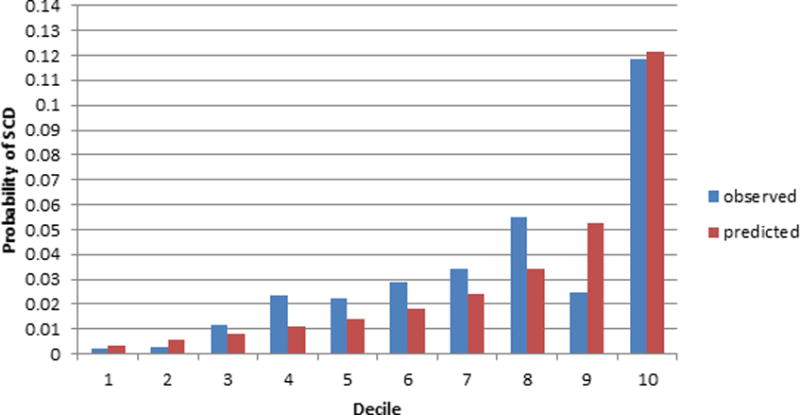
The x-axis refers to deciles of predicted SCD risk. Each bar in the graph represents the average observed and predicted SCD risk.
The SCD prediction equation appeared to discriminate SCD risk similarly in African Americans and Whites (Figure 3). In ARIC, SCD prediction appeared stronger in women compared to men; however, the limited number of events prevents a rigorous evaluation of differences across subgroups.
Figure 3.
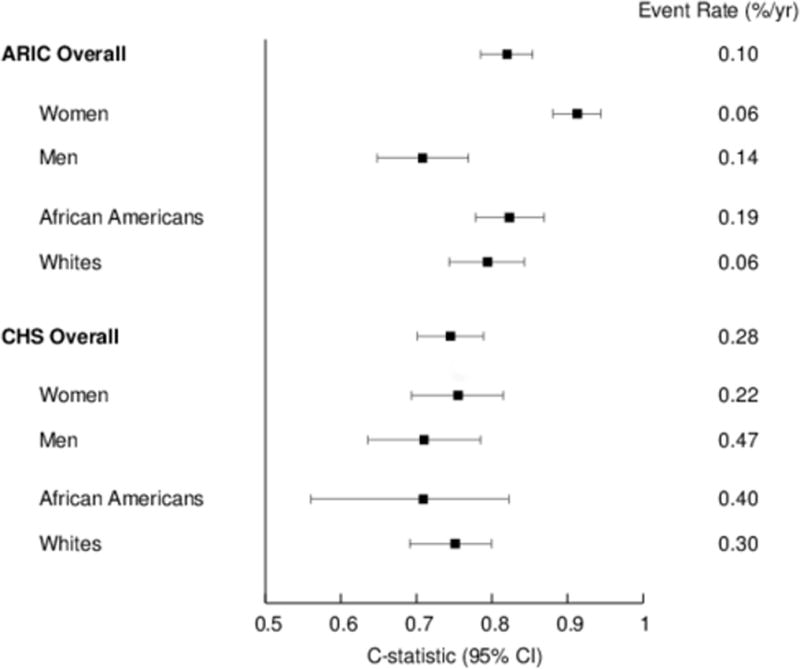
SCD Prediction stratified by Sex and Race
Left Ventricular Ejection Fraction Analysis
Of the 5,299 participants with echocardiographic data available, only 57 (1.1%) had an EF less than 50% or described as abnormal. The mean duration of follow-up after the initial echocardiographic study was 13.0 years in ARIC and 9.3 years in CHS. The significant majority of participants in our study, both with and without SCD events, had a normal LVEF. The addition of LVEF to the baseline prediction model did not result in any significant enhancement of the c-statistic in either ARIC or CHS.
Biomarker Analysis
In CHS, NT-pro BNP and high sensitivity troponin T were associated with SCD risk in unadjusted analysis. However, when CRP, NT-pro BNP, and high sensitivity troponin T were added individually to the final multivariable model, none was significant in the adjusted analysis (Table 4).
Table 4.
Association of Biomarkers with Sudden Cardiac Death in CHS
| Biomarker Quintiles
|
Linear model
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Per Doubling | P-value | |
|
|
|
||||||
| CRP | |||||||
| CRP Range (mg/L) | <1.05 | 1.05–1.99 | 2.00–3.18 | 3.19–5.68 | >5.68 | ||
| Unadjusted; HR (95% CI) | 1.00 (ref) | 0.77 (0.45, 1.32) | 1.08 (0.66, 1.78) | 1.14 (0.69, 1.89) | 1.50 (0.92, 2.46) | 1.10 (0.93, 1.30) | 0.275 |
| Adjusted*; HR (95% CI) | 1.00 (ref) | 0.75 (0.44, 1.29) | 1.04 (0.63, 1.72) | 0.92 (0.54, 1.56) | 1.19 (0.71, 1.99) | 1.03 (0.87, 1.23) | 0.712 |
| NT-pro BNP | |||||||
| NT-pro BNP Range (pg/dL) | <51 | 51–91 | 92–156 | 157–298 | >298 | ||
| Unadjusted; HR (95% CI) | 1.00 (ref) | 1.13 (0.63, 2.01) | 1.62 (0.93, 2.81) | 1.88 (1.09, 3.27) | 2.96 (1.65, 5.33) | 1.18 (1.00, 1.39) | 0.045 |
| Adjusted*; HR (95% CI) | 1.00 (ref) | 1.10 (0.61, 1.96) | 1.51 (0.86, 2.66) | 1.56 (0.87, 2.79) | 1.83 (0.95, 3.50) | 1.14 (0.90, 1.28) | 0.418 |
| High Sensitivity troponin T | |||||||
| High Sensitivity troponin T Range (pg/mL) | <3.00 | 3.00–5.44 | 5.45–8.16 | 8.17–12.94 | >12.94 | ||
| Unadjusted; HR (95% CI) | 1.00 (ref) | 0.53 (0.25, 1.14) | 1.51 (0.86, 2.64) | 1.97 (1.16, 3.37) | 3.75 (2.24, 6.26) | 1.50 (1.25, 1.81) | <0.001 |
| Adjusted*; HR (95% CI) | 1.00 (ref) | 0.41 (0.19, 0.89) | 0.96 (0.54, 1.72) | 1.03 (0.58, 1.83) | 1.39 (0.76, 2.55) | 1.14 (0.88, 1.49) | 0.327 |
The final multivariable model selected based on the summary estimates from the derivation cohort: age, male sex, African American race, current smoker, systolic blood pressure, use of antihypertensive medication, diabetes, potassium, albumin, HDL, eGFR, and QTC.
Discussion
In this combined analysis from two large US-based cohorts with over 17,000 adults without a clinical history of cardiovascular disease, we derived and validated a SCD prediction model that identified a gradient of risk across the general population. In ARIC, approximately three-fourths of the participants had a 10-year SCD risk of less than 1%. The highest decile of risk, however, approached 5% over 10 years, suggesting that this panel of risk factors can distinguish a large gradient in SCD risk among middle-aged adults. Similarly, a gradient of risk was observed in CHS with the lowest and highest deciles having predicted SCD risks of approximately 1.5 and 11% respectively over a 10-year follow-up period. Our study is the first to derive and validate a generalizable risk score that provides well-calibrated, absolute risk estimates across different risk strata in an adult population without a clinical diagnosis of cardiovascular disease. It is important to delineate these risks as the significant majority of SCD cases arise from this subgroup of the population.8, 9, 40 Finally, the SCD rates identified in the highest decile are significantly lower than the current clinical thresholds for ICD implantation in the primary prevention of SCD (annualized SCD rate approximately 3%).4, 41 Our findings, however, provide a strong step toward distinguishing SCD risk across the general population and can help target future non-ICD strategies aimed at SCD prevention for the highest risk subgroups in the community.
The SCD prediction model outperformed the 2013 ACC/AHA CVD Pooled Cohort risk equations especially in providing calibrated predictions of SCD risk in both community-based cohorts. The newly derived prediction model contained nearly all the variables present in the 2013 ACC/AHA CVD Pooled Cohort risk equation plus additional, arrhythmia-specific variables. In particular, both potassium and the corrected QT interval were selected as risk markers for SCD prediction. In addition, estimated GFR, which has strong, independent associations with SCD was also selected as an independent risk factor.42–44 The 2013 ACC/AHA CVD Pooled Cohort risk equations were developed to provide 10-year risk estimates of developing a first cardiovascular event defined as a nonfatal MI or CHD death or fatal or nonfatal stroke among individuals free from CVD at the beginning of the evaluation period. Although SCD events comprised a portion of CHD deaths, there were other cardiovascular events, both fatal and nonfatal, present in the combined endpoint that were used to generate the 2013 ACC/AHA CVD Pooled Cohort risk equation and likely limit its performance for SCD prediction.
To the best of our knowledge, our study is one of the first to identify low albumin concentration as an independent SCD risk factor in both cohorts suggesting that it is an important marker for arrhythmic mortality. Prior studies have demonstrated an association between low serum albumin and increased cardiovascular morbidity including coronary heart disease, myocardial infarction, and heart failure.43–46 Experimental models have also demonstrated that low serum albumin reflects the overall inflammatory burden.46 In our study of over 17,000 participants without coronary heart disease, prevalent heart failure, or stroke, low serum albumin levels may be a marker of subclinical cardiac dysfunction that subsequently increases the risk for arrhythmias and SCD.
The overwhelming majority of participants in both cohorts had a normal LVEF. As such, an echocardiographic-based assessment of systolic function among individuals in the general population provides limited insight from the standpoint of SCD risk stratification, largely because of the low prevalence of systolic dysfunction. In addition, the preserved LVEF group comprised the majority of the SCD cases that occurred during the approximately 10 years of follow-up after the echocardiogram. Recent data from the Oregon Sudden Unexpected Death Study also support that the majority of patients who died suddenly did not have a depressed LVEF documented prior to the cardiac arrest.40
Our findings from these prospective cohort studies support previous community-based surveillance studies from the early 1990’s conducted in Seattle and Chicago that demonstrated the presence of racial disparities in SCD risk after adjusting for service-related variables including cardiopulmonary resuscitation, response times of emergency medical services, and the likelihood of being hospitalized after the cardiac arrest.47, 48 A recent autopsy-based study in San Francisco found a more than 3-fold higher incidence of SCD among African Americans compared to Whites.49 In addition, the prediction model performed similarly in African Americans and Whites.
Several limitations should be considered when interpreting our findings. First, individuals in the ARIC study were younger than CHS participants at the time of their initial assessment for baseline characteristics. Evaluation of potential risk factors at a later period in life may enhance SCD prediction in the elderly population. The variables assessed in our study were obtained at only one time point. Our study does not evaluate how changes in risk factors affect SCD risk, nor can we discern whether they are modifiable risk factors. In addition, the events in our analyses are not contemporary as formal adjudication protocols for SCD ended in 2001 in the ARIC study and 2006 in CHS. As a result, ongoing adjudication protocols in these cohorts and a larger number of SCD cases will enhance the predictive accuracy of the SCD equation especially across the different deciles of risk. Further, the echocardiograms were obtained in only 30% of participants, and a detailed assessment of different LVEF categories as predictors of SCD could not be performed. Finally, the three biomarkers in our analysis were not measured at baseline in ARIC participants. As a result, their lack of improvement for SCD prediction is based on CHS data only.
In conclusion, our study is one of the first to develop and validate a prediction model for SCD risk across middle aged and elderly populations in the United States. After a systematic evaluation of demographic, clinical, laboratory, electrocardiographic, echocardiographic, and biological markers, we identified a substantially elevated 10-year risk of SCD among participants in the highest decile of predicted SCD risk. These findings allow identification of high-risk individuals appropriate for targeted interventions designed to reduce the burden of SCD.
Supplementary Material
Clinical Perspective.
What is new?
This project provides the first, generalizable risk score for sudden cardiac death in adults without a history of cardiovascular disease.
Using 17,884 adults 45 years of age and older without a history of cardiovascular disease across 2 large population-based studies, we derived and validated a SCD prediction model that outperformed the 2013 ACC/AHA Pooled Cohort risk equations for the prediction of SCD.
The SCD prediction model included the following risk factors: age, male sex, African American race, current smoking, systolic blood pressure, use of antihypertensive medication, diabetes, serum potassium, serum albumin, HDL, estimated GFR, and QTc interval.
What are the clinical implications?
Our findings provide a strong step toward distinguishing SCD risk across the general population and can help target future non-ICD strategies aimed at SCD prevention for the highest risk subgroups of the general population.
A low left ventricular ejection fraction was present in only 1% of participants and did not enhance SCD prediction.
Acknowledgments
The authors thank the staff and participants of the ARIC and CHS cohorts for their important contributions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Sources. Dr. Deo is supported by the National Institutes of Health grant K23DK089118. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This study was additionally supported by grants RC1-HL099452, R01HL116747, and R01HL111089 from the National Heart, Lung, and Blood Institute and 16EIA26410001 from the American Heart Association.
This research was also supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/PI.htm.
Footnotes
Disclosures: None
References
- 1.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen P-S, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125:620–637. doi: 10.1161/CIRCULATIONAHA.111.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, Schatzkin A. Sudden death: lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–149B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 7.Josephson M, Wellens HJ. Implantable defibrillators and sudden cardiac death. Circulation. 2004;109:2685–2691. doi: 10.1161/01.CIR.0000129322.97266.F3. [DOI] [PubMed] [Google Scholar]
- 8.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990’s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 9.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 10.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 11.IOM (Insttitute of Medicine) Strategies to improve cardiac arrest survival: A time to act. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 12.Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM, Jr, Chen PS, Chugh SS, Costantini O, Exner DV, Kadish AH, Lee B, Lloyd-Jones D, Moss AJ, Myerburg RJ, Olgin JE, Passman R, Stevenson WG, Tomaselli GF, Zareba W, Zipes DP, Zoloth L. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014;129:516–526. doi: 10.1161/CIRCULATIONAHA.113.007149. [DOI] [PubMed] [Google Scholar]
- 13.Deyell MW, Krahn AD, Goldberger JJ. Sudden cardiac death risk stratification. Circ Res. 2015;116:1907–1918. doi: 10.1161/CIRCRESAHA.116.304493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, Defilippi C, See V, Deo R, Siscovick D, Stein PK, Lloyd-Jones D. Inflammation and sudden cardiac death in a community-based population of older adults: The Cardiovascular Health Study. Heart Rhythm. 2013;10:1425–1432. doi: 10.1016/j.hrthm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Patton KK, Sotoodehnia N, DeFilippi C, Siscovick DS, Gottdiener JS, Kronmal RA. N-terminal pro-B-type natriuretic peptide is associated with sudden cardiac death risk: the Cardiovascular Health Study. Heart Rhythm. 2011;8:228–233. doi: 10.1016/j.hrthm.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, Defilippi C, Dickfeld T, Deo R, Siscovick D, Stein PK, Lloyd-Jones D. Cardiomyocyte Injury Assessed by a Highly Sensitive Troponin Assay and Sudden Cardiac Death in the Community: The Cardiovascular Health Study. J Am Coll Cardiol. 2013;22:2112–2120. doi: 10.1016/j.jacc.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 18.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 19.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. New Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 23.Havranek EP, Emsermann CD, Froshaug DN, Masoudi FA, Krantz MJ, Hanratty R, Estacio RO, Dickinson LM, Steiner JF. Thresholds in the relationship between mortality and left ventricular hypertrophy defined by electrocardiography. J Electriocardiol. 2008;41:342–350. doi: 10.1016/j.jelectrocard.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241–e250. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
- 25.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e235–e240. doi: 10.1161/CIRCULATIONAHA.108.191095. [DOI] [PubMed] [Google Scholar]
- 26.Skelton TN, Andrew ME, Arnett DK, Burchfiel CM, Garrison RJ, Samdarshi TE, Taylor HA, Hutchinson RG. Echocardiographic left ventricular mass in African-Americans: the Jackson cohort of the Atherosclerosis Risk in Communities Study. Echocardiography. 2003;20:111–120. doi: 10.1046/j.1540-8175.2003.03000.x. [DOI] [PubMed] [Google Scholar]
- 27.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Jr, Ribeiro LG, Miller RR. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 28.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 29.Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet. 2001;358:439–444. doi: 10.1016/s0140-6736(01)05620-3. [DOI] [PubMed] [Google Scholar]
- 30.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 31.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O’Leary D, Manolio TA. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 32.Soliman EZ, Prineas RJ, Case LD, Russell G, Rosamond W, Rea T, Sotoodehnia N, Post WS, Siscovick D, Psaty BM, Burke GL. Electrocardiographic and clinical predictors separating atherosclerotic sudden cardiac death from incident coronary heart disease. Heart. 2011;97:1597–1601. doi: 10.1136/hrt.2010.215871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;160:464–470. doi: 10.1016/j.ahj.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried L, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B. Vitamin D, Parathyroid Hormone and Sudden Cardiac Death: Results from the Cardiovascular Health Study. Hypertension. 2011;58:1021–1028. doi: 10.1161/HYPERTENSIONAHA.111.179135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deo R, Katz R, de Boer IH, Sotoodehnia N, Kestenbaum B, Mukamal KJ, Chonchol M, Sarnak MJ, Siscovick D, Shlipak MG, Ix JH. Fibroblast growth factor 23 and sudden versus non-sudden cardiac death: the Cardiovascular Health Study. Am J Kidney Dis. 2015;66:40–46. doi: 10.1053/j.ajkd.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 37.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P, Group CHDRP Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 38.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 39.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 40.Narayanan K, Reinier K, Uy-Evanado A, Teodorescu C, Chugh H, Marijon E, Gunson K, Jui J, Chugh SS. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–1738. doi: 10.1161/CIRCULATIONAHA.113.002539. [DOI] [PubMed] [Google Scholar]
- 41.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 42.Deo R, Sotoodehnia N, Katz R, Sarnak MJ, Fried LF, Chonchol M, Kestenbaum B, Psaty BM, Siscovick DS, Shlipak MG. Cystatin C and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes. 2010;3:159–164. doi: 10.1161/CIRCOUTCOMES.109.875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillum RF, Makuc DM. Serum albumin, coronary heart disease, and death. Am Heart J. 1992;123:507–513. doi: 10.1016/0002-8703(92)90667-k. [DOI] [PubMed] [Google Scholar]
- 44.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Tang WW, Methvin A, Smith AL, Bauer DC, Newman AB, Kim L, Harris TB, Kritchevsky SB, Butler J, Health ABCS. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. Am Heart J. 2010;160:279–285. doi: 10.1016/j.ahj.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djousse L, Rothman KJ, Cupples LA, Levy D, Ellison RC. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation. 2002;106:2919–2924. doi: 10.1161/01.cir.0000042673.07632.76. [DOI] [PubMed] [Google Scholar]
- 46.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 47.Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, Barrett J. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. New Eng J Med. 1993;329:600–606. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- 48.Cowie MR, Fahrenbruch CE, Cobb LA, Hallstrom AP. Out-of-hospital cardiac arrest: racial differences in outcome in Seattle. Am J Public Health. 1993;83:955–959. doi: 10.2105/ajph.83.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinhaus DA, Vittinghoff E, Moffatt E, Hart AP, Ursell P, Tseng ZH. Characteristics of sudden arrhythmic death in a diverse, urban community. Am Heart J. 2012;163:125–131. doi: 10.1016/j.ahj.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


