Abstract
Objective
To describe characteristics of Parkinson’s disease (PD) by sex among a large, population-based cohort of patients and determine if differences in disease progression exist.
Design
Longitudinal, observational study.
Setting
Twenty-one international National Parkinson Foundation (NPF) Centers of Excellence.
Participants
Patients (N=4,679; 63% men and 37% women with idiopathic PD.
Methods
Demographic and clinical data at enrollment and after one year were collected. We defined progression as a one-year change in functional health outcome measures: a) Health related quality of life (Parkinson’s disease questionnaire-39; PDQ-39); b) Timed up and go (TUG); c) Cognitive function; d) Number of medications. We compared baseline characteristics between men and women. Then, linear regression models were built to assess the independent contribution of sex to progression while controlling for potential confounders.
Results
At baseline, women were significantly more likely to be older and have greater disease severity and more co-morbidities than men despite similar duration of disease. This finding corresponded to worse function as assessed by PDQ-39 and TUG but not number of medications and cognitive function. After one year, declines across all functional measures except delayed recall occurred. No significant changes in PDQ-39, TUG, number of medications or verbal fluency between men and women occurred. Women did have a more significant improvement in delayed recall than men.
Conclusion
Numerous, small, baseline differences between men and women with PD occurred, although differences in markers of progression were few. Findings suggest clinical manifestations and prognosis appear similar by sex under the same treatment conditions.
Keywords: Gender, observational, Parkinsonism, outcomes
Précis
While there are numerous, small, baseline clinical differences between men and women with Parkinson’s disease, differences in markers of one-year progression are few.
Researchers have described sex differences across a wide spectrum in Parkinson’s disease (PD). These differences include risk, clinical presentation, and health outcomes. While women have been reported to have a lower risk of PD, they have a higher mortality and earlier nursing home placement than men (Safarpour et al., 2015; Willis et al., 2012; Wooten, Currie, Bovbjerg, Lee, & Patrie, 2004). Some of these differences are hypothesized to be related to differences in sex hormone exposure and sex chromosome effects (Smith & Dahodwala, 2014). However, additional factors such as differences in access to specialty care, socioeconomic differences and gender bias may also have a role (Saunders-Pullman, Wang, Stanley, & Bressman, 2011). Unfortunately, the degree to which each factor contributes to sex differences is unknown. Additionally, there has been little study of interventions to reduce sex disparities in PD.
Clinical motor and non-motor features are one area where sex differences have been described in PD. However, results have been conflicting partly due to differences in sample characteristics and sizes, as well as measurement tools used. In a recent, large cross-sectional study of subjects enrolled in a randomized-clinical treatment trial, no significant differences were seen in health-related quality of life or disease severity between men and women (Augustine et al., 2015). However, investigators did find that women performed better on tests of cognitive function than men. On the other hand, in another large cross-sectional study of more than 1000 subjects seen at single site, researchers found that at baseline assessment women with PD had greater disease severity and disability when compared to men (Baba, Putzke, Whaley, Wszolek, & Uitti, 2005). In previous, clinic-based studies, researchers also described more frequent levodopa-induced dyskinesias and greater non-motor symptoms among women when compared to men (Baba et al., 2005; Haaxma et al., 2007; Lyons, Hubble, Troster, Pahwa, & Koller, 1998; Picillo et al., 2013; Solla et al., 2012).
Several, small longitudinal studies of PD progression been used to compare clinical features by sex with mixed results. In an older study of 47 men and 23 women followed for six years, researchers did not find any differences in disability, dyskinesias, or dementia between groups (Diamond, Markham, Hoehn, McDowell, & Muenter, 1990). Similarly, in a community-based study of 237 subjects, researchers did not find any differences in motor progression between men and women with PD (Louis et al., 1999). However, in a clinic-based study of men and women at varying stages of PD and follow-up time, the investigators reported that men had a more rapid decline in non-motor complaints and disability (Jankovic & Kapadia, 2001). In a five-year study of disease progression among 129 subjects, investigators found that men with PD had more severe motor impairment than women, but this was not associated with worse quality of life (Velseboer et al., 2013).
Defining the exact role of sex in the course of PD has important implications. If sex is linked to disease progression, then it is possible that hormonal-based therapies may have therapeutic benefits. It is important to study this question in an appropriately powered sample and under similar treatment conditions to reduce the effects of differential access to care on health outcomes. In an effort to better understand the characteristics of sex differences in the motor and non-motor manifestations of PD, we sought to describe the demographic and clinical characteristics of PD by sex among a large, population-based cohort of patients seen at movement disorders clinics worldwide and determine if there are sex differences in clinical progression of patient-centered outcome measures.
Methods
Study Design and Sample
The present study was a secondary analysis of data already drawn from the longitudinal, observational study of PD patients enrolled in the National Parkinson Foundation Parkinson’s Outcomes Project (NPF-POP). The authors are all investigators involved in this project. The NPF-POP is intended to examine the quality of care provided at NPF Centers of Excellence for Parkinson’s disease and to determine which factors improve health outcomes for their patients in order to increase accessibility to the best treatments for people with PD. Participants are followed annually at 21 international NPF Centers of Excellence located in Canada, Netherlands, Israel and the United States. Subject enrollment began in 2009 and new subjects continue to be enrolled every year; there is no anticipated end to this project. Only subjects with a physician diagnosis of idiopathic Parkinson’s disease and at least one year of follow-up data were included in the analysis. The one-year follow-up criterion for this analysis was selected to minimize the effects of differential loss to follow-up by sex. The sample size of 4679 subjects will allow us to detect a one point difference in mean quality of life scores between groups with a probability (power) 0.80. There is no remuneration for participation in this project. All measures selected for use in this study are widely-used within PD research and described in detail below. Approval from the Institutional Review Board at each site was obtained prior to the start of subject recruitment. We obtained written informed consent from all subjects.
Data Collection
Annual patient and caregiver data are collected during a regular clinical visit. Patient data include demographics, number of co-morbidities, medications, disease duration, Hoehn and Yahr (H&Y) stage (Goetz et al., 2004), five-word recall and verbal fluency (Chou et al., 2010; Nasreddine et al., 2005), Timed up and go (TUG) (Morris, Morris, & Iansek, 2001), and Parkinson’s Disease Questionnaire-39 (PDQ-39). The PDQ-39 is a disease-specific health related quality of life scale (Peto, Jenkinson, & Fitzpatrick, 1998). Markers of disease progression were defined as one-year change in PDQ-39 and subscales, TUG, number of medications, verbal fluency score, and five-word recall.
Hoehn and Yahr Stage Scale
The Hoen and Yahr Stage Scale ranges from 1 to 5 (1 = unilateral involvement only; 2 = bilateral or midline involvement with normal balance; 3 = mild to moderate bilateral disease with impaired postural reflexes; 4 = severe disability, still able to walk or stand unassisted; 5 = wheelchair bound or bedridden).
Timed up and go (TUG)
The TUG is a widely-used, reliable and valid measure of mobility performance in people with PD. Longer performance times are associated with decreased mobility and may predict falls (Morris et al., 2001; Stegemöller et al, 2014).
Parkinson’s Disease Questionnaire-39 (PDQ-39)
The PDQ-39 consists of an overall summary score and 8 subdomain scores (mobility, activities of daily living (ADLs), emotional well-being, stigma, social support, cognition, communication and bodily discomfort) that range from 0–100 with lower scores indicating better quality of life. It has been validated in several countries including France, China, Singapore, and the United Kingdom (Auquier et. al., 2002; Peto et al., 1995; Stegemöller et. al., 2014; Zhang & Chan, 2012).
Five-word recall and verbal fluency
Two tests of cognitive function were employed. Participants were asked to recall five words after a 90 second delay as a test of memory drawn from the Montreal Cognitive test (Nasreddine et al., 2005). They were also asked to list as many animals as possible in 60 seconds as a test of verbal fluency (Gladsjo et al., 1999). For both tests, higher numbers indicate better performance. Both impaired memory and executive function are associated with cognitive impairment in Parkinson’s disease (Levy et. al., 2002).
Analysis
Descriptive statistics were used to characterize the sample. The TUG scores were standardized to z-scores for analysis. To compare demographic and clinical characteristics at baseline by sex, we performed ANOVA analysis for continuous variables and Chi-square test for categorical variables. Then, linear multivariate models were conducted for the one-year change in all markers of disease progression adjusting for sex, age, H&Y stage, disease duration and number of comorbidities as covariates. A two-sided p-value cutoff < 0.05 was used to determine statistical significance.
Results
A total of 4,679 (63% men and 37% women) with idiopathic Parkinson’s disease and at least one-year of follow-up data were included in the study. Men had a mean age of 65.5 (SD 9.7) years and women had a mean age of 66.9 (SD 9.7) years. Men had PD for an average of 8.7 (SD 6.0) years, and women were similar with a mean of 8.9 (SD 6.6) years disease duration. A majority of the men (63%; n=1844) and a smaller proportion of women (59%; n=1030) were in early stage disease (H&Y stage 1–2), whereas only 5% (n=146) of men and 7% (n=126) of women were in late stage disease (H&Y stages 4–5). Men had a mean of 1.7 (SD 1.3) comorbidities, and women had a slightly higher mean of 1.8 (SD 1.3) comorbidities. Table 1 describes the baseline demographic and clinical characteristics of the cohort by sex. While differences between men and women were small in magnitude, there were several statistically significant differences. Notably, women were slightly older, had a greater proportion of H&Y stage 4 and 5, worse overall PDQ-39 scores, worse TUG and better delayed recall than men.
Table 1.
Comparison of baseline demographic and clinical characteristics among the National Parkinson Foundation Parkinson’s Outcomes Project cohort
| Variable | Men (N=2,938) | Women (N=1,741) |
p- value |
|
|---|---|---|---|---|
| Mean age in years (SD) | 65.5 (9.7) | 66.9 (9.7) | <0.001 | |
| Disease duration in years (SD) | 8.7 (6.0) | 8.9 (6.6) | 0.176 | |
| % Hoehn and Yahr stage (n)a | 1 | 9.7 (285) | 13.6 (236) | |
| 2 | 53.1 (1559) | 45.6 (794) | ||
| 3 | 26.2 (770) | 25.8 (450) | ||
| 4–5 | 5.0 (146) | 7.2 (126) | <0.001 | |
| Mean # of co-morbidities (SD) | 1.7 (1.3) | 1.8 (1.3) | 0.005 | |
| Mean PDQ-39 (SD) | Sum score | 23.1 (14.8) | 25.2 (15.9) | <0.001 |
| Mobility | 11.0 (10.3) | 13.9 (11.5) | <0.001 | |
| ADL | 7.2 (5.5) | 7.0 (6.0) | 0.267 | |
| Emotional | 5.3 (4.5) | 6.5 (4.9) | <0.001 | |
| Stigma | 2.8 (3.2) | 3.1 (3.4) | 0.009 | |
| Social support | 1.2 (1.8) | 1.4 (2.0) | 0.015 | |
| Cognition | 4.2 (3.1) | 4.0 (3.1) | 0.136 | |
| Pain | 3.6 (2.7) | 4.5 (2.9) | <0.001 | |
| Mean # of medications (SD) | 2.3 (1.2) | 2.3 (1.2) | 0.189 | |
| Mean standardized TUG (SD) | −0.2 (1.0) | 0.0 (1.0) | <0.001 | |
| Mean verbal fluency (SD) | 18.6 (6.5) | 18.5 (6.5) | 0.517 | |
|
Mean 5 word delayed recall (SD) |
2.9 (1.4) | 3.3 (1.4) | <0.001 |
178 values missing for men and 126 missing for women;
PDQ-39 = Parkinson’s disease questionnaire; ADL = Activities of Daily Living; TUG = timed up and go
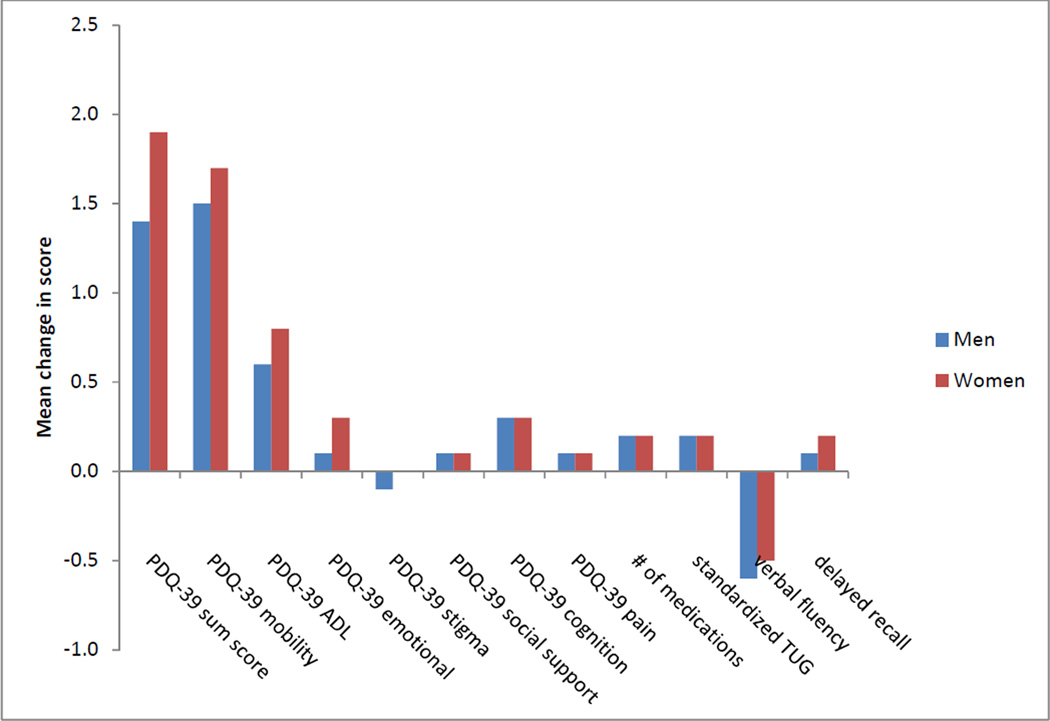
After one year, men and women declined across all measures of disease progression, except the PDQ-39 stigma sub-score. In bivariate analysis, only a decline in the PDQ-39 ADL sub-score was significantly different between men and women (0.6 vs. 0.8; p=0.035). Figure 1 illustrates the change in each marker of disease progression between men and women.
Figure 1.
Bivariate comparison of change in disease progression
In multivariate analysis, age, H&Y stage, disease duration and number of co-morbidities were independently associated with numerous markers of disease progression (Table 2). However, only the one-year change in 5-word delayed recall was associated with sex (coefficient of change women compared to men = 0.094, SE 0.042; p-value = 0.025).
Table 2.
Independent predictors of disease progression markers.
| Outcome 1 year change in: |
Women (ref: Men) |
Coefficient of change [standard error] | Disease duration | # of comorbidities |
|||
|---|---|---|---|---|---|---|---|
| Hoehn and Yahr (ref: Stage 1) | Age | ||||||
| 4&5 | 3 | 2 | |||||
| PDQ-39 | |||||||
| Summary score | 0.396 [0.322] | −1.555 [0.842] | 0.393 [0.589] | −0.120 [0.515] | 0.068 *** [0.017] | −0.004 [0.027] | −0.269* [0.121] |
| Mobility | 0.293 [0.228] | −2.648 ***[0.595] | 0.200 *** [0.416] | 0.226 ***[0.364] | 0.067 *** [0.012] | 0.050** [0.019] | −0.199 [0.086] |
| ADL | 0.255 [0.132] | −0.052 [0.344] | 0.203 [0.241] | −0.138 [0.211] | 0.030 *** [0.007] | −0.006 [0.011] | −0.080 [0.050] |
| Emotional well-being | 0.128 [0.118] | −0.277 [0.310] | 0.277 [0.216] | 0.153 [0.189] | 0.026*** [0.006] | 0.009 [0.010] | −0.095* [0.045] |
| Stigma | 0.009 [0.087] | −0.30 [0.227] | 0.002 [0.159] | 0.015 [0.139] | 0.006 [0.005] | −0.11 [0.007] | 0.004 [0.033] |
| Social support | 0.039 [0.056] | −0.358* [0.147] | 0.030* [0.103] | −0.041* [0.090] | 0.003 [0.003] | 0.000 [0.005] | −0.010 [0.021] |
| Cognition | 0.059 [0.081] | −0.287 [0.213] | 0.137 [0.148] | −0.036 [0.130] | 0.009* [0.004] | −0.006 [0.007] | −0.029 [0.031] |
| Pain | −0.004 [0.077] | −0.251 [0.202] | −0.299 [0.141] | −0.199 [0.124] | 0.002 [0.004] | −0.014* [0.006] | −0.042 [0.029] |
| # of medications | 0.004 [0.027] | −0.130** [0.071] | −0.097** [0.050] | −0.032**[0.044] | 0.001 [0.001] | −0.014***[0.002] | −0.011 [0.010] |
| Standardized TUG | −0.048 [0.025] | −0.151* [0.070] | 0.051* [0.045] | 0.052* [0.039] | 0.003* [0.001] | 0.008 *** [0.002] | 0.005 [0.010] |
| Verbal fluency | 0.155 [0.155] | −0.885* [0.407] | −0.429* [0.282] | −0.058* [0.247] | −0.018* [0.008] | −0.032* [0.013] | −0.062 [0.058] |
| Delayed recall | 0.094* [0.042] | −0.129 [0.110] | −0.015 [0.076] | 0.058 [0.067] | −0.008 ***[0.002] | −0.004 [0.004] | −0.037* [0.016] |
Note. Values represent coefficient of change for each predictor variable. Each model controls for all other listed variables.
p < 0.05;
p < 0.01;
p < 0.001
Discussion
A cross-sectional comparison of a large cohort of men and women cared for at Parkinson’s disease specialty centers revealed small but statistically significant differences across many different measures of health status. However, in prospective follow-up, very few differences were seen in disease progression after one year. Women demonstrated a greater improvement in delayed recall after one year when compared to men. The root causes of sex differences in PD are important to understand in order to disentangle the differential effects of biological and social determinants of health.
Pevious, cross-sectional and longitudinal studies of sex differences have had mixed findings. The small sex differences in disease severity that we observed could reflect previous treatment differences prior to receipt of care at the study sites (Saunders-Pullman et al., 2011) or the overall older age of women when compared to men in our sample. This finding is supported by the findings by Augustine et al.: no differences in disease severity between men and women were observed as part of a large, clinical trial in which individuals were on similar treatment regiments before enrollment and were in the early stages of disease (Augustine et al., 2015). It is also possible that despite similar disease durations, women have a more severe disease course than men. In the current study, our team addressed this concern within the second, longitudinal part of the analysis. Similar to two prior studies, we did not find any clinically meaningful differences in disease progression between men and women with PD (Diamond et al., 1990; Louis et al., 1999). In contrast, two other groups of investigators found more rapid decline among men than women (Jankovic & Kapadia, 2001; Velseboer et al., 2013). One potential reason for our disparate findings is the differential loss to follow-up between men and women with PD. As women with PD become more disabled they are more likely to enter nursing homes than men, which would lead to the appearance of greater disease severity among men still followed in PD clinics (Safarpour et al., 2015). Alternatively, longer periods of follow-up may have allowed sufficient time to observe differences in disease progression. The average length of follow-up in these studies ranged from 5–6 years. While we only followed participants for one year, we hypothesized that this shorter length of time limited selection bias due to loss to follow-up.
In addition, our sample was the largest clinical cohort of PD subjects that were derived from many different clinic populations globally, which increased the generalizability of our findings. Furthermore, since all patients were treated at centers of excellence for Parkinson’s disease, the treatment conditions for all patients were assumed to be relatively similar. Requirements to be an NPF Center of Excellence include the following: (a) employ a multi-disciplinary team model for care delivery; (b) manage a high volume of patients with PD; (c) conduct research; (d) provide support services for patients and families; (e) educate patients and the community about PD; and (f) provide access to the latest therapies including neurosurgeons experienced with PD surgical options and experimental therapies through clinical trials.
Unfortunately, not all individuals living with PD are able to access specialty centers for movement disorders, and it is possible that those who cannot may experience a different disease prognosis. As the world’s population is projected to increase in numbers and in age, the prevalence of PD will exponentially increase (Dorsey et al., 2007). Furthermore, the corresponding number of care providers to meet this need is limited (Dorsey, George, Leff, & Willis, 2013). The potential for advanced practice nurses to help fill this gap is immense. In England, investigators examined the effect of community-based nurse specialists on health outcomes in PD and found that the involvement of nurse specialists had a positive effect on patients’ sense of wellbeing (Jarman, Hurwitz, Cook, Bajekal, & Lee 2002). Similarly, in a qualitative study of 19 women with PD, particular note was made of the benefits of a nurse specializing in Parkinson’s disease to provide additional support and reduce feelings of isolation (Fleming, Tolson, & Schartau, 2004). Given the complexity of PD motor and non-motor symptoms and its chronic, progressive course, a growing need for nurses to act as case managers in leading the team of multi-disciplinary providers (e.g., physician, social worker, physical therapy, speech therapist) in care coordination (van der Marck et al., 2009). Policymakers must begin to develop and test strategies to provide this high quality specialty care to all PD patients.
A few limitations to this study are important to consider. While we examined one of the largest cohorts of PD patients, the possibility of selection bias among those who chose not to participate in the registry or were lost to follow-up was still present. Furthermore, we only followed subjects for one year. It is possible that additional variables influence the relationship between sex and outcomes that were not accounted for in this analysis (for example, measures of socioeconomic status such as education or income). However, as substantial sex differences were not found, it became less important to include these variables. Lastly, this study focused on the collection of a limited set of outcome measures to improve feasibility and retention for this large, longitudinal study. However, there are other measures or outcomes that may differ by sex that were not captured by this study (e.g. dyskinesias, sexual function). Future analyses will include longer follow-up time and additional clinical indicators of outcomes that will be newly added to the annual assessment.
In closing, the findings of this study fill some important gaps in our knowledge in regards to PD progression and differences between men and women. While there were numerous, small baseline differences between men and women with PD, very few differences in markers of progression were identified. Sex differences in PD risk and long-term outcomes may still exist, but in the setting of similar treatment conditions, our findings support the conclusion that clinical manifestations and prognosis are similar over one year.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no conflict of interest or relevant financial relationships.
Callouts.
The biological and social determinants of sex differences in Parkinson’s disease risk, progression, and outcomes are poorly understood. (page 1)
Our findings showed few differences in progression of motor and non-motor symptoms between men and women during the course of one year. (page 7)
The comparable course between women and men with Parkinson’s disease suggests that the prognosis is similar when there is equal access to specialty care. (page 9)
Contributor Information
Nabila Dahodwala, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Qinglin Pei, Department of Biostatistics, University of Florida, Gainesville, FL.
Peter Schmidt, Chief Mission Officer for the National Parkinson Foundation Inc, Miami, FL.
References
- Augustine EF, Perez A, Dhall R, Umeh CC, Videnovic A, Cambi F, Suchowersky O. Sex differences in clinical features of early, treated reated Parkinson's disease. PLoS One. 2015;10(7):e0133002. doi: 10.1371/journal.pone.0133002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auquier P, Sapin C, Ziegler M, Tison F, Destee A, Dubois B, Peto V. Validation of the French language version of the Parkinson's Disease Questionnaire - PDQ-39. Revue Neurologique (Paris) 2002;158(1):41–50. [PubMed] [Google Scholar]
- Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson's disease phenotype. Journal of Neurology. 2005;252(10):1201–1205. doi: 10.1007/s00415-005-0835-7. [DOI] [PubMed] [Google Scholar]
- Chou KL, Amick MM, Brandt J, Camicioli R, Frei K, Gitelman D, Levin B. A recommended scale for cognitive screening in clinical trials of parkinson's disease. Movement Disorders. 2010;25(15):2501–2507. doi: 10.1002/mds.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD. An examination of male-female differences in progression and mortality of Parkinson's disease. Neurology. 1990;40(5):763–766. doi: 10.1212/wnl.40.5.763. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Tanner CM. Projected number of people with parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. doi: 01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, George BP, Leff B, Willis AW. The coming crisis: Obtaining care for the growing burden of neurodegenerative conditions. Neurology. 2013;80(21):1989–1996. doi: 10.1212/WNL.0b013e318293e2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming V, Tolson D, Schartau E. Changing perceptions of womanhood: Living with Parkinson’s disease. International Journal of Nursing Studies. 2004;41(5):515–524. doi: 10.1016/j.ijnurstu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6(2):147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Wenning GK. Movement disorder society task force report on the Hoehn and Yahr staging scale: Status and recommendations the movement disorder society task force on rating scales for parkinson's disease. Movement Disorders. 2004;19(9):1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Horstink MW. Gender differences in Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(8):819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Archives of Neurology. 2001;58(10):1611–1615. doi: 10.1001/archneur.58.10.1611. [DOI] [PubMed] [Google Scholar]
- Jarman B, Hurwitz B, Cook A, Bajekal M, Lee A. Effects of community based nurses specialising in Parkinson’s disease on health outcomes and costs: Randomised controlled trial. British Medical Journal. 2002;324(7345):1072–1075. doi: 10.1136/bmj.324.7345.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G, Jacobs DM, Tang MX, Cote LJ, Louis ED, Alfaro B, Marder K. Memory and executive function impairment predict dementia in parkinson's disease. Movement Disorders. 2002;17(6):1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K. Progression of parkinsonian signs in Parkinson disease. Archives of Neurology. 1999;56(3):334–337. doi: 10.1001/archneur.56.3.334. [DOI] [PubMed] [Google Scholar]
- Lyons KE, Hubble JP, Troster AI, Pahwa R, Koller WC. Gender differences in Parkinson's disease. Clinical Neuropharmacology. 1998;21(2):118–121. [PubMed] [Google Scholar]
- Makoutonina M, Iansek R, Simpson P. Optimizing care of residents with Parkinsonism in supervised facilities. Parkinsonism and Related Disorders. 2010;16(5):351–355. doi: 10.1016/j.parkreldis.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the timed "up & go" test in people with parkinson disease. Physical Therapy. 2001;81(2):810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H. The montreal cognitive assessment, moca: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. doi: JGS53221 [pii] 10.1111/j.1532-5415.2005.53221.x [doi] [DOI] [PubMed] [Google Scholar]
- Olsson M, Stafström L, Söderberg S. Meanings of fatigue for women with Parkinson’s disease. Qualitative Health Research. 2013;23(6):741–748. doi: 10.1177/1049732313482398. [DOI] [PubMed] [Google Scholar]
- Pavon JM, Whitson HE, Okun MS. Parkinson’s disease in women: A call for improved clinical studies and for comparative effectiveness research. Maturitas. 2010;65(4):352–358. doi: 10.1016/j.maturitas.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: A review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. Journal of Neurology. 1998;245(Suppl 1(1)):S10–S14. doi: 10.1007/pl00007730. [DOI] [PubMed] [Google Scholar]
- Picillo M, Amboni M, Erro R, Longo K, Vitale C, Moccia M, Pellecchia MT. Gender differences in non-motor symptoms in early, drug naive Parkinson's disease. Journal of Neurology. 2013;260(11):2849–2855. doi: 10.1007/s00415-013-7085-x. [DOI] [PubMed] [Google Scholar]
- Safarpour D, Thibault DP, DeSanto CL, Boyd CM, Dorsey ER, Racette BA, Willis AW. Nursing home and end-of-life care in Parkinson disease. Neurology. 2015;85(5):413–419. doi: 10.1212/WNL.0000000000001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders-Pullman R, Wang C, Stanley K, Bressman SB. Diagnosis and referral delay in women with Parkinson's disease. Gender Medicine. 2011;8(3):209–217. doi: 10.1016/j.genm.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Dahodwala N. Sex differences in Parkinson's disease and other movement disorders. Experimental Neurology. 2014;259:44–56. doi: 10.1016/j.expneurol.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Solla P, Cannas A, Ibba FC, Loi F, Corona M, Orofino G, Marrosu F. Gender differences in motor and non-motor symptoms among Sardinian patients with Parkinson's disease. Journal of the Neurological Sciences. 2012;323(1–2):33–39. doi: 10.1016/j.jns.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Stegemoller EL, Nocera J, Malaty I, Shelley M, Okun MS, Hass CJ. Timed up and go, cognitive, and quality-of-life correlates in Parkinson's disease. Archives of Physical Medicine and Rehabilitation. 2014;95(4):649–655. doi: 10.1016/j.apmr.2013.10.031. [DOI] [PubMed] [Google Scholar]
- van der Marck MA, Kalf J, Sturkenboom IH, Nijkrake MJ, Munneke M, Bloem BR. Multidisciplinary care for patients with Parkinson's disease. Parkinsonism & related disorders. 2009;15:S219–S223. doi: 10.1016/S1353-8020(09)70819-3. [DOI] [PubMed] [Google Scholar]
- Velseboer DC, Broeders M, Post B, van Geloven N, Speelman JD, Schmand B, de Bie RM. Prognostic factors of motor impairment, disability, and quality of life in newly diagnosed PD. Neurology. 2013;80(7):627–633. doi: 10.1212/WNL.0b013e318281cc99. [DOI] [PubMed] [Google Scholar]
- Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of survival in patients with Parkinson disease. Archives of Neurology. 2012;69(5):601–607. doi: 10.1001/archneurol.2011.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? Journal of Neurology, Neurosurgery & Psychiatry. 2004;75(4):637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JL, Chan P. Reliability and validity of PDQ-39: a quality-of-life measure for patients with PD in China. Quality of Life Research. 2012;21(7):1217–1221. doi: 10.1007/s11136-011-0026-1. [DOI] [PubMed] [Google Scholar]