Abstract
Introduction
Bowel-associated dermatosis-arthritis syndrome is rare systemic diseases characterized by a prodrome of fever, chills, and influenza-like symptoms with subsequent skin eruptions, myalgias, and polyarthralgias. It is reported to be occurred in Intestinal bypass surgery and inflammatory bowel disease.
Case description
Herein, we described a 29-years-old man with Bowel-associated dermatosis-arthritis syndrome. He had no history of gastrointestinal surgery and inflammatory bowel disease. Distribution of the gut bacterial flora showed small intestinal bacterial overgrowth.
Discussion and Evaluation
It is rarely form as Non-intestinal bypass road and non-inflammatory bowel disease was induced by small intestinal bacteria overgrowth.
Conclusions
We concluded that Immuno-inflammatory response to overgrowth of intestinal bacterial antigen induce the clinical symptoms of bowel-associated dermatosis-arthritis syndrome.
Keywords: Dermatosis, Arthritis, Inflammatory bowel disease
Background
Bowel-associated dermatosis-arthritis syndrome (BADAS) was first described by Ely in 1980s with characteristics of fever, chills, and influenza-like symptoms with subsequent skin eruptions, myalgias, and polyarthralgias (Ely PH.1980). It is called as short-bowel syndrome since the occurrence rate among patients of jejuno-ileum bypass bariatric surgery is over 20 %. BADAS is reported to be occurred in ileoanal anastomosis, biliopancreatic diversion as well as Billroth II gastrectomy. Jorizzo described four cases (Jorizzo et al. 1983), these patients had the same clinical manifestations with short-bowel syndrome, however, they had never received any operations which mentioned above. The only common point of these patients was that they all had gastrointestinal disease and similar cases had been continuously reported afterwards (Delaney et al. 1989; Kemp and Gin 1990; Geary et al. 1999; Cox and Palmer 2003). Scholars believed that it was a complication of inflammatory bowel disease and put forward the concept of bowel-associated dermatosis-arthritis syndrome (BADAS) to cover the non-intestinal bypass short-bowel syndrome. A number of literatures reported that SIBO was involved in the pathogenesis of the disease. Short-bowel syndrome predisposes the patient to Small Intestine Bacterial Overgrowth (SIBO) (Goulet and Joly 2010). It has been shown that patients with Crohn’s disease (CD) have a higher risk of SIBO development (Greco et al. 2015). Herein, we described a patient with BADAS induce by small intestinal bacterial overgrowth.
Case presentation
A 29-year-old man complained of joint pain, fever, rash and intermittent diarrhea for about a year. One year ago, he had arthralgia and swelling in the ankles, knees, wrists and digital joints, together with fever (>38.7 °C) and erythematous nodular rashes with diameter of about 1 cm turn up on his limbs,These rashes have vague boundaries with the normal skin nearby (Fig. 1). The watery and soft stools occurred intermittently, with 3–4 bouts a day and 4–5 days a month. He had been diagnosed with reactive arthritis. After taking non-steroidal anti-inflammatory drugs (NSAIDs) such as celecoxib, arthritis, fever and rash were relieved for a short time before recurrence. He didn’t undergo gastrointestinal surgery. After being admitted to our hospital, his blood cell count, urinary analysis and stool routine with occult blood test were normal. The erythrocyte sedimentation rate (ESR) 104 mm/h (0–21 mm/h), C-reactive protein (CRP) 94.7 mg/L (0–8 mg/l), interleukin-6(IL-6) 123.8 ng/ml (0–7 ng/ml) were all increased. HLA-B27 was negative. No abnormalities were found in the procalcitonin, blood culture, Multiple virus antibody, T-SPOT.TB, tumor marker, anti-nuclear antibody (ANA), anti-dsDNA antibody (AdsDNA), anti-ENA antibodies, rheumatoid factor (RF), anti-CCP, and anti-neutrophil cytoplasmic antibodies (ANCA). Electronic gastrointestinal endoscope (FUJINON EVE 400 SERIES VIDEO SYSTEM), results of abdomen CT and bone marrow aspiration cytology were normal. Distribution of the gut bacterial flora showed dysbacteriosis with 10 % klebsiellapneumoniae, 80 % E. coli and 10 % peptostreptococcus. The result of SIBO test (hydrogen-methane breath test with lactulose as the substrate) was positive (Fig. 2). A further skin biopsy was taken from a rash on the left lower extremity and Low power microscope shows lymphocyte and neutrophile granulocyte around deep blood vessels of dermis (Fig. 3). He was diagnosed with BADAS (caused by small intestinal bacterial overgrowth). The patient was improved dramatically after treatment with doxycycline 0.2 g/day and methylprednisolone 40 mg/day for a week. Glucocorticoid tapered gradually to withdrawal. The patient was followed up for 12 months without recurrent symptoms.
Fig. 1.
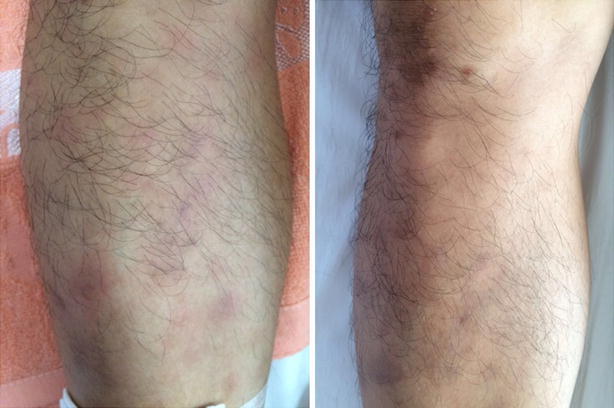
The picture of red tubercular rashes on both lower limbs. Note: The patient has red tubercular rashes on both lower limbs,erythematous nodular rashes with diameter of about 3-10 mm and vague boundaries on his limbs
Fig. 2.
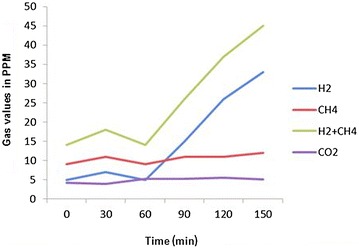
The result of small intestinal bacterial overgrowth (SIBO). Note: The result of small intestinal bacterial overgrowth (SIBO) test is positive. Hydrogen-methane breath test with glucose as the substrate, after taking glucose for 1 h, the concentration of H2 reached the peak value which implied the bacterial overgrowth in the ileum
Fig. 3.
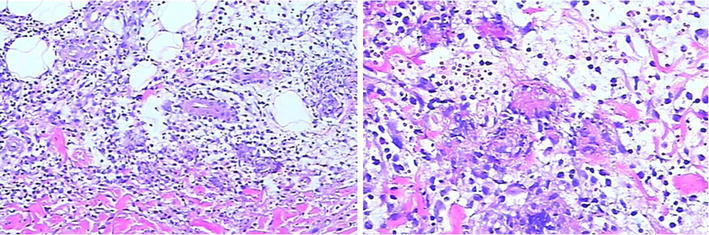
The dermal pathology at rashes. Note: The dermal pathology at rashes manifests lymphocyte and neutrophile granulocyte around the deep blood vessels of dermis (rash skin of left limb, HE staining, original magnification 200–400)
Discussion and evaluation
BADAS is a non-infectious neutrophilic dermatosis with prominent of features of rashes and joint pain. Typical rashes are red spots with diameter of 3–10 mm and vague boundaries. The rashes gradually harden in the following 1 or 2 days and turn to papulopustule with diameter of 2–4 mm. The process of this disease could last for 2–8 days and showed as a self-limited process (Patton et al. 2009; Slater et al. 2004; Kawakami et al. 2006). The rashes are mainly distributed on the body and limbs (Jorizzo et al. 1983), and they may be featured by pruritus, pain or no subjective symptom (Slater et al. 2004). Polyarthritis may be involved in patients which had peripheral joints like IP joints and wrist joints, however, but there is no imageological change, joint destruction or deformity (Utsinger 1980). Laboratory indexes such as RF, immune globulin and uric acid are all within normal limits, however, some patients may have elevated cryoglobulin (Patton et al. 2009). In microbiological examination, both of blood culture and rash fester culture results are negative (Truchuelo et al. 2013). The dermal pathology of BADAS is usually the same as acute febrile neutrophilic dermatosis (SWEET) syndrome. It mainly shows infiltration of mature neutrophile granulocyte in dermis and sometimes edema of dermal papilla. It had no fibrinoid necrosis or blood vessel infarct, which is the main difference from leukocytoclastic vasculitis (Patton et al. 2009; Ashok and Kiely 2007). However, other research showed that the dermal pathology changes with the development of the disease is not an essential condition for diagnosing the BADAS (Patton et al. 2009). In the course of clinical diagnosis and treatment, BADAS also must be distinguished from extra-intestinal manifestations of inflammatory bowel disease (Adams and Eksteen 2006; Trikudanathan et al. 2012; Marineaţă et al. 2014; Brown et al. 2015).
As the cause is not clear, it is now generally accepted that it is an abnormal immune response of residual intestine after intestinal survey or abnormal intestine of inflammatory bowel disease to the bacterial overgrowth. SIBO is defined as nonpathogenic bacteria increase over 105cfuin 1 ml of small intestine content. Due to the lack of specific symptoms, SIBO is often misdiagnosed. In fact, SIBO occurrence is fairly frequent. SIBO might present in more than 60 % patients with abdominal pain (Siniewicz-Luzenczyk et al. 2015). In systemic sclerosis, 38 % patientswith intestinal complaints were diagnosed with SIBO (Tauber et al. 2014). The formation of immune complex, access to blood and deposition in tissues and organs are crucial in the occurrence of disease which could induce various related clinical symptoms (Slater et al. 2004; Utsinger 1980; Dicken 1986; Jorizzo et al. 1984) and this theory can be indirectly proved by the curative effect of antibiotics and glucocorticoid to the disease. Glucocorticoid is as the main medicine for this disease and antibiotics (metronidazole, tetracycline and sulfonamides) are also used with different effects. Resuming the normal anatomy of intestine or curing potential gastrointestinal disease also has certain mitigative effect (Ashok and Kiely 2007).
About 20 % of patients of jejunoileum bypass bariatric surgery are likely to have the symptoms of rash or joint pain (Ely 1980). Other non-bypass surgery or disease of digestive tract such as inflammatory bowel disease has had already been continuously reported, which is secondary to the BADAS (Cox and Palmer 2003; Patton et al. 2009). In our case, the clinical manifestation and dermal pathology of rashes are the same with those diseases mentioned above, however, there is no intestinal surgery, nor any evidence of inflammatory bowel disease. Only the hydrogen-methane breath test with lactulose as the substrate indicates SIBO. The disease may not merely occur among patients of jejuno-ileum bypass bariatric surgery and inflammatory bowel disease as mentioned in previous literature. Immuno-inflammatory response to bacterial antigen may be caused by any factors which caused the overgrowth of intestinal bacteria, mediate formation of immune complex and hence induce the clinical symptoms of BADAS. Therefore, whether the patients had specific digestive system surgery or medical history, as organic or functional factor which may cause intestinal bacterial overgrowth should be taken into consideration during the clinical diagnosis of physician. Patient with only SIBO, non-intestinal bypass road and non-inflammatory bowel disease could also be diagnosed as BADAS.
Conclusion
In clinical practice, rheumatologists can easily recognize BADAS patients with jejunoileum bypass bariatric surgery or inflammatory bowel disease, and more attention should be paid to bacteria translocation, flora imbalance and SIBO which may also result in BADAS. Correctly understanding the factors that influence the occurrence of BADAS will help us getting to understand more about diagnosing this relatively rare disease.
Authors’ contributions
HZ: Management of the case and manuscript writing and correction. LZ, HL, LD and YY: Management of the case and manuscript redaction and correction. WS: Cutaneous clinicopathological analysis and manuscript correction. YL: Management of the case and manuscript correction and redaction of the illustrations. XZ: Manuscript correction. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to thank their colleagues of the Departments of Rheumatology and Immunology, Xiangya Hospital, Central South University.
Competing interests
HL received grants from Natural Science Foundation of Hunan Province and Hunan Provincial Science and Technology Department for Scientifi Research. The authors declare that they have no competing interests.
Consent
Written informed consent was obtained from the patient and his brother for publication of this case presentation and any accompanying image.
Abbreviations
- BADAS
bowel-associated dermatosis-arthritis syndrome
- SIBO
small intestinal bacterial overgrowth
- CD
Crohn’s disease
- NSAIDs
non-steroidal anti-inflammatory drugs
- ESR
erythrocyte sedimentation rate
- CRP
C-reactive protein
- IL-6
interleukin-6
- HLA-B27
human leukocyte antigen B27
- T-SPOT.TB
T-cell spot of TB test
- AdsDNA
anti-dsDNA antibody
- anti-ENA antibodies
anti-extractable nuclear antigen antibodies
- RF
rheumatoid factor
- anti-CCP
anti-cyclic peptide containing citrulline
- ANCA
anti-neutrophil cytoplasmic antibodies
- SWEET syndrome
acute febrile neutrophilic dermatosis syndrome
- ANA
anti-nuclear antibody
- IP
interphalangeal
Contributor Information
Hongjun Zhao, Email: hongjunzhao2015@sina.com.
Lijuan Zhao, Email: 1542587599@qq.com.
Wei Shi, Email: shiwei201501@sina.com.
Hui Luo, Email: huiluo2015@sina.com.
Liping Duan, Email: duanliping2015@sina.com.
Yunhui You, Email: youyunhui2015@sina.com.
Yisha Li, Email: liyisha2012@sina.com.
Xiaoxia Zuo, Email: zuoxiaoxia2015@sina.com.
References
- Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6(3):244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- Ashok D, Kiely P. Bowel-associated dermatosis–arthritis syndrome: a case report. J Med Case Reports. 2007;1:81. doi: 10.1186/1752-1947-1-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Gibson PR, Hart A, Kaplan GG, Kachroo S, Ding Q, Hautamaki E, Fan T, Black CM, Hu X, Beusterien K. Long-term outcomes of colectomy surgery among patients with ulcerative colitis. Springerplus. 2015;4:573. doi: 10.1186/s40064-015-1350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox NH, Palmer JG. Bowel -associated dermatosis-arthritis syndrome associated with ileoanal pouch anastomosis, and treatment with mycophenolate. Br J Dermatol. 2003;149(6):1296–1297. doi: 10.1111/j.1365-2133.2003.05664.x. [DOI] [PubMed] [Google Scholar]
- Delaney TA, Clay CD, Randell PL. The bowel-associated dermatosis-arthritis syndrome. Australas J Dermataol. 1989;30:23–27. doi: 10.1111/j.1440-0960.1989.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Dicken CH. Bowel-associated dermatosis-arthritis syndrome: bowel bypass syndrome without bowel bypass. J Am Acad Dermatol. 1986;14(5 Pt 1):792–796. doi: 10.1016/S0190-9622(86)70095-9. [DOI] [PubMed] [Google Scholar]
- Ely PH. The bowel bypass syndrome: a response to bacterial peptidoglycans. J Am Acad Dermatol. 1980;2:473–487. doi: 10.1016/S0190-9622(80)80148-4. [DOI] [PubMed] [Google Scholar]
- Geary RJ, Long LL, Mutasim DF. Bowel bypass syndrome without bowel bypass. Cutis. 1999;63:17–20. [PubMed] [Google Scholar]
- Goulet O, Joly F. Intestinal microbiota in short bowel syndrome. Gastroenterol Clin Biol. 2010;34(Suppl 1):S37–S43. doi: 10.1016/S0399-8320(10)70019-1. [DOI] [PubMed] [Google Scholar]
- Greco A, Caviglia GP, Brignolo P, Ribaldone DG, Reggiani S, Sguazzini C, Smedile A, Pellicano R, Resegotti A. Glucose breath test and Crohn’s disease: diagnosis of small intestinal bacterial overgrowth and evaluation of therapeutic response. Scand J Gastroenterol. 2015;50(11):1376–1381. doi: 10.3109/00365521.2015.1050691. [DOI] [PubMed] [Google Scholar]
- Jorizzo JLAP, Subrt P, Hebert AA, Henry JC, Raimer SS, Dinehart SM, Reinarz JA. Bowel—bypass syndrome without bowel bypass: bowel-associated dermatosis-arthritis syndrome. Arch Intern Med. 1983;143:457–461. doi: 10.1001/archinte.1983.00350030071013. [DOI] [PubMed] [Google Scholar]
- Jorizzo JL, Schmalstieg FC, Dinehart SM, Daniels JC, Cavallo T, Apisarnthanarax P, Rudloff HB, Gonzalez EB. Bowel-associated dermatosis-arthritis syndrome. Immune complex-mediated vessel damage and increased neutrophil migration. Arch Intern Med. 1984;144(4):738–740. doi: 10.1001/archinte.1984.00350160088016. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Saga K, Hida T, Jimbow K, Takahashi H. Fulminant bowel-associated dermatosis–arthritis syndrome that clinically showed necrotizing fasciitis-like severe skin and systemic manifestations. J Eur Acad Dermatol Venereol. 2006;20(6):751–753. doi: 10.1111/j.1468-3083.2006.01530.x. [DOI] [PubMed] [Google Scholar]
- Kemp DR, Gin D. Bowel-associated dermatosis-arthritis syndrome. Med J Aust. 1990;1(152):43–45. doi: 10.5694/j.1326-5377.1990.tb124429.x. [DOI] [PubMed] [Google Scholar]
- Marineaţă A, Rezuş E, Mihai C, Prelipcean CC. Extra intestinal manifestations and complications in inflammatory bowel disease. Rev Med Chir Soc Med Nat Iasi. 2014;118(2):279–288. [PubMed] [Google Scholar]
- Patton T, Jukic D, Juhas E. Atypical histopathology in bowel-associated dermatosis–arthritis syndrome: a case report. Dermatol Online J. 2009;15(3):3. [PubMed] [Google Scholar]
- Siniewicz-Luzenczyk K, Bik-Gawin A, Zeman K, Bąk-Romaniszyn L. Small intestinal bacterial overgrowth syndrome in children. PrzGastroenterol. 2015;10(1):28–32. doi: 10.5114/pg.2014.47494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater GH, Kerlin P, Georghiou PR, Fielding GA. Bowelassociated dermatosis–arthritis syndrome after biliopancreatic diversion. Obes Surg. 2004;14:133–135. doi: 10.1381/096089204772787446. [DOI] [PubMed] [Google Scholar]
- Tauber M, Avouac J, Benahmed A. Prevalence and predictors of small intestinal bacterial overgrowth in systemic sclerosis patients with gastrointestinal symptoms. Clin Exp Rheumatol. 2014;32(6):S82–S87. [PubMed] [Google Scholar]
- Trikudanathan G, Venkatesh PG, Navaneethan U. Diagnosis and therapeutic management of extra-intestinal manifestations of inflammatory bowel disease. Drugs. 2012;72(18):2333–2349. doi: 10.2165/11638120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Truchuelo MT, Alcántara J, Vano-Galván S, Jaén P, Moreno C. Bowel-associated dermatosis–arthritis syndrome: another cutaneous manifestation of inflammatory intestinal disease. Int J Dermatol. 2013;52(12):1596–1598. doi: 10.1111/j.1365-4632.2011.05149.x. [DOI] [PubMed] [Google Scholar]
- Utsinger PD. Systemic immune complex disease following intestinal bypass surgery: bypass disease. J Am Acad Dermatol. 1980;2(6):488–495. doi: 10.1016/S0190-9622(80)80149-6. [DOI] [PubMed] [Google Scholar]


