Abstract
Objective
It has been thought that the depletion of insulin is responsible for the catabolic consequences of diabetes; however, evidence suggests that glucagon also plays a role in diabetes pathogenesis. Glucagon suppression by glucagon receptor (Gcgr) gene deletion, glucagon immunoneutralization, or Gcgr antagonist can reverse or prevent type 1 diabetes in rodents suggesting that dysregulated glucagon is also required for development of diabetic symptoms. However, the models used in these studies were rendered diabetic by chemical- or immune-mediated β-cell destruction, in which insulin depletion is incomplete. Therefore, it is unclear whether glucagon suppression could overcome the consequence of the complete lack of insulin.
Methods
To directly test this we characterized mice that lack the Gcgr and both insulin genes (GcgrKO/InsKO).
Results
In both P1 pups and mice that were kept alive to young adulthood using insulin therapy, blood glucose and plasma ketones were modestly normalized; however, mice survived for only up to 6 days, similar to GcgrHet/InsKO controls. In addition, Gcgr gene deletion was unable to normalize plasma leptin levels, triglycerides, fatty acids, or hepatic cholesterol accumulation compared to GcgrHet/InsKO controls.
Conclusion
Therefore, the metabolic manifestations associated with a complete lack of insulin cannot be overcome by glucagon receptor gene inactivation.
Keywords: Mice, Type 1 diabetes, Insulin, Glucagon, Glucose metabolism, Lipid metabolism
Abbreviations: Gcgr, glucagon receptor; Het, heterozygous; Ins1, insulin 1; Ins2, insulin 2; InsKO, insulin knockout; KO, knockout; P, post-natal day; STZ, streptozotocin; WT, wildtype
Highlights
-
•
Gcgr gene deletion does not promote survival of InsKO mice.
-
•
Gcgr gene deletion modestly reduces blood glucose and ketones in InsKO mice.
-
•
Gcgr gene deletion does not normalize perturbed lipid metabolism in InsKO mice.
-
•
Gcgr gene deletion does not prevent diabetes in the complete absence of insulin.
1. Introduction
Hyperglucagonemia is present in many forms of diabetes [1], [2] and contributes to elevated blood glucose by promoting glycogenolysis, gluconeogenesis, and ketogenesis while inhibiting glycogen synthesis. Remarkably, mice with whole body Gcgr gene deletion (GcgrKO) are protected from streptozotocin (STZ) induced diabetes [3], [4]. While GcgrWT mice developed severe hyperglycemia, hyperketonemia, and cachexia and had to be euthanized 6 weeks post-STZ treatment, GcgrKO mice remained healthy [3], [4]. Moreover, other studies have shown that glucagon suppression by Gcgr gene deletion, glucagon immunoneutralizing antibody, or Gcgr antagonism can reduce fasting and fed blood glucose and improve glucose tolerance in healthy and diabetic models [5], [6], [7], [8]. These studies support the idea that elevated glucagon action is required for hyperglycemia in insulin-deficient type 1 diabetes. However, those studies used immune- or chemical-mediated destruction of β-cells that reduce but do not eliminate insulin. In those models, animals retain islet insulin immunoreactivity and circulating insulin, and survive for weeks without treatment [3], [4], [5], [6], [8]. As mice with knockout of both insulin genes (Ins1KO/Ins2KO, herein referred to as InsKO mice) survive for <2 days after birth [9] and approximately 1 day in adulthood following cessation of insulin therapy [10], it is evident that these other models are not insulin-free. Therefore, we aimed to determine whether loss of glucagon action improves glucose metabolism and promotes survival in the complete absence of insulin. To achieve this, we characterized mice with Gcgr, Ins1 and Ins2 gene deletions.
2. Material and methods
2.1. Animals
Mice with global Gcgr knockout, originally described by Gelling et al. [7], were supplied by Dr. Daniel Drucker. Male GcgrKO/Ins1KO/Ins2Het and female GcgrHet/Ins1KO/Ins2Het mice were bred to generate male and female experimental and control mice. Mice were genotyped using primers found in Supplemental Table 1. For pup characterization, InsKO pups were not treated with insulin. For young adult characterization, InsKO mice were treated twice daily with subcutaneous injections of Lantus insulin glargine (Sanofi-Aventis Inc., Laval, Canada). On P13-15, InsKO mice underwent a transplantation of ∼130 islets into the anterior chamber of the eye from ∼20 week old Ins1−/− donors with 0–2 alleles of Gcgr and 1–2 alleles of Ins2. At 4 weeks of age mice underwent enucleation and were carefully monitored for rapid weight loss, hunching, piloerection, lethargy, and non-responsiveness in order to euthanize any animals that reached humane endpoint. Mice were housed on a 14:10 h light–dark cycle with ad libitum access to food (5053, PicoLab Rodent Diet 20, LabDiet) and water. All experiments were approved by the University of British Columbia Animal Care Committee and performed in accordance with the Canadian Council on Animal Care guidelines.
2.2. Metabolic measurements
All parameters were measured in the ad libitum fed state. Blood glucose was measured via the tail vein using an OneTouch Ultra Glucometer (LifeScan) with a detection limit of 1.1–33.3 mM. In Figure 3G & H, one glucometer reading was above the limit of detection and assigned a value of 33.3 mM and statistical analysis was not performed. Plasma β-hydroxybutyrate (β-Hydroxybutyrate LiquiColor Test, Stanbio, Boerne, TX), glucagon (Glucagon ELISA, Mercodia, Salem, NC), leptin (Mouse Leptin ELISA, Crystal Chem, Downers Grove, IL), triglycerides and glycerol (Serum Triglyceride Determination Kit, Sigma–Aldrich, Oakville, Canada), free fatty acids (HR Series NEFA HR [2] Kit, Wako Diagnostics, Richmond, VA), and cholesterol (Cholesterol E, Wako Diagnostics, Richmond, VA) were measured from trunk samples in pups or cardiac puncture samples in young adult mice which were collected as mice reached humane endpoint. Hepatic lipid extraction was performed as previously described [11].
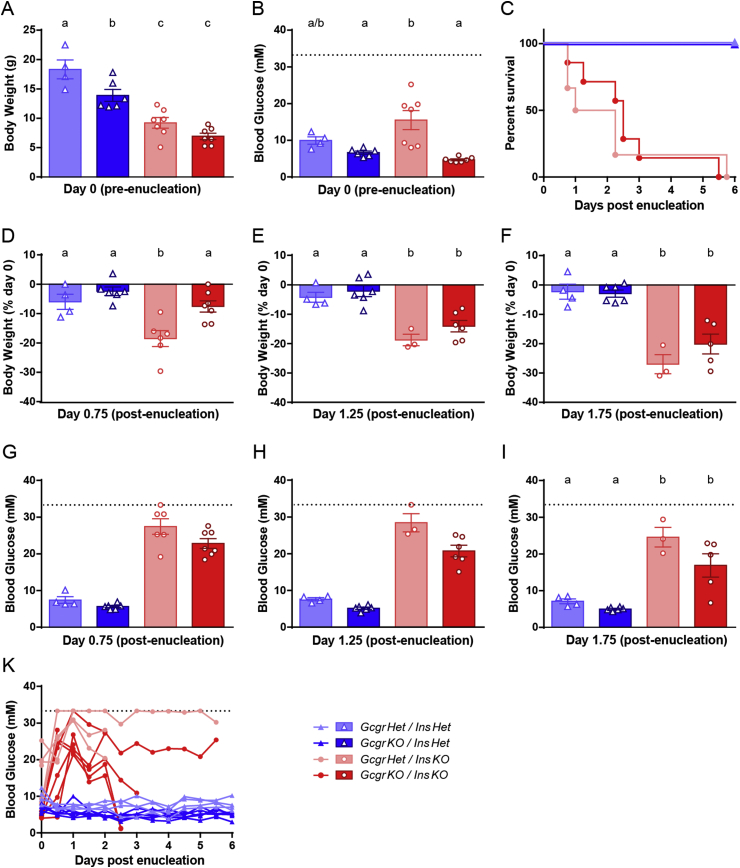
Figure 3.
Gcgr gene deletion modestly lowers blood glucose but does not promote survival in adult InsKO mice. InsKO mice with and without Gcgr gene deletion were kept alive to 4 weeks of age using insulin injections and an islet transplant into the eye. On day 0 of the study, the eye containing islets was enucleated rendering the mice completely insulin deficient. Day 0 absolute body weight (A) and blood glucose levels (B) prior to enucleation. Following enucleation, survival (C) and day 0.75 (D), 1.25 (E) and 1.75 (F) body weight loss were measured. Blood glucose at day 0.75 (G), 1.25 (H), 1.75 (I) and throughout the study (H) are graphed. Different superscripts (a, b, c) are significantly different from each other within each graph. Statistical analysis was not performed on G and H as one blood glucose value fell above the limit of detection and was assigned a value of 33.3 mM, or in K because the n value decreased over time. Data are graphed as mean ± SEM (A, B, D–I), % survival (C), or individual mice (K), n = 4–7, throughout study InsKO mice reached humane endpoint therefore n is reduced in E, F, H, and I.
2.3. Statistics
Data were analyzed using a 1-way or 2-way ANOVA with Bonferroni post-hoc testing for bar and line graphs respectively, or a Gehan–Breslow–Wilcoxon test for survival curves. Statistical analysis was performed using GraphPad Prism 6.05 (La Jolla, CA). Significance was set at P < 0.05.
3. Results
3.1. Effect of Gcgr gene deletion on survival in InsKO pups
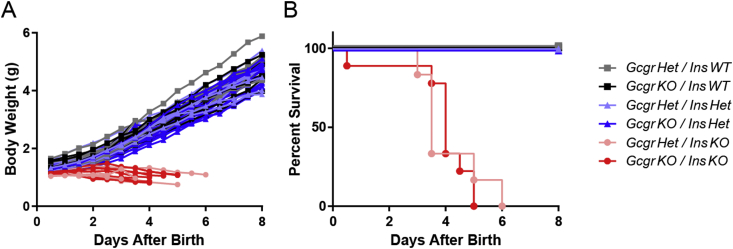
First, we aimed to determine whether Gcgr gene deletion would extend survival of InsKO pups. All male and female pups compared had 0 Ins1 alleles, 0–1 Gcgr alleles and 0–2 Ins2 alleles. GcgrHet/InsWT, GcgrKO/InsWT, GcgrHet/InsHet, and GcgrKO/InsHet pups were used as controls, while GcgrHet/InsKO and GcgrKO/InsKO pups tested the effect of Gcgr gene deletion on InsKO pups. All controls survived and gained weight throughout the first week of life, while both GcgrHet/InsKO and GcgrKO/InsKO failed to gain mass and died at a similar rate, surviving no longer than 6 days (Figure 1A & B). Therefore Gcgr gene deletion does not promote survival of InsKO pups.
Figure 1.
Gcgr gene deletion does not promote survival in InsKO pups. Ins1KO pups with 0–1 alleles of Gcgr and 0–2 alleles of Ins2 were tracked twice daily for body weight (A) and survival (B) for 8 days after birth. For A statistical analysis was not performed, for B no statistical differences were observed between GcgrHet/InsKO and GcgrKO/InsKO pups. Data are graphed as individual pups (A) and % survival (B), n = 6–17.
3.2. Effect of Gcgr gene deletion on glucose and lipid metabolism in InsKO pups
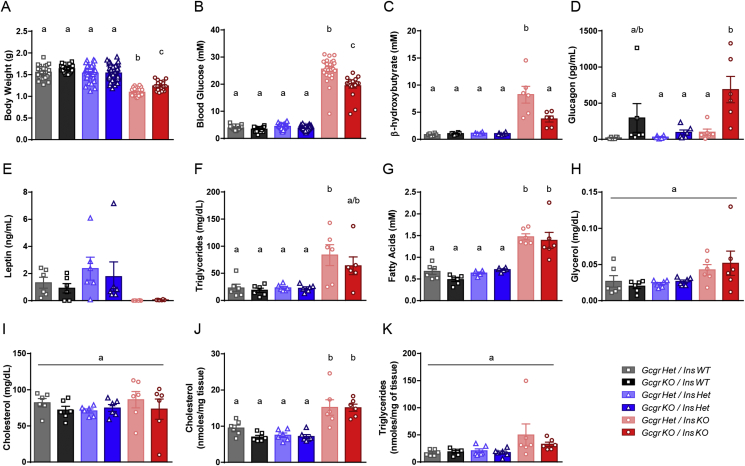
We assessed if loss of glucagon action in InsKO pups improved metabolism by analyzing metabolic parameters associated with glucose metabolism in P1 pups. Results were variable, likely a reflection of litter size, when the animals last fed, and the time of day the samples were collected. Gcgr gene deletion with the InsWT or Het genotype did not affect any of the parameters measured. At P1 GcgrHet/InsKO pups weighed 30% less than controls, which was marginally increased in GcgrKO/InsKO pups but still 20% less than the control groups (Figure 2A). As expected, GcgrHet/InsKO pups were hyperglycemic compared to controls. While still hyperglycemic, this was modestly decreased by 24% in GcgrKO/InsKO pups (Figure 2B) relative to GcgrHet/InsKO pups. Similarly, plasma β-hydroxybutyrate levels were 9-fold higher in GcgrHet/InsKO pups compared to controls and reduced in GcgrKO/InsKO pups to 4-fold that of controls (Figure 2C). It has been published that adult GcgrKO mice exhibit elevated glucagon levels [3], [7]. At P1, glucagon levels were significantly higher than controls in GcgrKO/InsKO pups (Figure 2D). Therefore Gcgr gene deletion modestly improved hyperglycemia and hyperketonemia in InsKO pups.
Figure 2.
Gcgr gene deletion modestly improves body weight, blood glucose and plasma ketones but does not normalize plasma leptin, triglycerides, fatty acids or hepatic cholesterol accumulation in InsKO pups. On P1, Ins1KO pups with 0–1 alleles of Gcgr and 0–2 alleles of Ins2 were harvested to measure body weight (A), trunk blood was collected to measure blood glucose (B) and plasma β-hydroxybutyrate (C), glucagon (D), leptin (E), triglycerides (F), fatty acids (G), glycerol (H), and cholesterol (I), and liver was collected to quantify hepatic cholesterol (J) and triglycerides (K). Different superscripts (a, b, c) are significantly different from each other within each graph. Statistical analysis was not performed on leptin measurements (D) as some samples were below the limit of detection. Data are mean ± SEM, n = 19–59 for A & B, n = 6 for C–K.
Since type 1 diabetes is associated with aberrant lipid metabolism, we characterized lipid homeostasis in P1 pups. As expected, due to the positive effect of insulin on adipogenesis and leptin production [12], GcgrHet/InsKO pups had undetectable plasma leptin levels which were unchanged due to Gcgr gene deletion (Figure 2E). Plasma triglycerides and free fatty acids were increased in GcgrHet/InsKO pups relative to controls and Gcgr gene deletion did not alter this (Figure 2F & G). Plasma glycerol and cholesterol in GcgrHet/InsKO pups were similar to controls and unchanged due to the loss of the Gcgr (Figure 2H & I). Finally, hepatic cholesterol accumulation was unaffected by Gcgr gene deletion, and hepatic triglyceride levels in InsKO pups were comparable to that of controls (Figure 2J & K). Therefore, Gcgr gene deletion does not normalize lipid metabolism in InsKO pups.
3.3. Effect of Gcgr gene deletion on survival of adult InsKO mice
We have shown that GcgrKO/InsKO pups do not have extended survival; however, because insulin is involved in growth and development [13], we sought to determine the effect of glucagon suppression in adult InsKO mice. As previously published, male and female InsKO mice were maintained to adulthood using insulin injections and islet transplantation into the eye [10]. GcgrKO/InsKO mice failed to grow normally following weaning despite post-transplant euglycemia (Figure 3A & B). Despite receiving the same number of islets, GcgrKO/InsKO mice had normal blood glucose levels compared to hyperglycemic GcgrHet/InsKO mice, suggesting that the loss of Gcgr aids in improving hyperglycemia when insulin is present. On day 0 of the study, the eye containing the exogenous islets was enucleated to render the InsKO mice insulin deficient; InsHet mice underwent enucleation to control for the effect of surgery. Following enucleation, all InsKO mice rapidly lost weight, were hunched, displayed piloerection, were lethargic or non-responsive, and reached humane endpoint at a similar rate, by day 6 of the study (Figure 3C). As shown in Figure 3D–F, the kinetics of this weight loss was similar between GcgrHet/InsKO and GcgrKO/InsKO mice. Therefore in young adult mice, Gcgr gene deletion was unable to prevent body weight loss and death associated with a complete loss of insulin.
3.4. Effect of Gcgr gene deletion on glucose and lipid metabolism in adult InsKO mice
We next sought to determine whether Gcgr gene deletion improved blood glucose levels in young adult InsKO mice. InsKO mice rapidly became hyperglycemic upon removal of the graft. At day 0.75, 1.25, and 1.75, blood glucose of GcgrKO/InsKO mice was modestly lower than that of GcgrHet/InsKO mice (Figure 3G–K). Interestingly, at humane endpoint, 3 of the GcgrKO/InsKO mice were hypoglycemic (<1.3 mM), which was not observed in the GcgrHet/InsKO mice (Figure 3K and data not shown). In conclusion, Gcgr gene deletion modestly reduced blood glucose throughout the study and even resulted in hypoglycemia in some mice, but did not permit survival in young adult InsKO mice.
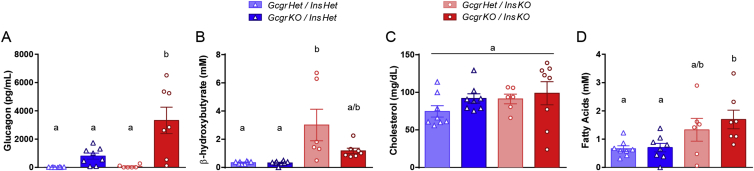
Finally, we measured various metabolic parameters in young adult InsKO mice with and without Gcgr gene deletion. Similar to P1 pups, GcgrKO/InsKO mice had substantially elevated plasma glucagon levels compared to all other groups, while the GcgrKO/InsHet group trended towards increased levels of glucagon compared to GcgrHet groups (Figure 4A). As expected β-hydroxybutyrate levels were substantially elevated in GcgrHet/InsKO mice. The GcgrKO/InsKO mice trended (P = 0.09) to have lower β-hydroxybutyrate levels, which were similar to that of controls, however there was large variability in ketones levels in the GcgrHet/InsKO mice (Figure 4B). Plasma cholesterol levels were similar between controls and InsKO mice, and fatty acids levels were increased in GcgrKO/InsKO compared to controls (Figure 4C & D).
Figure 4.
Gcgr gene deletion increases plasma glucagon, normalizes plasma ketones, and does not affect plasma cholesterol or fatty acids in adult InsKO mice. At humane endpoint (InsKO mice) or the end of the study (controls), cardiac blood was collected to measure plasma glucagon (A), β-hydroxybutyrate (B), cholesterol (C), and fatty acids (D). Different superscripts (a, b, c) are significantly different from each other within each graph. Data are mean ± SEM, n = 6–8.
4. Discussion
Recent evidence suggests that glucagon itself causes the lethal catabolic consequences of insulin deficiency [14]. Remarkably, despite a 90% reduction in insulin, GcgrKO mice remained euglycemic and healthy compared to controls, which required euthanization 6 weeks post-STZ [3], [6]. However, we found that with 100% elimination of insulin, Gcgr gene deletion in mice does not extend survival. At both P1 and in early adulthood, GcgrKO/InsKO mice failed to gain mass or lost a substantial amount of weight and survived no longer than GcgrHet/InsKO controls. In a previous study, we generated InsKO mice on a GcgrWT background and found that these mice survived for ∼1 day following islet graft removal, less than the 6 days observed in the current study. While it may appear that the reduction of one Gcgr allele may have had a slight protective effect on InsKO mice, we believe this is unlikely, because it has been shown that GcgrWT and GcgrHet mice display similar Gcgr function and metabolic phenotypes. Indeed, liver membranes prepared from GcgrWT and GcgrHet mice exhibit identical responses to glucagon stimulation as measured by cAMP production and these mice exhibit similar blood glucose elevations in response to i.p. injection of glucagon, suggesting that the loss of 1 allele does not significantly alter the function of glucagon receptor in the mouse [7]. In addition, in another strain of GcgrKO mice, it has been shown that gross appearance, body weight, visceral fat, liver and pancreas weight, and random glucose are not different between GcgrWT and GcgrHet mice [15]. Therefore, we believe that the difference in days of survival between GcgrWT and GcgrHet mice between studies may be due to differences between background strain, age, diet, and facility. Nonetheless, it is clear that deletion of both Gcgr alleles cannot promote long-term survival in mice completely lacking insulin as all reached humane endpoint within 1 week of birth.
The loss of glucagon action in the complete absence of insulin does moderately improve aspects of metabolism. Glucagon plays a role not only in the fasting state but also in the fed state, which is evident as Gcgr gene deletion can normalize fed blood glucose levels and glucose tolerance [3], [4], [6]. In our study, GcgrKO/InsKO mice only experienced a partial improvement in the fed blood glucose levels in both P1 pups and young adults. Furthermore, although we saw modest blood glucose reduction throughout the study in the fed state, at euthanasia, some of the GcgrKO/InsKO were hypoglycemic, a state not observed in the GcgrHet/InsKO mice. The feeding state of the mice at the time of euthanization is unclear. Therefore, we speculate that if we had fasted the GcgrKO/InsKO mice, they may have experienced a larger decrease in blood glucose due to insufficient endogenous glucose production. Furthermore, it has been shown that Gcgr gene deletion normalizes ketones [3]. Since GcgrHet/InsKO mice had highly variable ketones, levels only trended towards improvement in GcgrKO/InsKO adult mice, and although we observed a significant improvement in ketones in P1 pups, levels in GcgrKO/InsKO mice still remained 4× higher than controls. Interestingly, in both pups and adults, loss of the Gcgr gene did not improve aberrant lipid metabolism in the InsKO mice. Thus, while loss of glucagon action can improve some aspects of metabolism, metabolism is not normalized in the absence of both insulin and glucagon action.
While several reports indicate that loss of glucagon action can protect rodents from diabetes [3], [4], [5], [6], [8], some studies suggest a less substantial role for glucagon in diabetes pathogenesis [16], [17]. Meek et al. suppressed glucagon action in STZ-diabetic rats using a glucagon-like peptide 1 receptor agonist or a glucagon neutralizing antibody, both of which did not change blood glucose levels, but lowered ketones levels, suggesting that hyperglucagonemia is required for ketosis but not hyperglycemia [16]. Their report only partially agrees with our data as we observed minor improvements in both blood glucose and ketones. Furthermore, Steenberg et al. report that ablation of glucagon expressing cells using GCG-DTR mice does not improve glucose tolerance but modestly prevents further deterioration in STZ-diabetic mice [17]. In addition, a recent study by Damond et al. attempted to maximize insulin blockage in GcgrKO mice through combined STZ treatment and insulin receptor antagonism or diphtheria toxin induced β-cell loss [18]. Interestingly, although insulin-depleted GcgrKO mice were hyperglycemic compared to untreated GcgrKO mice, these mice had modestly improved blood glucose levels compared to GcgrHet mice treated with the same insulin-blocking regimen [18]. This effect is similar to the results of our paper, strengthening the conclusion that Gcgr gene deletion can only modestly reduce blood glucose levels in the absence of insulin but cannot prevent diabetes. Furthermore, in the report by Damond et al., although GcgrKO treated mice had decreased survival compared to untreated GcgrKO mice, they survived 3 times longer than GcgrHet mice with the same insulin-blocking regimen [18]. This is in contrast to our results in which both GcgrKO/InsKO and GcgrHet/InsKO mice survived for only 6 days. This difference may reflect the incomplete depletion of insulin by Damond et al., compared to the complete lack of insulin in our model.
Collectively, we have shown that the absence of glucagon signaling can modestly improve diabetic symptoms in insulin knockout mice, but this improvement was insufficient in promoting survival. Therefore, we have provided unequivocal evidence that it is not possible to protect from the catabolic consequences due to the complete loss of insulin by eliminating glucagon action.
Author contributions
U.H.N. and T.J.K. designed the experiments. U.H.N. and J.S.S.H. performed experiments. M.M. performed islet transplant and enucleation surgeries. U.H.N. analyzed data and wrote the initial manuscript. M.J.C. generated the GcgrKO mice. All authors were involved in the discussion and revision of the manuscript. T.J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
We thank Shannon O'Dwyer for technical assistance and Dr. Daniel Drucker for supplying the GcgrKO mice. This work was supported by CIHR. U.H.N. was supported by NSERC, J.S.S.H. was supported by the Kinsley Brotherton McLeod Endowment and the Florence & George Heighway Endowment Fund, and M.M. was supported by JDRF.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.05.014.
Contributor Information
Ursula H. Neumann, Email: ursulahneumann@gmail.com.
Jessica S.S. Ho, Email: h.jessica1@gmail.com.
Majid Mojibian, Email: mojibian@mail.ubc.ca.
Scott D. Covey, Email: scott.covey@ubc.ca.
Maureen J. Charron, Email: maureen.charron@einstein.yu.edu.
Timothy J. Kieffer, Email: tim.kieffer@ubc.ca.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Muller W.A., Faloona G.R., Unger R.H. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. The American Journal of Medicine. 1973;54:52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- 2.Reaven G.M., Chen Y.D., Golay A., Swislocki A.L., Jaspan J.B. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. The Journal of Clinical Endocrinology and Metabolism. 1987;64:106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y., Wang M.Y., Du X.Q., Charron M.J., Unger R.H. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y., Berglund E.D., Wang M.Y., Fu X., Yu X., Charron M.J. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14972–14976. doi: 10.1073/pnas.1205983109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M.Y., Yan H., Shi Z., Evans M.R., Yu X., Lee Y. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2503–2508. doi: 10.1073/pnas.1424934112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jun L.S., Millican R.L., Hawkins E.D., Konkol D.L., Showalter A.D., Christe M.E. Absence of glucagon and insulin action reveals a role for the GLP-1 receptor in endogenous glucose production. Diabetes. 2015;64:819–827. doi: 10.2337/db14-1052. [DOI] [PubMed] [Google Scholar]
- 7.Gelling R.W., Du X.Q., Dichmann D.S., Romer J., Huang H., Cui L. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand C.L., Jorgensen P.N., Svendsen I., Holst J.J. Evidence for a major role for glucagon in regulation of plasma glucose in conscious, nondiabetic, and alloxan-induced diabetic rabbits. Diabetes. 1996;45:1076–1083. doi: 10.2337/diab.45.8.1076. [DOI] [PubMed] [Google Scholar]
- 9.Duvillie B., Cordonnier N., Deltour L., Dandoy-Dron F., Itier J.M., Monthioux E. Phenotypic alterations in insulin-deficient mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5137–5140. doi: 10.1073/pnas.94.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann U.H., Denroche H.C., Mojibian M., Covey S.D., Kieffer T.J. Insulin knockout mice have extended survival but volatile blood glucose levels on leptin therapy. Endocrinology. 2016;157:1007–1012. doi: 10.1210/en.2015-1890. [DOI] [PubMed] [Google Scholar]
- 11.Huynh F.K., Levi J., Denroche H.C., Gray S.L., Voshol P.J., Neumann U.H. Disruption of hepatic leptin signaling protects mice from age- and diet-related glucose intolerance. Diabetes. 2010;59:3032–3040. doi: 10.2337/db10-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrann C.D., Rosen E.D. New insights into adipocyte-specific leptin gene expression. Adipocyte. 2012;1:168–172. doi: 10.4161/adip.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laron Z. Insulin–a growth hormone. Archives of Physiology and Biochemistry. 2008;114:11–16. doi: 10.1080/13813450801928356. [DOI] [PubMed] [Google Scholar]
- 14.Unger R.H., Cherrington A.D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. Journal of Clinical Investigation. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu R., Dhall D., Nissen N.N., Zhou C., Ren S.G. Pancreatic neuroendocrine tumors in glucagon receptor-deficient mice. PLoS One. 2011;6:e23397. doi: 10.1371/journal.pone.0023397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meek T.H., Dorfman M.D., Matsen M.E., Fischer J.D., Cubelo A., Kumar M.R. Evidence that in uncontrolled diabetes, hyperglucagonemia is required for ketosis but not for increased hepatic glucose production or hyperglycemia. Diabetes. 2015;64:2376–2387. doi: 10.2337/db14-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steenberg V.R., Jensen S.M., Pedersen J., Madsen A.N., Windelov J.A., Holst B. Acute disruption of glucagon secretion or action does not improve glucose tolerance in an insulin-deficient mouse model of diabetes. Diabetologia. 2016;59:363–370. doi: 10.1007/s00125-015-3794-2. [DOI] [PubMed] [Google Scholar]
- 18.Damond N., Thorel F., Moyers J.S., Charron M.J., Vuguin P.M., Powers A.C. Blockade of glucagon signaling prevents or reverses diabetes onset only if residual beta-cells persist. eLife. 2016;5 doi: 10.7554/eLife.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.