Abstract
CD4+ T cells are the primary HIV-1 target cell, with the vast majority of these cells residing within lymphoid tissue compartments throughout the body. Predictably, HIV-1 infection, replication, localization, reservoir establishment and persistence, as well as associated host immune and inflammatory responses and disease pathology principally take place within the tissues of the immune system. By virture of the fact that the virus-host struggle is played out within lymphoid and additional tissues compartments in HIV-1 infected individuals it is critical to understand HIV-1 infection and disease within these relevant tissue sites; however, there are obvious limitations to studying these dynamic processes in humans. Nonhuman primate (NHP) research has provided a vital bridge between basic and preclinical research and clinical studies, with experimental SIV infection of NHP models offering unique opportunities to understand key processes of HIV-1 infection and disease that are either not practically feasible or ethical in HIV-1 infected humans. In this review we will discuss current approaches to studying the tissue based immunopathogenesis of AIDS virus infection in NHPs, including both analyses of tissues obtained at biopsy or necropsy and complementary non-invasive imaging approaches that may have practical utility in monitoring HIV-1 disease in the clinical setting.
Introduction
HIV-1 pathogenesis and disease progression result from the cumulative effects of complex and dynamic host-viral interactions that begin during the early acute stage of infection and in the absence of therapeutic intervention continue through end-stage disease. Upon viral transmission to a susceptible host, host innate and adaptive immune responses are induced and amplified within infected tissues. However, some of these responses, intended to limit or clear the infection, can also have deleterious effects, potentially enhancing viral replication and inducing inflammation that can lead to tissue damage and immunopathology. This host-viral interplay is exemplified in the gastrointestinal (GI) tract, where extensive early viral replication results in massive depletion of lamina propria CD4+ T cells, loss of important mucosal immune cell subsets (i.e. CD103+ DCs, TH17 cells, and IL-22+ lymphocytes) [1] and GI tract mucosal damage leading to microbial translocation and resultant systemic inflammation and pathological immune activation [2,3]. Because HIV-1 replication, host responses and resultant disease pathology occurs within tissues (i.e. lymphoid tissues, GI tract, CNS, etc.) of infected humans it is critical to understand the disease process within these tissues where the host and virus interact. Nonhuman primate (NHP) research provides a vital bridge between basic and preclinical research and clinical studies. Experimental SIV infection of NHP models offer unique opportunities that are either not practically feasible or ethical in HIV infected humans, such as: i) examining the critical early stages of infection following a known time of viral transmission, with a defined virus, particularly for mucosal transmission; ii) pathogenesis studies with longitudinal tissue sampling; and iii) testing novel preventative, intervention and “cure” strategies that are conceptually unproven and/or potentially harmful. NHPs provide the most physiologically relevant HIV-1 models, compared to alternative small animal models such as humanized mice, with the additional advantage of longitudinal tissue sampling, up to and including tissues obtained at scheduled necropsy, allowing a level of extensive immunopathologic tissue based analysis that is not possible studying HIV-infected humans [4–7]. In this review we will cover current approaches to studying the tissue based immunopathogenesis of HIV/SIV virus infection in NHPs, including both analyses of tissues obtained at biopsy or necropsy and complementary non-invasive imaging approaches.
SIV Pathogenesis: Imaging GI tract damage and LT Fibrosis
Tissue based analyses in SIV infected NHPs focusing on tissue compartments relevant to immunopathogenesis have been essential in understanding key aspects and processes that drive lentiviral disease progression. Given that the intestinal immune system is considered the largest single immunologic organ in the body, containing upwards of 40% of all CD4+ T lymphocytes [8,9], the preferred cellular target for the virus [10], it is not surprising that this organ system is impacted early and severely by HIV/SIV infections and plays a key role in disease progression. Tissue based histological imaging studies have demonstrated that damage to the GI tract epithelial barrier shortly after SIV infection leads to local translocation of microbial constituents from the lumen of the intestine into the lamina propria and distal dissemination into systemic tissue compartments [3]. Deep sequencing analysis of bacterial DNA isolated from tissues of infected animals revealed a preference for bacterial translocation of the phylum Proteobacteria, suggesting that these bacteria preferentially translocate into the host [11]. These observations provided compelling direct evidence for GI tact pathology, in particular, alterations in intestinal structural integrity and function leading to translocation of bacteria with putative pathogenic bacterial species that may represent an even greater potential for immune activation [11]. Collectively, these data strongly suggest that GI tract damage plays a key role in contributing to the heightened state of chronic inflammation and pathological immune activation, as well as progressive immune deficiency and immune deregulation during SIV infection.
However, direct evidence that GI tract damage leading to microbial translocation independently causes systemic inflammation and immune activation was still lacking. To this end, we developed a NHP model of GI tract damage in the absence of SIV infection to directly determine the connection between GI tract damage, microbial translocation and systemic inflammation and immune activation as an important independent driver of characteristic pathologic features observed in HIV/SIV infections [12]. We developed a NHP model of chemically induced colitis, utilizing dextran sulfate sodium (DSS), in SIV uninfected rhesus macaques (RMs) and demonstrated that damage to the integrity of the GI tract mucosal barrier, in the absence of SIV infection, caused local and systemic microbial translocation, with corresponding inflammation and immune activation similar in scope and character to that seen in chronically SIV-infected animals [12]. Importantly, sustained GI tract damage recapitulated hallmark pathological features of the SIV disease, including fibrosis of secondary lymphoid tissues [12]. This study directly demonstrated that GI tract damage leading to microbial translocation is independently sufficient to drive local and systemic inflammation and immune activation in the absence of SIV infection, and highlights GI tract damage as a key pathological feature of HIV/SIV disease where adjunctive therapy to modulate these processes could provide clinical benefit, independent of any direct impact on viral replication.
While histological imaging was necessary to demonstrate that GI tract damage leading to microbial translocation drives local and systemic inflammation and immune activation and associated tissue pathology; given the limited accessibility of tissue samples in patients, developing novel non-invasive clinically relevant techniques to monitor and image these pathogenic processes in HIV infected individuals is essential. Thus, we explored the utility of using an FDA approved gadolinium-based magnetic resonance imaging (MRI) contrast agent (gadofosveset trisodium; Ablavar®) that reversibly binds to serum albumin and has been used clinically for magnetic resonance angiography to diagnose vascular disease [13] to monitor and quantify GI tract inflammation in our SIV-negative NHP colitis model [12]. We longitudinally imaged RMs, acquiring fat saturated T1-weighted MRIs following sequential DSS treatment cycles (which induced histologically documented sustained chronic colitis, inflammation, immune activation, and microbial translocation) and performed quantitative MRI scoring, assessing the severity of colitis using clinically relevant MRI criteria for bowel inflammation [14]. In this NHP model of chemically induced colitis we demonstrated the utility of utilizing a novel non-invasive MRI approach for evaluating the severity of colitis longitudinally, with findings corroborated by parallel histopathological analyses, suggesting its potential usefulness in the clinical setting (Figure 1). While these studies have obvious translatability to HIV-1 disease, these results may also have broad applicability in noninvasive monitoring of other diseases like IBD and colon cancer in human patients.
Figure 1. Imaging GI tract inflammation by MRI.
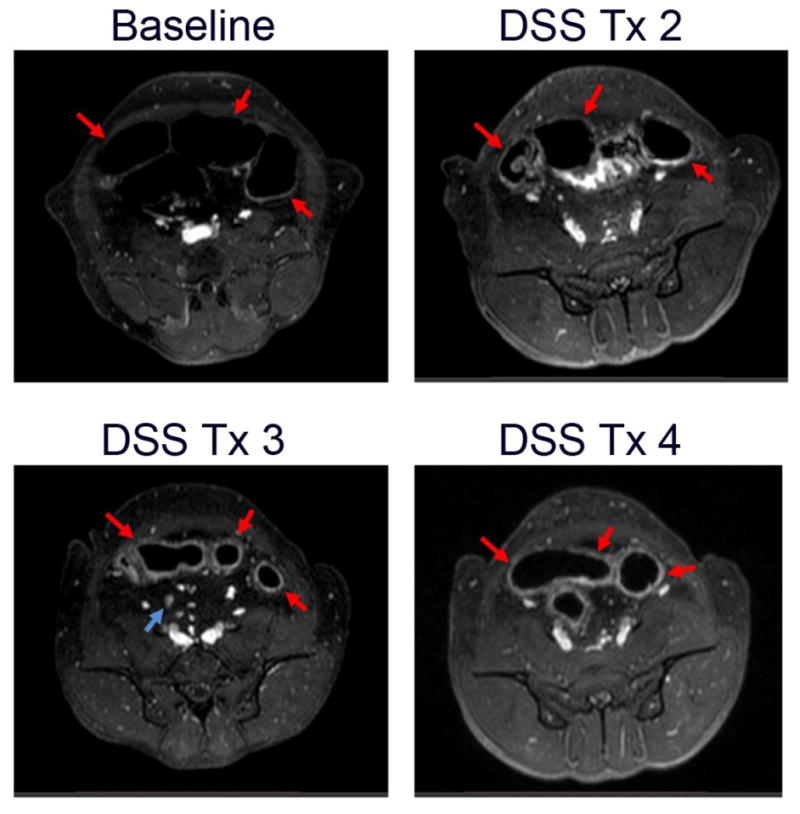
(A) Baseline axial Ablavar® enhanced MRI shows normal sigmoid colon segments (red arrows). After 2 (B), 3 (C) and 4 (D) treatment cycles of DSS (10–14 days DSS followed by 14 days off DSS equals 1 cycle) MRI reveals progressively increased thickening of the sigmoid segments with prominent inflammatory changes visualized as enhancement of the bowel wall with Ablavar® secondary to induced bowel inflammation (red arrows) and mesenteric adenopathy (blue arrow).
The central feature of HIV-1 disease is the progressive loss of CD4+ T cells. While monitoring CD4+ T cell numbers in the peripheral blood has provided key insights into HIV-1 disease and is a clinically validated surrogate marker for staging HIV-1 infection that allows convenient monitoring of patients, it is clear that the peripheral blood compartment represents only a small fraction of the body’s total CD4+ T cell population [15]. Secondary lymphoid tissues (i.e. lymph nodes, spleen, gut associated lymphoid tissue (GALT), etc.) harbor most of the body’s CD4+ T cells and play an essential role in their survival and maintenance. CD4+ T cell loss in HIV-1 infection is a dynamic and progressive process of cell death due to infection, activation, and loss of needed survival signals that is played out within lymphoid tissues (LTs) [12,16–19]. A variety of different mechanisms have been invoked to account for the progressive loss of CD4+ T cells, but progressive inflammation related structural damage to secondary LTs has been demonstrated to play a significant role in this process [2,3,12,20–23].
Studies in SIV infected NHPs have been essential in understanding this process and demonstrated that multiple pathological changes in the morphology, structure and cellular composition of LTs begin shortly after SIV acquisition and are progressive throughout the infection [24–28], confirming and extending findings from HIV-infected humans [29–35] (Figure 2). Histological imaging studies in SIV infected NHPs have been important to understanding not only the timing and progressive nature of LT pathological damage and its causal relationship to CD4+ T cell loss, but also potential mechanisms and underlying pathways leading to these pathological outcomes [23,36,37]. Collectively, these studies have demonstrated that LT fibrosis, characterized by extensive deposition of collagen and other extracellular matrix proteins, is a hallmark pathological feature of HIV-1 and pathogenic SIV infections that disrupts trophic microenviroments in secondary LTs required for maintenance of CD4+ T cells, contributing to loss of these cells and ultimately to disease progression [23,35,36,38,39]. Because LT fibrosis persists and CD4+ T cells in LTs of HIV-infected individuals remain depleted during cART, using an SIV NHP model we sought to determine if antifibrotic therapy would decrease LT fibrosis resulting in improved reconstitution of peripheral and LT CD4+ T cells [40]. We demonstated that combining the antifrotic drug pirfenidone with an ART regimen was associated with greater preservation/restoration of CD4+ T cells in peripheral blood and LTs than ART alone, suggesting a potential role for antifibrotic drug treatment as adjunctive therapy with ART to improve immune reconstitution [40]. While these NHP studies have been informative, given the challenges of collecting longitudinal LT samples from HIV-infected patients, development of new non-invasive clinically relevant techniques to monitor and image LT pathological damage and the impact of therapeutic interventions on these processes in HIV-infected individuals will represent a useful advance.
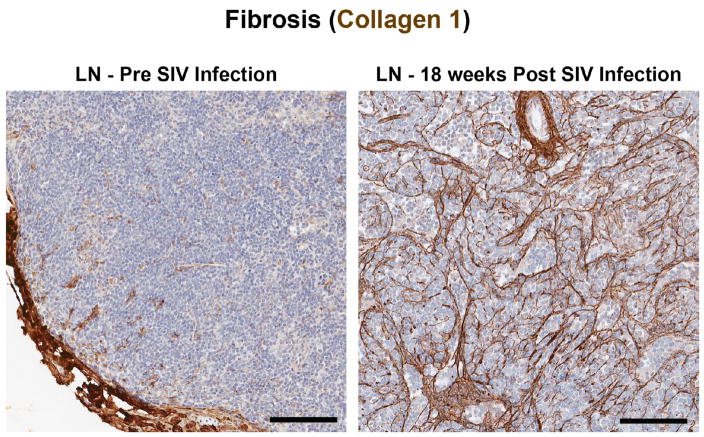
Figure 2. Imaging lymph node fibrotic damage during SIV-infection.
Histological images of collagen 1 stained axillary lymph nodes showing the lack of fibrosis and normal architecture at baseline before (left panel) and the significantly increased fibrotic damage (right panel) following SIV infection (18 weeks post infection). Scale bar = 100 μm.
Reservoirs
With the advent of potent and safe combination antiretroviral drugs, the landscape of HIV-1 disease has changed in the developed world, with HIV-1 infected patients having a near normal life expectancy. While in most cases combination antiretroviral therapy (cART) leads to reductions in viral replication to undetectable levels in the blood by current methods, it is quite clear that cART does not eradicate the virus from the infected host. Replication competent HIV-1 (and SIV) persists in reservoirs throughout the body, despite seemingly effective suppressive cART, with viral recrudescence inevitably occurring when cART is halted [41–43]. Thus, in order to realize a sterilizing HIV-1 cure, as was achieved in the Berlin patient, ultimately eliminating all viral reservoirs harboring replication competent virus appears necessary. Prior to developing practical strategies for reservoir eradication for clinical use it will be important to identify all relevant cellular and anatomic tissue reservoirs and sources of replication competent virus in the infected host on suppressive cART. While latently infected resting CD4+ T cells have been shown to be a major HIV-1 reservoir [44–46], recent work has shed additional insight into how and where the virus persists under “effective” cART with one controversial recent study suggesting that virus may also persist by actively replicating in LTs during cART [47]. In addition, extracellularly trapped HIV-1 on follicular dendritic cells (FDCs) within B cell follicles has been demonstrated to harbor replication competent virus [48,49]. Recent work in both humans and NHPs highlight B cell follicles as an important sanctuary site for viral persistence during cART and host immune viral control [50,51]. Collectively, these findings point to the importance of tissue compartments, particularly secondary LTs, in viral persistence and reservoir maintenance.
Imaging approaches for understanding reservoirs
Understanding the tissue compartments and cellular populations that comprise viral reservoirs will likely be important to design specific strategies to eliminate these potential sources of infectious virus. In this regard, the development of novel and sensitive approaches for the imaging of reservoirs and virus in situ has provided new important insight into reservoir biology and viral persistence. In 2012, Wang F. et al. [52] reported a new approach of in situ RNA hybridization (termed RNAscope) for host mRNAs with the sensitivity approaching single-RNA molecule visualization in individual cells, which was possible because of the assays exceptional specificity resulting in extremely low-to-no visible background. Because of its promise for detecting low abundant host RNA transcripts, we developed and optimized an RNAscope platform for the detection of HIV-1 and SIV RNA in tissue sections [53]. Utilizing this highly sensitive and specific approach, we documented the ability to sensitively detect vRNA in tissue sections, including individual virions bound on the follicular dendritic cell network (FDCn) both before (Figure 3) and even during, at greatly reduced levels, cART [53].
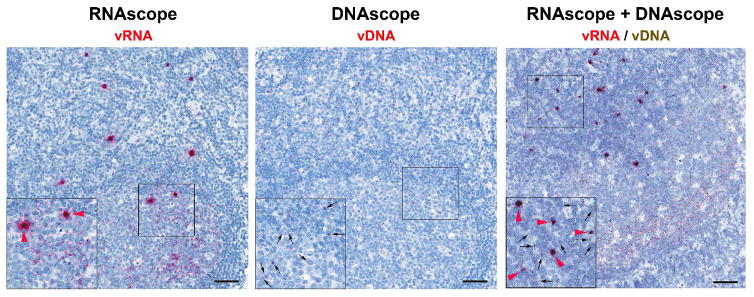
Figure 3. In situ detection of virus and infected cells in lymphoid tissue during SIV-infection.
Lymph node sections showing the sensitive dection of vRNA by RNAscope alone (left panel), vDNA by DNAscope alone (middle panel) and duplex dection of both vRNA and vDNA in the same tissue section by a combination RNAscope/DNAscope approach (right panel) in a chronically SIV infected rhesus macaque. Insets show a magnified region of the lymph node indicated by the black square. Black arrows point to vDNA+ cells, and red arrowheads point to productively infected vRNA+ cells. Individual viral particles bound to the FDC network are also evident but only in the RNAscope images (not highlighted). Scale bar = 50 μm.
Due to its ability to detect individual viral particles in situ, we reasoned that this approach could be modified and optimized for the detection of viral DNA in infected cells (termed DNAscope). Sensitive and specific detection of vDNA+ cells in situ in tissues would provide important insight into the biology of HIV/SIV reservoirs, including latently infected cells [54]. We demonstrated that DNAscope in situ hybridization is remarkably more rapid and reproducible than previous reported approaches to detect vDNA+ cells in formalin-fixed paraffin embedded (FFPE) tissues (i.e. in situ PCR) and detected vDNA+ cells (Figure 3) to nearly the level of sensitivity seen with quantitative PCR methods before and during cART [53]. Combining DNAscope with immunofluorescent analysis allowed us to phenotypically characterize cells harboring vDNA in situ, and confirmed that CD4+ T cells are the cell type that harbor the vast majority of vDNA in SIV+ RMs [53]. Collectively, using these new next-generation in situ hybridization approaches we determined that the B cell follicle was an important anatomical compartment that harbored vDNA+ and vRNA+ cells, as well as low levels of trapped virions on the FDCn during cART [53].
The ability to unambiguously identify and quantify latently HIV/SIV infected cells that are transcriptionally silent in their native in vivo environment could provide needed insight into reservoir persistence and maintenance. To this end, we developed an approach that combined RNAscope with DNAscope in order to visualize vRNA expression and vDNA+ cells in the same section of tissue (Figure 3). This approach may be valuable in understanding the frequency and location of truly latently infected (vDNA+/vRNA−) cells in their native tissue environments, and tissue responses to interventions intended to induce vRNA expression in latently infected cells.
The capacity to perform detailed quantitative immunophenotypic analysis in situ was significantly enhanced with the advent of an approach, termed “histo-cytometry”, that combines the quantitative expression analyses of multiple phenotypic markers typically provided by flow cytometry of disassociated single cell suspensions, with the valuable anatomical location information provided by confocal microscopy [55]. Due to the fact that this new analytical approach achieves quantitatively similar results to flow cytometry, while retaining detailed cellular positional information in situ, merging the highly sensitive RNAscope and DNAscope approaches with histo-cytometry holds great promise in obtaining new detailed immunophenotypic information of viral reservoirs in vivo. Like most current approaches utilized to quantify the HIV/SIV reservoir, these in-situ approaches can not discriminate between replication competent genomes that are capable of leading to infectious virions and de novo infections from defective genomes that result in non-infectious virions. The inability to determine the capacity of an infected cell that is either actively transcribing viral RNA (vDNA+/vRNA+) or transcriptionally silent (vDNA+/vRNA−) to produce de novo infectious virions in situ remains a major limition in the characterization and quantification of HIV/SIV reservoirs.
Recently, a new non-invasive technique was explored in an SIV infected NHP model to visualize SIV-infected cells in the entire body using an immunoPET/CT approach (SIV Env specific antibody-targeted positron emission tomography with computed tomography) [56]. Despite the disadvantage of using radioactive immune tracers, limited spatial resolution, background associated with antibody based imaging methods and need for improved dynamic range of specific immune contrast uptake over background, PET/CT potentially provides a unique approach to visualize compartments throughout the entire body that harbor virus and/or productively infected cells. This approach allowed localization of SIV and/or productively infected cells that expressed SIV Env on their surface in multiple tissues of chronically viremic, cART-treated and elite controller (EC) animals. In chronically viremic animals, before initiation of cART, administration of 64Cu-labeled SIV Gp120–specific antibody led to readily detectable signals in the gastrointestinal and respiratory tract, secondary lymphoid tissues and reproductive organs, with signal being significantly reduced after 34 weeks of cART, but still measurable above background, in the colon, spleen, male genital tract, NALT and individual lymph nodes of some animals [56]. These results demonstrate the potential ability of the immunoPET/CT approach to detect and localize virus and/or cells that are actively replicating virus throughout the body. While the authors suggest that this approach would be easily applicable to the clinic to monitor the effect of cART and to localize the potential residual virus infection during treatment, further work needs to be done to specifically compare this approach with other established quantitative measures of viral persistence in tissue compartments and to ascertain its feasibility in the clinic.
Summary
While most studies that focus on understanding HIV/SIV infections and disease are performed on conveniently obtained blood samples, it is important to remember that these infections are primarily diseases of tissues, in particular LTs [e.g., lymph nodes, spleen, mucosal associated lymphoid tissue (MALT), etc.], which are highly complex and compartmentalized organ systems. Thus, the opportunities afforded for tissue analysis in NHP SIV models offer unique opportunities to understand the processes that lead to disease and viral persistence in an infected host in the very tissues where the infection is located. Imaging techniques provide a powerful additional dimension to monitoring, quantifying and understanding the processes of tissue pathology and disease progression leading to immune breakdown and failure, as well as providing key insights into the location and phenotypes of viral reservoirs within important anatomical tissue sites. Exploring viral reservoirs and persistence in tissue compartments should be a priority in order to better define where potential infectious virus is hiding and the types of cells harboring virus in the infected host, which could provide important insight for the development of therapeutic “cure” strategies to specifically target these potentially diverse reservoirs located throughout the body.
Nonhuman primate (NHP) research has provided a vital bridge between basic and preclinical research and clinical studies.
-
Histological and noninvasive imaging approaches of SIV NHP models have afforded unique insights into key processes of HIV-1 infection and disease that are either not practically feasible or ethical in HIV-1 infected humans, including demonstrating:
direct and unequivocal evidence that GI tract damage leading to microbial translocation is an important and independent driver of local and systemic inflammation and immune activation in the absence of SIV infection;
lymphoid tissue fibrosis is a hallmark pathological feature of lentiviral infections that contribute to loss of CD4+ T cells and ultimately to disease progression;
the importance of secondary lymphoid tissue compartments, in particular the B cell follicle, in viral persistence during cART.
Acknowledgments
We would like to thank Jeffrey D. Lifson for his careful reading and thoughtful comments in the preparation of this review. This project has been funded with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 3.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fennessey CM, Keele BF. Using nonhuman primates to model HIV transmission. Curr Opin HIV AIDS. 2013;8:280–287. doi: 10.1097/COH.0b013e328361cfff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keckler MS, Hodara VL, Parodi LM, Giavedoni LD. Novel application of nonhuman primate tethering system for evaluation of acute phase SIVmac251 infection in rhesus macaques (Macaca mulatta) Viral Immunol. 2007;20:623–634. doi: 10.1089/vim.2007.0068. [DOI] [PubMed] [Google Scholar]
- 6.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schieferdecker HL, Ullrich R, Hirseland H, Zeitz M. T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J Immunol. 1992;149:2816–2822. [PubMed] [Google Scholar]
- 9.MacDonald TT, Spencer J. Ontogeny of the gut-associated lymphoid system in man. Acta Paediatr Suppl. 1994;83:3–5. doi: 10.1111/j.1651-2227.1994.tb13219.x. [DOI] [PubMed] [Google Scholar]
- 10.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klase Z, Ortiz A, Deleage C, Mudd JC, Quinones M, Schwartzman E, Klatt NR, Canary L, Estes JD, Brenchley JM. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol. 2015;8:1009–1020. doi: 10.1038/mi.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Hao XP, Lucero CM, Turkbey B, Bernardo ML, Morcock DR, Deleage C, Trubey CM, Smedley J, Klatt NR, Giavedoni LD, et al. Experimental colitis in SIV-uninfected rhesus macaques recapitulates important features of pathogenic SIV infection. Nat Commun. 2015;6:8020. doi: 10.1038/ncomms9020. This study reports the development of an SIV-negative NHP model of colitis and demonstrates that GI tract damage leading to local and systemic microbial translocation, and associated immune activation, are important determinants of AIDS pathogenesis. Furthermore, this study reports for the first time the use of an unique non-invasive MRI approach to monitor and quantify GI tract inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis M, Yanny S, Malcolm PN. Advantages of blood pool contrast agents in MR angiography: a pictorial review. J Med Imaging Radiat Oncol. 2012;56:187–191. doi: 10.1111/j.1754-9485.2012.02347.x. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich C, Fajfar A, Pawlik M, Hoffstetter P, Rennert J, Agha A, Jung EM, Ott C, Stroszczynski C, Schreyer AG. Magnetic resonance enterography with and without biphasic contrast agent enema compared to conventional ileocolonoscopy in patients with Crohn’s disease. Inflamm Bowel Dis. 2012;18:1842–1848. doi: 10.1002/ibd.22843. [DOI] [PubMed] [Google Scholar]
- 15•.Di Mascio M, Paik CH, Carrasquillo JA, Maeng JS, Jang BS, Shin IS, Srinivasula S, Byrum R, Neria A, Kopp W, et al. Noninvasive in vivo imaging of CD4 cells in simian-human immunodeficiency virus (SHIV)-infected nonhuman primates. Blood. 2009;114:328–337. doi: 10.1182/blood-2008-12-192203. Using in vivo SPECT imaging, this study provides new measures of the total body CD4+ T cell population size and suggests that the peripheral blood only contains between 0.3% and 0.5% of the CD4+ T cells that are localized within lymphoid tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doitsh G, Greene WC. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe. 2016;19:280–291. doi: 10.1016/j.chom.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan ES, Micci L, Fromentin R, Paganini S, McGary CS, Easley K, Chomont N, Paiardini M. Loss of Function of Intestinal IL-17 and IL-22 Producing Cells Contributes to Inflammation and Viral Persistence in SIV-Infected Rhesus Macaques. PLoS Pathog. 2016;12:e1005412. doi: 10.1371/journal.ppat.1005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi A, Sedano M, Beauchamp B, Punke EB, Mulla ZD, Meza A, Alozie OK, Mukherjee D, Garg H. HIV-1 Env Glycoprotein Phenotype along with Immune Activation Determines CD4 T Cell Loss in HIV Patients. J Immunol. 2016;196:1768–1779. doi: 10.4049/jimmunol.1501588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Wang X, Malam N, Lackner AA, Veazey RS. Persistent Simian Immunodeficiency Virus Infection Causes Ultimate Depletion of Follicular Th Cells in AIDS. J Immunol. 2015;195:4351–4357. doi: 10.4049/jimmunol.1501273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, Hamann D, Prins M, Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 21.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010;3:387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchetti G, Bellistri GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, Morace G, Gori A, Monforte AD. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 23.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdhury A, Del Rio Estrada PM, Tharp GK, Trible RP, Amara RR, Chahroudi A, Reyes-Teran G, Bosinger SE, Silvestri G. Decreased T Follicular Regulatory Cell/T Follicular Helper Cell (TFH) in Simian Immunodeficiency Virus-Infected Rhesus Macaques May Contribute to Accumulation of TFH in Chronic Infection. J Immunol. 2015;195:3237–3247. doi: 10.4049/jimmunol.1402701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demberg T, Mohanram V, Musich T, Brocca-Cofano E, McKinnon KM, Venzon D, Robert-Guroff M. Loss of marginal zone B-cells in SHIVSF162P4 challenged rhesus macaques despite control of viremia to low or undetectable levels in chronic infection. Virology. 2015;484:323–333. doi: 10.1016/j.virol.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijewardana V, Bouwer AL, Brown KN, Liu X, Barratt-Boyes SM. Accumulation of functionally immature myeloid dendritic cells in lymph nodes of rhesus macaques with acute pathogenic simian immunodeficiency virus infection. Immunology. 2014;143:146–154. doi: 10.1111/imm.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong JJ, Villinger F, Courtney CL. PAS-positive extracellular deposits within germinal centers of hyperplastic follicles during SIV infection in a rhesus macaque. J Med Primatol. 2014;43:374–377. doi: 10.1111/jmp.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estes JD. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol Rev. 2013;254:65–77. doi: 10.1111/imr.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez R, Mouradian J, Metroka C, Davis J. The prognostic value of histopathology in persistent generalized lymphadenopathy in homosexual men. N Engl J Med. 1983;309:185–186. doi: 10.1056/NEJM198307213090314. [DOI] [PubMed] [Google Scholar]
- 30.Biberfeld P, Porwit-Ksiazek A, Bottiger B, Morfeldt-Mansson L, Biberfeld G. Immunohistopathology of lymph nodes in HTLV-III infected homosexuals with persistent adenopathy or AIDS. Cancer Res. 1985;45:4665s–4670s. [PubMed] [Google Scholar]
- 31.Vago L, Antonacci MC, Cristina S, Parravicini C, Lazzarin A, Moroni M, Negri C, Uberti-Foppa C, Musicco M, Costanzi G. Morphogenesis, evolution and prognostic significance of lymphatic tissue lesions in HIV infection. Appl Pathol. 1989;7:298–309. [PubMed] [Google Scholar]
- 32.Baroni CD, Uccini S. Lymph nodes in HIV-positive drug abusers with persistent generalized lymphadenopathy: histology, immunohistochemistry, and pathogenetic correlations. Prog AIDS Pathol. 1990;2:33–50. [PubMed] [Google Scholar]
- 33.Biberfeld P, Ost A, Porwit A, Sandstedt B, Pallesen G, Bottiger B, Morfelt-Mansson L, Biberfeld G. Histopathology and immunohistology of HTLV-III/LAV related lymphadenopathy and AIDS. Acta Pathol Microbiol Immunol Scand A. 1987;95:47–65. doi: 10.1111/j.1699-0463.1987.tb00009_95a.x. [DOI] [PubMed] [Google Scholar]
- 34.Pantaleo G, Graziosi C, Demarest JF, Cohen OJ, Vaccarezza M, Gantt K, Muro-Cacho C, Fauci AS. Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection. Immunol Rev. 1994;140:105–130. doi: 10.1111/j.1600-065x.1994.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 35.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol. 2008;20:181–186. doi: 10.1016/j.smim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, Milush JM, Lifson JD, Sodora DL, Carlis JV, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–561. doi: 10.1086/510852. This study provides a plausbile mechanistic link of lymphoid tissue fibrosis to early augmented and sustained expression of TGFβ by Treg populations. [DOI] [PubMed] [Google Scholar]
- 38.Diaz A, Alos L, Leon A, Mozos A, Caballero M, Martinez A, Plana M, Gallart T, Gil C, Leal M, et al. Factors associated with collagen deposition in lymphoid tissue in long-term treated HIV-infected patients. AIDS. 2010;24:2029–2039. doi: 10.1097/QAD.0b013e32833c3268. [DOI] [PubMed] [Google Scholar]
- 39.Estes JD. Role of collagen deposition in lymphatic tissues and immune reconstruction during HIV-1 and SIV infections. Curr HIV/AIDS Rep. 2009;6:29–35. doi: 10.1007/s11904-009-0005-0. [DOI] [PubMed] [Google Scholar]
- 40•.Estes JD, Reilly C, Trubey CM, Fletcher CV, Cory TJ, Piatak M, Jr, Russ S, Anderson J, Reimann TG, Star R, et al. Antifibrotic therapy in simian immunodeficiency virus infection preserves CD4+ T-cell populations and improves immune reconstitution with antiretroviral therapy. J Infect Dis. 2015;211:744–754. doi: 10.1093/infdis/jiu519. This study demonstrated that combining the antifibrotic drug pirfenidone with an ART regimen resulted in greater preservation/restoration of CD4+ T cells in the peripheral blood and lymphoid tissue than ART alone, supporting a potential role for antifibrotic drug treatment as an adjunctive therapy with ART to improve immune reconstitution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, Robles YP, Davis BT, Li JZ, Heisey A, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161:319–327. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chun TW, Justement JS, Murray D, Hallahan CW, Maenza J, Collier AC, Sheth PM, Kaul R, Ostrowski M, Moir S, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS. 2010;24:2803–2808. doi: 10.1097/QAD.0b013e328340a239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le T, Farrar J, Shikuma C. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS. 2011;25:871–872. doi: 10.1097/QAD.0b013e32834490b1. author reply 872–873. [DOI] [PubMed] [Google Scholar]
- 44.Lee GQ, Lichterfeld M. Diversity of HIV-1 reservoirs in CD4+ T-cell subpopulations. Curr Opin HIV AIDS. 2016;11:383–387. doi: 10.1097/COH.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siliciano JM, Siliciano RF. The Remarkable Stability of the Latent Reservoir for HIV-1 in Resting Memory CD4+ T Cells. J Infect Dis. 2015;212:1345–1347. doi: 10.1093/infdis/jiv219. [DOI] [PubMed] [Google Scholar]
- 46.Chomont N, DaFonseca S, Vandergeeten C, Ancuta P, Sekaly RP. Maintenance of CD4+ T-cell memory and HIV persistence: keeping memory, keeping HIV. Curr Opin HIV AIDS. 2011;6:30–36. doi: 10.1097/COH.0b013e3283413775. [DOI] [PubMed] [Google Scholar]
- 47••.Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Kosakovsky Pond SL, Chung YS, Penugonda S, Chipman JG, Fletcher CV, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530:51–56. doi: 10.1038/nature16933. This recent study demonstrates that on going viral replication occurs in patients with undetectable levels of virus in their bloodstream by showing virus evolution and trafficking between tissue compartments during cART. These data suggest that HIV-1 can continue to replicate within tissues and replenish the viral reservoir despite efficient antiretroviral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith-Franklin BA, Keele BF, Tew JG, Gartner S, Szakal AK, Estes JD, Thacker TC, Burton GF. Follicular dendritic cells and the persistence of HIV infectivity: the role of antibodies and Fcgamma receptors. J Immunol. 2002;168:2408–2414. doi: 10.4049/jimmunol.168.5.2408. [DOI] [PubMed] [Google Scholar]
- 49.Heesters BA, Lindqvist M, Vagefi PA, Scully EP, Schildberg FA, Altfeld M, Walker BD, Kaufmann DE, Carroll MC. Follicular Dendritic Cells Retain Infectious HIV in Cycling Endosomes. PLoS Pathog. 2015;11:e1005285. doi: 10.1371/journal.ppat.1005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Deleage C, Wietgrefe WS, Del Prete G, Morcock DR, Hao XP, Piatak M, Jr, Bess J, Anderson JL, Perkey KE, Reilly C, McCune JM, Haase AT, Lifson JD, Schacker TW, Estes JD. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun. 2016;1:68–106. doi: 10.20411/pai.v1i1.100. This paper details a new next-generation in situ hybridization approach for the detection of vRNA and vDNA, alone and in combination, with higher sensitivity and greater speed of analysis compared to traditional in situ hybridization approaches. Using these new approaches the authors demonstrate that B cell follicles are an important anatomical compartment for both latent and active viral persistence during treatment in an SIV NHP model. These new techniques provide a powerful new approach to study HIV/SIV reservoirs in tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicol A, Nuovo GJ. Detection of HIV-1 provirus and RNA by in situ amplification. Methods Mol Biol. 2005;304:171–182. doi: 10.1385/1-59259-907-9:171. [DOI] [PubMed] [Google Scholar]
- 55.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37:364–376. doi: 10.1016/j.immuni.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Santangelo PJ, Rogers KA, Zurla C, Blanchard EL, Gumber S, Strait K, Connor-Stroud F, Schuster DM, Amancha PK, Hong JJ, et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods. 2015;12:427–432. doi: 10.1038/nmeth.3320. This study reports the use of a non-invasive a whole body PET/CT approach to determine the anatomical location of virus and/or infected cells throughout the body in viremic and aviremic NHPs. [DOI] [PMC free article] [PubMed] [Google Scholar]