Abstract
Pigment epithelium-derived factor (PEDF) encoded by serpinf1 is a potent anti-angiogenic factor found in a wide variety of fetal and adult tissues. Several reports have shown that lack of PEDF leads to osteogenesis imperfecta (OI) type VI whose hallmark is a defect in mineralization that leads to excessive osteoid build up that fails to mineralize. Because PEDF is antiangiogenic factor it would pose serious consequences on bone development and healing of fractures. To understand possible mechanisms by which PEDF plays a role in bone development and regulation of matrix mineralization, we determined the effects of exogenous PEDF on vascular endothelial growth factor (VEGF) expression by human mesenchymal stem cells (hMSCs) and mechanisms of its regulation by PEDF. Human MSCs incubated in normal medium supplemented with exogenous PEDF increased VEGF expression; this increase was also seen when PEDF was added to hMSCs undergoing osteogenic differentiation. MSCs maintained in osteogenic medium increased synthesis of both VEGF and PEDF but both factors were maintained relatively in balance during differentiation. To understand mechanisms by which exogenous PEDF regulated VEGF expression, hMSCs exposed to PEDF activated Erk signaling pathway in MSCs; inhibition of Erk signaling reduced VEGF mRNA expression as well as protein production suggesting that PEDF regulates VEGF expression in MSCs via Erk signaling pathway. In conclusion, PEDF increases VEGF expression by MSCs suggesting that regulation of VEGF by PEDF may be part of the mechanisms by which PEDF regulates osteoblastic mineralization.
Keywords: Pigment epithelium derived factor, Vascular endothelial growth factor, MSCs, mineralization, Erk signaling
Introduction
Pigment epithelium-derived factor (PEDF) is a potent anti-angiogenic factor found in a wide variety of fetal and adult tissues; it is believed to play a role in the regulation of angiogenesis during development [1,2,3]. PEDF was originally isolated from human fetal retinal pigment epithelial cells, it has now been shown to be synthesized by a variety of other cell types including osteoblasts [4,5,6]. Several reports have shown that patients with a recessive form of osteogenesis imperfecta (OI) type VI lack expression of PEDF leading to severe bone defects and frequent fracturing [7,8,9,10]. Synthesis, posttranstional modification and secretion of type I collagen were reported to be normal. The major bone defect in these patients is presence of excessive osteoid build up that fails to mineralize [7–10]. We and others have shown that PEDF promotes mesenchymal stem cell differentiation and increases osteoblast mineralization [11,12]. In addition, we reported that PEDF reduced expression of sclerostin by osteocytes [13]. Mechanisms by which PEDF regulates osteogenesis and osteoblast mineralization however, remain unclear.
Most studies on PEDF have focused on its role in antiangiogenic activities and as a potential therapeutic factor for inhibiting cancer cell migration as well as survival, and potential application in some diabetic related pathologies like retinopathy [14,15,16]. PEDF has been reported to downregulate expression of VEGF in retinal cells but PEDF also inhibited VEGF binding to the type 2 VEGF receptor [17,18]. In bone, VEGF plays important role in bone development; there is also evidence that VEGF can directly promote osteoblast differentiation and or matrix mineralization [19,20]. Several reports indicate that a balanced VEGF/PEDF ratio a key factor in maintaining the function of ocular neovascularization and osteoblastgenesis [21,22]. Because VEGF plays important role in bone, we set out to determine if PEDF regulates VEGF expression in hMSCs. In this report, we examined effect of exogenous PEDF on VEGF expression by MSCs in normal medium as well as during mineralization. We also determined mechanisms by which PEDF regulates VEGF expression in hMSCs.
Materials and Methods
Human Mesenchymal stem cells (MSCs) isolation
Human MSCs were harvested from the reaming of patients bones undergoing hip surgery with approved IRB protocol by Penn State college of Medicine (ages 50–70). The cells were isolated using the methods described previously [23]. Briefly, bone reaming were washed extensively in sterile PBS by repeated centrifugation at 1000 rpm for 5 min. Pellets were suspended in DMEM supplemented with 10% FBS and plated in Petri dishes. The cells were maintained in culture in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin for 7 days. Bone chips and non-adherent cells were removed; adherent cells were maintained in culture with medium changes and passaged when they reached confluence. The cells were used at passages 2 or 3. Prior to use, cells were assessed for stem characteristics as we described previously [11,24]. hMSCs were treated with PEDF protein at 250ng/ml either in osteogenic or maintenance medium. PEDF used for the experiments was donated by Dr. Tombran-Tink who isolated and characterized it as described previously [25].
Western Blotting
Total protein from differentiating hMSCs either in presence or absence of PEDF at different time points was extracted with RIPA lysis buffer (1% Nonidet P-40, 150 mM NaCl, 50 mM Tris–HCl, pH 7.4, 1 mM EDTA, 50 mM sodium fluoride, 2 mM sodium vanadate, 0.1% BSA, and complete protease inhibitors) (Pierce, Rockford, IL) . The cell lysates were centrifuged and the supernatant was collected. The protein concentration of cell lysates was determined using a Protein Assay kit (Bio-Rad, USA). Total cell extracts containing equal amounts of protein were separated by 12% SDS-PAGE and electroblotted onto PVDF membranes (Bio-Rad Laboratories). Only proteins associated with cells were collected, proteins secreted in the medium were not assessed. The membranes were probed with anti-human antibodies to proteins of interest for analysis; PEDF (1:2500, SC-25594, Santa Cruz, CA), VEGF (1:1000, SC-7269, Santa Cruz, CA), total ERK1/2 (1:1000, # 9102, Cell Signaling Technology, CA) and phosphorylated ERK1/2 (1:1000, #9106, Cell Signaling Technology, CA). Mouse anti-human GAPDH (1:2500, SC-32233, Santa Cruz, CA) antibodies were also used for normalization. For visualization, horseradish peroxidase-conjugated secondary antibodies were used and the bands were developed by SuperSignal West Pico substrate (Thermo Scientific). Specific bands were quantified using the Bio-Rad Quantity One 1-D Analysis Software (Bio-Rad, USA).
Signaling pathways analysis
Human MSCs were pretreated with 10 mM of Erk docking domain inhibitor (294675-79-9, Sigma Aldrich, CA) or control solution (DMSO). This was followed by incubation of MSCs in a medium supplemented with 250 ng/ml of PEDF for 5,15, 30 min,1h, 2h, 4h,8h, and 24 h. Cells were extracted with RIPA buffer and the cell lysates were collected for analysis.
Quantitative Real-time PCR
Total RNA was extracted from cells using TRIzol (Invitrogen). Synthesis of cDNA was performed with 1 µg of total RNA at 37 °C for 50 min using oligo (dT) primers and reverse transcriptase (M-MLV; Invitrogen). cDNA was analyzed in a StepOnePlus™ Real-Time PCR system (Applied Biosystems, CA). Amplification was carried out in a total volume of 20 µl and PerfeCTa SYBR Green FastMix® ROX reagents (Quanta Bioscience, MD) with the following cycling conditions initial denaturation at 95 °C for 5 min, followed by 40 cycles at 95 °C for 3s, 60 °C for 30 s, and Melt Curve: 95°C, 15s (100%); 65°C,1min, 95°C 15s (100%), +0.3°C/circle‥ The data were calculated with 2-ΔΔCt. Primer sequences are as VEGF: forward, 5’-AGGAGGAGGGCAGAATCATC and reward 5’-ATGTCCACCAGGGTCTCGAT-3’, beta-actin, Forward5’-CGTCTTCCCCTCCATCG-3’, and Reward5’- CTCGTTAATGTCACGCAC-3’. All determinations were measured in triplicates. The cycle threshold (Ct) values corresponded to the PCR cycle number at which fluorescence emission in real time reached a threshold above the base-line emission and were analyzed. The Ct value of the PCR product of interest and a control mRNA (β-actin) was then used to calculate relative quantities of mRNA between samples.
Statistical Analysis
For statistical evaluations of the calculated relative expression variations, data were analyzed for significant differences by ANOVA using approximate post-hoc tests. A level of p<0.05 was considered statistically significant.
Results
Exogenous PEDF increases VEGF expression by hMSCs in maintenance medium
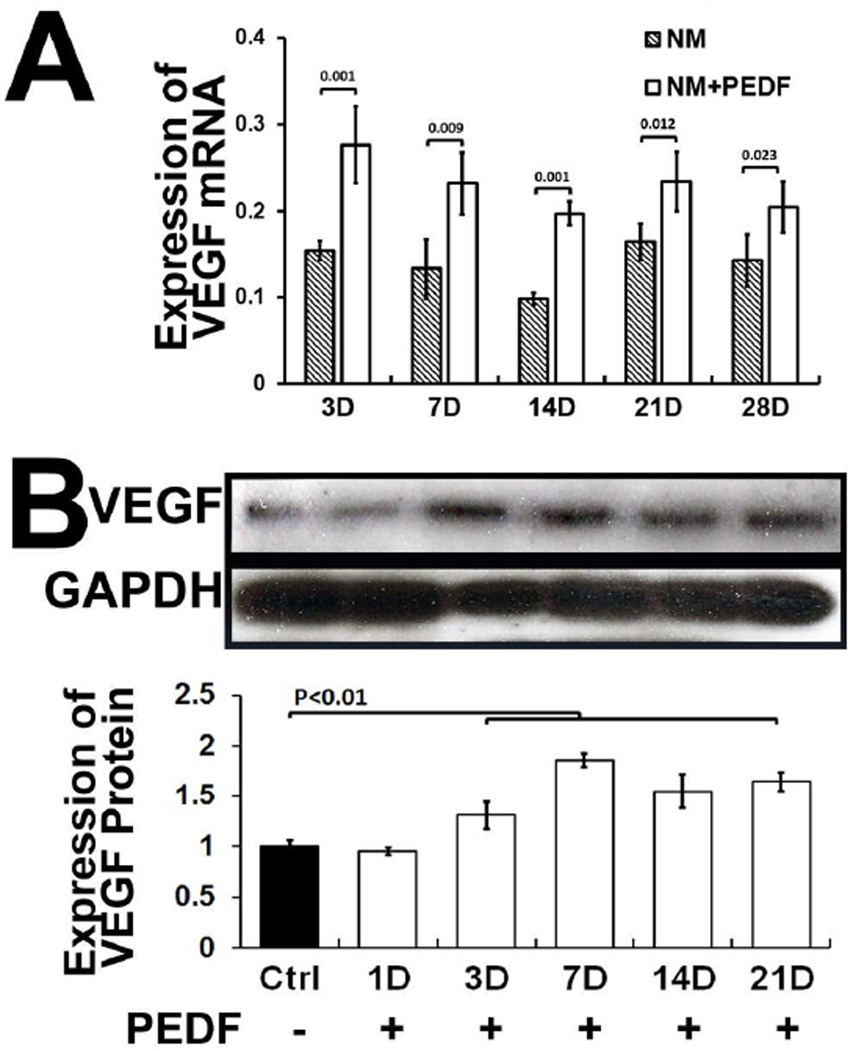
We first examined whether exogenous PEDF had an effect on VEGF expression by hMSCs cultured in maintenance medium (DMEM. 10% FBS, 1% P/S v/v), at different days of culture. The results showed that MSCs exposed to PEDF exhibited increased VEGF expression compared to cells not incubated in presence of PEDF (Fig. 1). These data indicate that PEDF increases expression of PEDF by hMSCs under static conditions.
Figure 1. Exogenous PEDF increases synthesis of VEGF by MSCs in maintenance medium at different days.
A) hMSCs incubated in maintenance medium supplemented with exogenous PEDF increased expression of VEGF mRNA . B) Western blot showing VEGF synthesis by MSCs in maintenance medium at different days. Medium supplemented with PEDF showed increased synthesis of VEGF. NM=maintenance medium; NM+PEDF=maintenance medium supplemented with PEDF.
Exogenous PEDF increases VEGF expression in differentiating MSCs
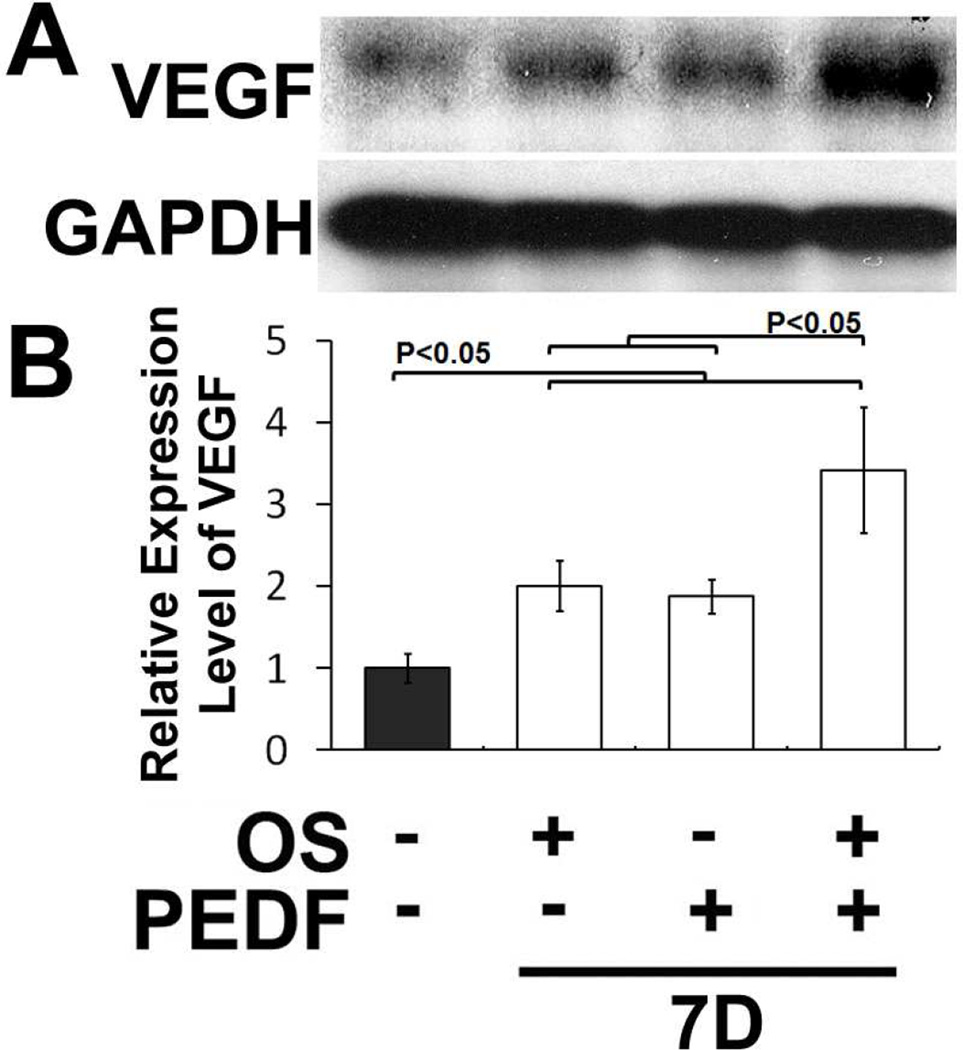
Next, we determined whether hMSC cultured in osteogenic medium supplemented with exogenous PEDF will increase VEG expression. The data showed that hMSCs incubated in osteogenic medium and supplemented with exogenous PEDF increased VEGF expression. The major increase was at day 7 of osteogenic differentiation (Fig. 2). These data indicate that PEDF increases expression of VEGF during osteoblastic differentiation. Because both PEDF and VEGF are synthesized by MSCs and they increase during differentiation (see below), the results imply that PEDF does not inhibit VEGF expression by differentiating MSCs but rather it promotes or regulates its expression.
Figure 2. Exogenous PEDF enhanced expression of VEGF by hMSCs in osteogenic medium.
A) A western blot showing increased VEGF synthesis by hMSCs incubated in osteogenic medium supplemented with exogenous PEDF. B) Quantitation of VEGF synthesis by hMSCs maintained in osteogenic medium shows cultures supplemented with exogenous PEDF increased expression of VEGF by day 7 of culture in osteogenic medium.
hMSCs increase expression of PEDF and VEGF during osteoblastic differentiation
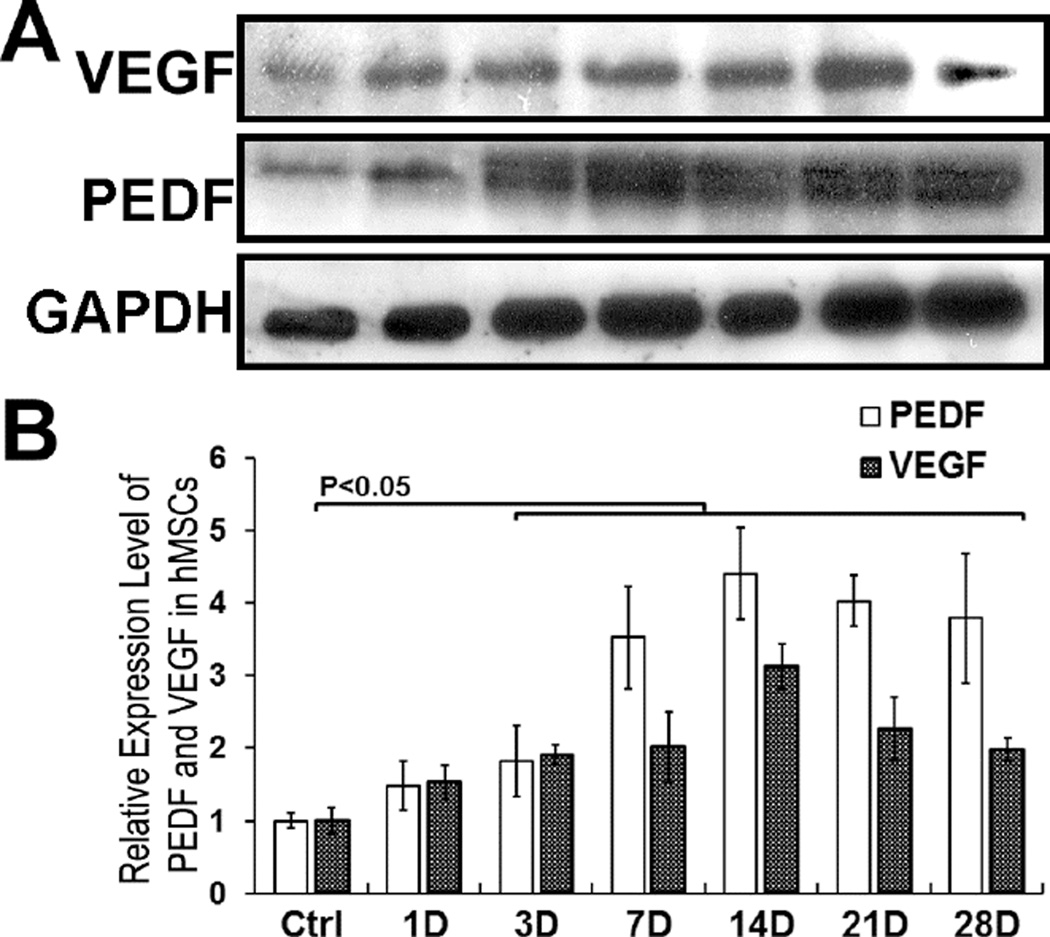
Because exogenous PEDF expression by differentiating MSCs cultured in osteogenic medium, we assessed we examined relative expressions of PEDF and VEGF during MSCs-osteoblastic differentiation. The results showed that both PEDF and VEGF increased in expression during MSCs-osteoblastic differentiation showing maximal expression at day 14 (Fig. 3). The data indicate that increase in PEDF synthesis did not affect VEGF synthesis during MSCs differentiation. Thus a balance between PEDF and VEGF is maintained in MSCs during osteoblastic differentiation suggesting that the two factors play a role in enhancing osteoblast differentiation and matrix mineralization.
Figure 3. hMSCs incubated in osteogenic medium increase synthesis of PEDF and VEGF during differentiation.
A) Western blot of VEGF and PEDF proteins synthesized by hMSCs incubated in osteogenic medium. B) Quantitative analysis of VEGF and PEDF synthesis during MSCs differentiation in osteogenic medium. The data indicate that that PEDF and VEGF are coordinately regulated during MSCs-osteoblastic differentiation, both VEGF and PEDF showed maximal expression at day 14.
PEDF increases VEGF expression in hMSCs via ERK signaling pathway
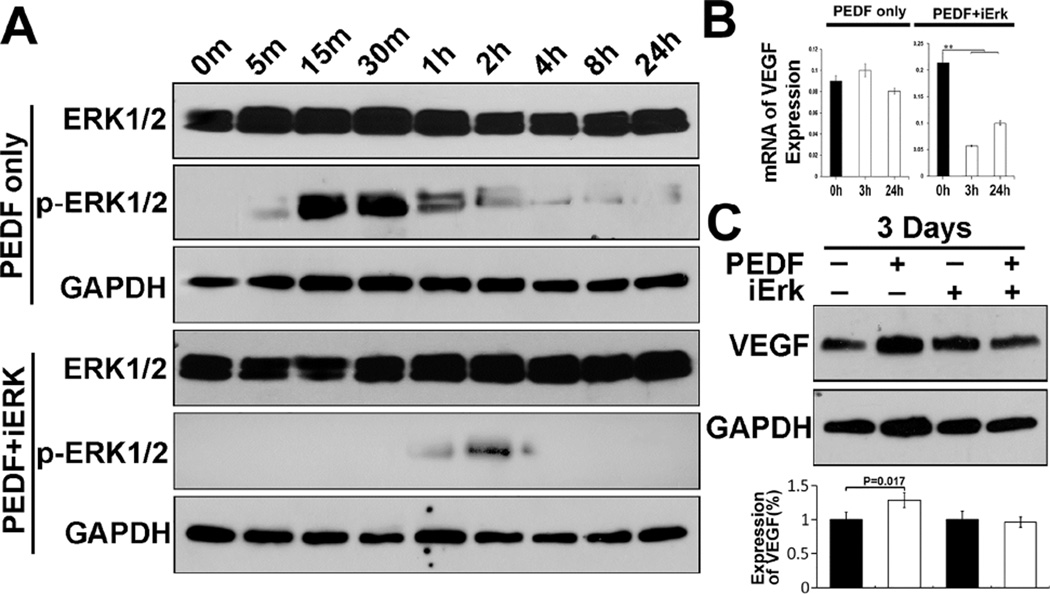
We reported previously that PEDF promotes expression of osteoblast associated genes via Erk signaling pathway [11]. In this report, we asked whether PEDF increases and regulates VEGF expression via similar mechanisms. Exposure of MSCs to PEDF activated Erk signaling pathway as indicated by Erk phosphorylation, (p-Erk1/2) (Fig. 4A). Treatment of MSCs with PEDF in presence of Erk inhibitor (iErk) suppressed Erk activation as indicated by reduction in Erk phosphorylation (Figure 4A). We then examined if inhibition of Erk activation reduced VEGF expression. Figure 4B shows that PEDF increases VEGF expression; treatment of MSCs with PEDF in presence of Erk inhibitor reduced VEGF mRNA expression (Fig. 4B). Following mRNA analysis, we determined if inhibiting Erk had an effect on VEGF protein synthesis by hMSCs. The data showed that within 3 days following incubation of MSCs with PEDF in presence of Erk inhibitor, VEGF synthesis was reduced suggesting that increased VEGF expression and regulation by PEDF is mediated via Erk signaling pathway (Fig. 4C).
Figure 4. Analysis of PEDF-mediated activation of ERK signaling pathway in hMSCs to regulate VEGF expression.
A) Incubation of hMSCs in presence of exogenous PEDF activated Erk signaling pathway (pERK1/2). Erk docking domain inhibitor (iERK) blocked activation of Erk following exposure of hMSCs to PEDF. B) VEGF gene expression was reduced by blocking Erk activation. C) Western blot indicated that increased expression of VEGF by exogenous PEDF was reduced by inhibition of Erk activation. These data indicate that PEDF regulates VEGF expression through Erk signaling pathway in hMSCs.
Discussion
Null mutations in serpinf1 gene that encodes PEDF lead to the development of OI type VI whose hallmark is presence of extensive osteoid build up that fails to mineralize [7,8,9,10]. Mechanisms by which PEDF regulates bone mineralization remain undefined. We and others showed that PEDF increased MSCs-osteoblastic mineralization; in addition, we showed that PEDF enhanced expression of genes that encode proteins which promote matrix mineralization [11,12]. In this report, we examined whether VEGF which plays important role in bone development is also regulated by PEDF. Because PEDF is known as a potent antiangiogenic factor its expression in bone would have undesirable consequences. Previous reports showed that VEGF can have a direct effect on osteoblast differentiation and bone mineralization [19,20,26]. VEGF plays important role in fracture healing through coupled angiogenesis and osteogenesis. The present report has established that VEGF expression was increased by differentiating murine mesenchymal stem cells incubated in osteogenic medium suggesting that it plays a role during osteoblastic differentiation. Interestingly, VEGF expression we examined was associated with cells not the medium. A report by Olsen and colleagues showed that intracellular VEGF was crucial for MSCs differentiation and matrix mineralization [27]. Our present data imply that PEDF may increase expression of intracellular VEGF by MSCs which then leads to the enhancement of MSCs differentiation and matrix mineralization. These assumptions remain to be further investigated.
The present findings may be unique to MSCs, because PEDF has been reported to reduce expression of VEGF in retinal capillary endothelia cells and muller cells [18]. Because increased synthesis of PEDF during differentiation by MSCs did not appear to have an effect on VEGF synthesis, the findings suggest that PEDF and VEGF are coordinately regulated in MSCs to maintain a balance between the two factors. Our findings also suggest that PEDF regulates VEGF in hMSCs differently in MSCs than in other cell types and that this may be part of the mechanisms by which PEDF regulates osteoblastic mineralization.
Acknowledgments
The authors would like to thank Dr. Charles Davis for supplying bone marrow for cell isolation. This work was supported in part by R21AR067473 (NIAMS) and by Penn State College of Medicine department of Orthopaedics and Rehabilitation.
Abbreviations
- PEDF
Pigment epithelium derived factor
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All Authors do not have any conflict of interest with data reported in the manuscript.
References
- 1.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 2.Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem. 2006;281:3604–3613. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 3.Chung C, Doll JA, Stellmach VM, Gonzales J, Surapureddi S, Cornwell M, Reddy JK, Crawford SE. Pigment epithelium-derived factor is an angiogenesis and lipid regulator that activates peroxisome proliferator-activated receptor alpha. Adv Exp Med Biol. 2008;617:591–597. doi: 10.1007/978-0-387-69080-3_61. [DOI] [PubMed] [Google Scholar]
- 4.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 5.Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993;90:1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho TC, Chen SL, Shih SC, Wu JY, Han WH, Cheng HC, Yang SL, Tsao YP. Pigment epithelium-derived factor is an intrinsic antifibrosis factor targeting hepatic stellate cells. Am J Pathol. 2010;177:1798–1811. doi: 10.2353/ajpath.2010.091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauch F, Husseini A, Roughley P, Glorieux FH, Moffatt P. Lack of circulating pigment epithelium-derived factor is a marker of osteogenesis imperfecta type VI. J Clin Endocrinol Metab. 2012;97:E1550–E1556. doi: 10.1210/jc.2012-1827. [DOI] [PubMed] [Google Scholar]
- 8.Venturi G, Gandini A, Monti E, Dalle Carbonare L, Corradi M, Vincenzi M, Valenti MT, Valli M, Pelilli E, Boner A, Mottes M, Antoniazzi F. Lack of expression of SERPINF1, the gene coding for pigment epithelium-derived factor, causes progressively deforming osteogenesis imperfecta with normal type I collagen. J Bone Miner Res. 2012;27:723–728. doi: 10.1002/jbmr.1480. [DOI] [PubMed] [Google Scholar]
- 9.Homan EP, Rauch F, Grafe I, Lietman C, Doll JA, Dawson B, Bertin T, Napierala D, Morello R, Gibbs R, White L, Miki R, Cohn DH, Crawford S, Travers R, Glorieux FH, Lee B. Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J Bone Miner Res. 2011;26:2798–2803. doi: 10.1002/jbmr.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, Giunta C, Bergmann C, Rohrbach M, Koerber F, Zimmermann K, de Vries P, Wirth B, Schoenau E, Wollnik B, Veltman JA, Hoischen A, Netzer C. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2011;88:362–371. doi: 10.1016/j.ajhg.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F, Song N, Tombran-Tink J, Niyibizi C. Pigment epithelium-derived factor enhances differentiation and mineral deposition of human mesenchymal stem cells. Stem Cells. 2013;31:2714–2723. doi: 10.1002/stem.1505. [DOI] [PubMed] [Google Scholar]
- 12.Gattu AK, Swenson ES, Iwakiri Y, Samuel VT, Troiano N, Berry R, Church CD, Rodeheffer MS, Carpenter TO, Chung C. Determination of mesenchymal stem cell fate by pigment epithelium-derived factor (PEDF) results in increased adiposity and reduced bone mineral content. FASEB J. 2013;27:4384–4394. doi: 10.1096/fj.13-232900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, Song N, Tombran-Tink J, Niyibizi C. Pigment epithelium derived factor suppresses expression of Sost/sclerostin by osteocytes: Implication for its role in bone matrix mineralization. J Cell Physiol. 2014 doi: 10.1002/jcp.24859. [DOI] [PubMed] [Google Scholar]
- 14.Aparicio S, Sawant S, Lara N, Barnstable CJ, Tombran-Tink J. Expression of angiogenesis factors in human umbilical vein endothelial cells and their regulation by PEDF. Biochem Biophys Res Commun. 2005;326:387–394. doi: 10.1016/j.bbrc.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Apte RS, Barreiro RA, Duh E, Volpert O, Ferguson TA. Stimulation of neovascularization by the anti-angiogenic factor PEDF. Invest Ophthalmol Vis Sci. 2004;45:4491–4497. doi: 10.1167/iovs.04-0172. [DOI] [PubMed] [Google Scholar]
- 16.Bai YJ, Huang LZ, Zhou AY, Zhao M, Yu WZ, Li XX. Antiangiogenesis Effects of Endostatin in Retinal Neovascularization. J Ocul Pharmacol Ther. 2013 doi: 10.1089/jop.2012.0225. [DOI] [PubMed] [Google Scholar]
- 17.Johnston EK, Francis MK, Knepper JE. Recombinant pigment epithelium-derived factor PEDF binds vascular endothelial growth factor receptors 1 and 2. In Vitro Cell Dev Biol Anim. 2015;51:730–738. doi: 10.1007/s11626-015-9884-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGFVEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Lin K, Chang J, Sun J. The stimulation of osteogenic differentiation of mesenchymal stem cells and vascular endothelial growth factor secretion of endothelial cells by beta-CaSiO3/beta-Ca3(PO4)2 scaffolds. J Biomed Mater Res A. 2014;102:2096–2104. doi: 10.1002/jbm.a.34880. [DOI] [PubMed] [Google Scholar]
- 20.Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126:509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong JP, Yao YF. Contribution of VEGF and PEDF to choroidal angiogenesis: a need for balanced expressions. Clin Biochem. 2006;39:267–276. doi: 10.1016/j.clinbiochem.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Fan W, Crawford R, Xiao Y. The ratio of VEGF/PEDF expression in bone marrow mesenchymal stem cells regulates neovascularization. Differentiation. 2011;81:181–191. doi: 10.1016/j.diff.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura K, Chu CR, Sobajima S, Robbins PD, Fu FH, Izzo NJ, Niyibizi C. Adenoviral-mediated transfer of TGF-beta1 but not IGF-1 induces chondrogenic differentiation of human mesenchymal stem cells in pellet cultures. Exp Hematol. 2005;33:865–872. doi: 10.1016/j.exphem.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song N, Armstrong AD, Li F, Ouyang H, Niyibizi C. Multipotent mesenchymal stem cells from human subacromial bursa: potential for cell based tendon tissue engineering. Tissue Eng Part A. 2014;20:239–249. doi: 10.1089/ten.tea.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gvritishvili AG, Leung KW, Tombran-Tink J. Codon preference optimization increases heterologous PEDF expression. PLoS One. 2010;5:e15056. doi: 10.1371/journal.pone.0015056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emad B, Sherif el M, Basma GM, Wong RW, Bendeus M, Rabie AB. Vascular endothelial growth factor augments the healing of demineralized bone matrix grafts. Int J Surg. 2006;4:160–166. doi: 10.1016/j.ijsu.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]