Abstract
Objective
The dorsomedial hypothalamus (DMH) has been considered an orexigenic nucleus, since the DMH lesion reduced food intake and body weight and induced resistance to diet-induced obesity. The DMH expresses feeding regulatory neuropeptides and receptors including neuropeptide Y (NPY), cocaine- and amphetamine-regulated transcript (CART), cholecystokinin (CCK), leptin receptor, and melanocortin 3/4 receptors. However, the principal neurons generating the orexigenic function in the DMH remain to be defined. This study aimed to clarify the role of the DMH GABAergic neurons in feeding regulation by using optogenetics and electrophysiological techniques.
Methods
We generated the mice expressing ChRFR-C167A, a bistable chimeric channelrhodopsin, selectively in GABAergic neurons of DMH via locally injected adeno-associated virus 2. Food intake after optogenetic activation of DMH GABAergic neurons was measured. Electrophysiological properties of DMH GABAergic neurons were measured using slice patch clamp.
Results
Optogenetic activation of DMH GABAergic neurons promoted food intake. Leptin hyperpolarized and lowering glucose depolarized half of DMH GABAergic neurons, suggesting their orexigenic property. Optical activation of axonal terminals of DMH GABAergic neurons at the paraventricular nucleus of hypothalamus (PVN), where anorexigenic neurons are localized, increased inhibitory postsynaptic currents on PVN neurons and promoted food intake.
Conclusion
DMH GABAergic neurons are regulated by metabolic signals leptin and glucose and, once activated, promote food intake via inhibitory synaptic transmission to PVN.
Keywords: Dorsomedial hypothalamus, GABAergic neuron, Feeding, Leptin, Glucose, Optogenetics
Highlights
-
•
Optogenetic activation of DMH-GABAergic neurons promotes food intake.
-
•
Leptin hyperpolarizes and low glucose depolarizes half of DMH-GABAergic neurons.
-
•
DMH-GABAergic neuron's inhibitory synaptic transmission to PVN promotes food intake.
1. Introduction
The dorsomedial hypothalamus (DMH) has been considered an orexigenic nucleus, since its lesion reduces food intake and body weight [1] and induces resistance against diet-induced obesity [2], [3]. The DMH lesion also impairs food-entrainable circadian rhythms [4], [5], [6]. The DMH expresses feeding regulatory neuropeptides including neuropeptide Y (NPY) [7], cocaine- and amphetamine-regulated transcript (CART) [8], and prolactin-releasing peptide (PrRP) [9]. It also expresses various receptors, including leptin receptor [10], melanocortin 3/4 receptors (MC3/4) [11], [12], Y1 receptor, Y5 receptor [13], and CCK receptor [14], [15]. Region specific knock down and overexpression studies demonstrated that NPY neurons in DMH, which are GABAergic and leptin insensitive [7], [16], play a role to promote food intake in rats [17], [18], [19], being consistent with the DMH-lesion studies. However, it was reported that the level of NPY expression is very low in mice fed with normal chow, questioning its physiological role, while it is increased in diet-induced obesity [20]. Hence, the principal orexigenic neuron in DMH under physiological conditions remains to be identified.
It was reported that the mice deficient in leptin receptor specifically in GABAergic neurons develop greater increases in food intake and body weight compared to the mice deficient in leptin receptor specifically in agouti-related protein (AgRP), proopiomelanocortin (POMC) or steroidogenic factor 1 (SF1) neurons [21]. These results suggested that GABAergic neurons including those in DMH could be a principal orexigenic neuron targeted by leptin [21].
In the present study, the role of GABAergic neurons in DMH in feeding regulation was analyzed using optogenetic and electrophysiological techniques. We found that DMH GABAergic neurons are hyperpolarized by leptin and depolarized by lowering glucose, and that their optogenetic activation elicits inhibitory synaptic transmission to the paraventricular nucleus of hypothalamus (PVN) where anorexigenic neurons are localized, and promotes food intake.
2. Materials and methods
2.1. Adeno-associated virus (AAV) 2 production
ChRFR-C167A, one of bistable variants of chimeric channelrhodopsins [22], was fused with Venus and cloned into AAV2-GAD1 promoter-WPRE-BGH-polyA vector. The AAV2 virus coded ChRFR-C167A-Venus was generated using GD1001-RV (Genedetect.com Ltd. Auckland, New Zealand). Titer of the virus was 1.1 × 1012 genomic particles/ml.
2.2. Animals
Male C57black6/J mice aged 8 weeks were maintained in a 12/12 h light/dark cycle. The AAV2-GAD1-ChRFR-C167A virus (50 nl/injection site) was injected stereotaxically to DMH at 1.4 mm caudal to the bregma in the midline, 0.2 mm lateral and 5.4 mm below the surface of the skull, under anesthesia with tribromoethanol (200 mg/kg). The optical fiber with 250 or 500 μm diameter was stereotaxically placed above DMH (at 1.4 mm caudal to the bregma in the midline, 0.5 mm lateral and 4.8 mm below the surface of the skull) or PVN (at 0.6 mm caudal to the bregma in the midline, 0.25 mm lateral and 4.4 mm below the surface of the skull). Mice were allowed to recover from the operation for 2 weeks. On the day of experiments, food was removed from cages at 15:30. The food was returned to cages at 19:30, and food intake at 0.5, 1, 2, 3, 6 h were measured. Exposure to blue laser (473 nm) followed by yellow (589 nm) laser (LUCIR, Tsukuba, Japan) was performed via optical fibers with 10 ms pulses, 50 Hz for 2 s, repeated every 5 s to the DMH or 3 s yellow pulse following 2 s blue pulse repeated every 10 s to the PVN for 3 h from 19:30 to 22:30. At the end of the experiments, the mice were perfused with 4% paraformaldehyde (PFA) in 0.1 M PB and the coronal sections of the hypothalamus were cut, to histologically verify the position of the virus infection and optical fiber.
The animal experiments for this study were carried out in a humane manner after receiving approval from the Institutional Animal experiment Committee of Jichi Medical University, and in accordance with the Institutional Regulation for Animal Experiments and Fundamental Guideline for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports and Technology.
2.3. Acute slice preparation
The brains were rapidly removed from C57BL/6J male mice infected AAV2-GAD1-ChRFR-C167A to DMH under anesthesia with tribromoethanol (200 mg/kg). The isolated brains were placed in ice-cold, carboxygenated (95% O2 and 5% CO2) high mannitol solution containing (in mM) 229 mannitol, 3 KCl, 6 MgCl2, 0.5 CaCl2, 1 NaH2PO4, 26 NaHCO3, and 10 glucose at pH 7.4 with 0.5 μM tetrodotoxin (osmolarity; 300–305 mOsm). A block of tissue containing the hypothalamus was isolated and coronal slices (300 μm) were cut on a vibratome. Following recovery for 1–2 h, slices were moved to a recording chamber mounted on BX51WI upright microscope (Olympus) equipped with video-enhanced infrared-differential interference contrast (DIC) and fluorescence. Slices were perfused with a continuous flow of carboxygenated aCSF containing (in mM) 127 NaCl, 2.5 KCl, 2 MgCl2, 2 CaCl2, 1.23 NaH2PO4, 26 NaHCO3, and 2.5–10 glucose at pH 7.4. Neurons were visualized with Olympus Optical 40× water-immersion lens.
2.4. Patch-clamp recording
Whole-cell current-clamp recordings were performed as previously reported [23]. Briefly, pipettes were used with 3–9 MΩ resistance after being filled with pipette solution. Pipettes were made of borosilicate glass (Narishige) using a PP-83 vertical puller (Narishige) or a Sutter micropipette puller (P-1000). The pipettes with 3–9 MΩ resistance after being filled with pipette solution were used. The composition of the pipette solution for current clamp recording was (in mM): 135 K-gluconate (for current clamp recording) or KCl (for IPSC recording), MgCl2 2, HEPES 10, EGTA 1.1, Mg-ATP 2.5, Na2-GTP 0.3, and Na2-phosphocreatine 10 at pH 7.3 with KOH (osmolarity; 290–295 mOsm). Axopatch 200B amplifier and Clampex 10 software (Axon Instruments) were used for data acquisition. Pclamp 10 (Axon Instruments) software was used for analysis. Liquid junction potential correction was performed off-line. Access resistance was continuously monitored during the experiments. Only those cells in which access resistance was stable (changes ∼30%) were included in the analysis. The data was analyzed by Clamp fit 10 (Axon instruments) software and GraphPad Prism6 software. If a change of membrane potential was at least twice the standard deviation of membrane potential for 2 min before addition of agents, it was considered the response.
Irradiation was carried out using power LEDs (each from Lumileds, San Jose, CA) emitting either blue light (peak, 460–490 nm, LXHL-NB98) or yellow light (peak, 587–597 nm, LXHL-NL98) controlled by a regulator (SLA-1000-2, Mightex, Toronto, Canada). If the cumulative distribution of IPSC amplitude for 20 s after light exposure was significantly larger than that before light exposure by kolomogrv-semirnov test, it was considered the induction of light-evoked IPSCs.
2.5. Statistical analysis
Data are expressed as means ± s.e.m. Two-way ANOVA followed by Sidak multiple range tests was used for food intake experiments and one-way ANOVA followed by Dunnet multiple range tests for membrane potential experiments.
3. Results
3.1. Optogenetic activation of GABAergic neurons in DMH promotes food intake
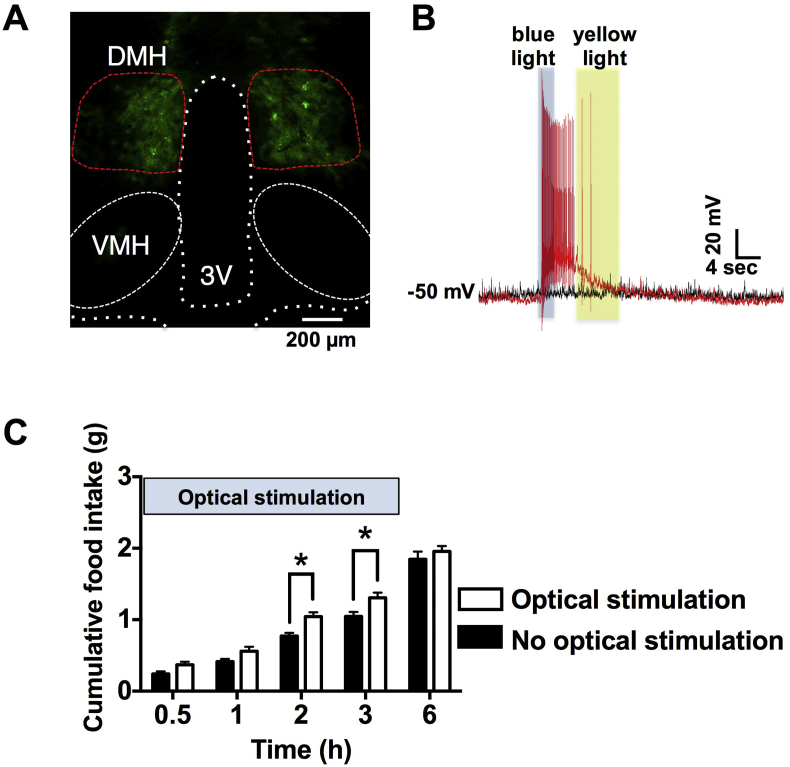
To selectively activate GABAergic neurons in DMH, AAV2 coded ChRFR-C167A-Venus under GAD1 promoter was infected to DMH. ChRFR-C167A, a bistable variant of chimeric channelrhodopsin, provides bimodal regulation: exposure to blue light (470 nm) induces long lasting opening, which is subsequently terminated by exposure to yellow light (592 nm) [22]. The mice expressing ChRFR-C167A-Venus in DMH GABAergic neurons were studied. Venus fluorescence was observed in DMH at 2 weeks after virus infection (Figure 1A). In acute slices including DMH under current clamp mode, the ChRFR-C167A-Venus expressing neurons were long lastingly depolarized by blue LED light exposure and repolarized by yellow LED light (Figure 1B). In these mice, food intake was measured with or without blue (473 nm) and yellow (589 nm) laser light exposure for 3 h via optical fiber inserted above DMH. Cumulative food intake at 2 and 3 h of light exposure was significantly greater than the corresponding values in mice without light exposure (Figure 1C). These data indicated that activation of DMH GABAergic neurons promoted food intake.
Figure 1.
Optogenetic activation of GABAergic neurons in DMH promotes food intake. A, Local infection of AAV2-GAD1-ChRFR-C167A virus expressing ChRFR-C167A in DMH. Scale bar indicates 200 μm. B, ChRFR-C167A expressing neuron in DMH was depolarized and repolarized by blue and yellow LED light exposure, respectively (red trace), while membrane potential was unchanged without light exposure (black trace). C, Cumulative food intake at 2 and 3 h was significantly increased by optical stimulation of DMH GABAergic neurons for 3 h. Optical stimulation started at the onset of dark phase (19:30). Optical stimulation group n = 3, non-optical stimulation group n = 4. * indicates p < 0.05, two-way ANOVA followed by Sidak multiple range test.
3.2. Leptin hyperpolarizes and lowering glucose depolarizes DMH GABAergic neurons
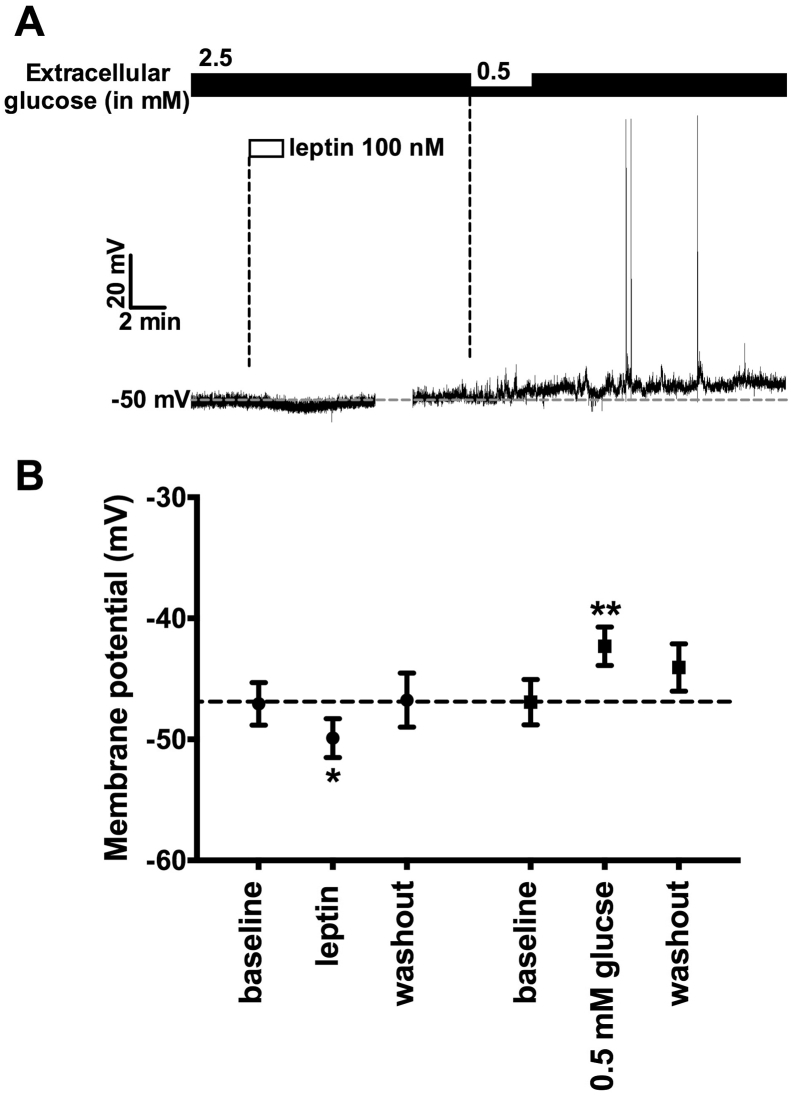
To further assess the feeding-related property of DMH GABAergic neurons, their responsiveness to systemic metabolic factors, anorexigenic leptin and orexigenic low glucose was examined. Neural activity of ChRFR-C167A expressing neurons in DMH against leptin and lowering glucose was measured in patch clamp experiments under current clamp mode. In 10 recordings from 4 mice, leptin hyperpolarized approximately 40% of ChRFR-C167A expressing neurons from −47.1 ± 1.8 mV to −49.9 ± 1.6 mV (Figure 2A,B). Lowering glucose from 2.5 to 0.5 mM depolarized approximately 60% of ChRFR-C167A expressing neurons from −46.93 ± 1.9 mV to −42.31 ± 1.6 mV (Figure 2A,B), and hyperpolarized 30% of them from −51.5 ± 2.2 mV to −55.1 ± 2.4 mV (data not shown). All the leptin-hyperpolarized neurons were depolarized by lowering glucose (Figure 2A). These data indicate that the DMH GABAergic neuron is under reciprocal regulation by leptin and lowering glucose and its activation promotes food intake.
Figure 2.
Leptin hyperpolarizes and lowering glucose depolarizes DMH GABAergic neurons. A, Representative trace of membrane potential recording on ChRFR-C167A expressing neuron. Leptin (100 nM) hyperpolarized and lowering glucose (from 2.5 to 0.5 mM) depolarized the neuron. B, averaged membrane potential before, during and after application of leptin (left) and lowering glucose (right). *p < 0.05, **p < 0.01. One-way ANOVA followed by Dunnet multiple range test.
3.3. DMH GABAergic neurons inhibit PVN neurons and promote food intake
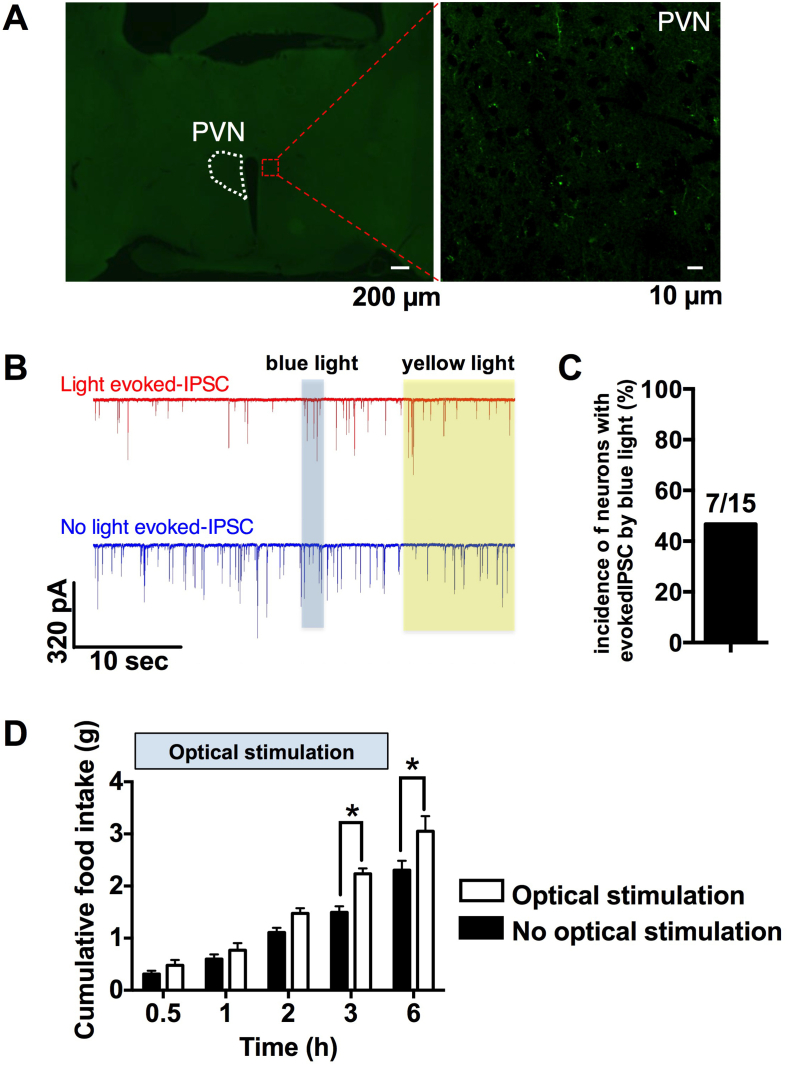
Axonal fibers and terminals of DMH ChRFR-C167A expressing neurons were detected by the Venus fluorescence fused with ChRFR-C167A in PVN (Figure 3A), where anorexigenic neuropeptides, oxytocin, CRH and nesfatin-1, are expressed [24]. To examine the functional connection between DMH ChRFR-C167A expressing neurons and PVN neurons, the effect of light exposure on IPSC onto PVN neurons was examined. In 15 recordings from 5 mice, blue light exposure increased amplitude of IPSC in 47% of PVN neurons (Figure 3B,C). This result indicated a functional connection of DMH-ChRFR-C167A expressing neurons to PVN neurons, and prompted us to examine whether this connection is linked to feeding behavior. Light exposure was performed via optical fiber inserted above PVN and food intake was measured. Blue (478 nm) and yellow (584 nm) laser light exposure for 3 h increased cumulative food intake for 3 h and 6 h significantly as well as a trend for increase for 0.5, 1 and 2 h (Figure 3D). These data indicated the DMH GABAergic neuron's inhibitory synaptic transmission to PVN, which promotes food intake.
Figure 3.
DMH GABAergic neurons inhibit PVN neurons and promote food intake. A, Axonal fibers and terminals of DMH GABAergic neurons projecting to PVN. Scale bar indicates 200 μm in low magnification (left) and 10 μm in high magnification images (right). B, Representative traces of IPSC onto PVN neurons that were evoked (upper) and unaltered (lower) by light. C, In 15 IPSC recordings on PVN neurons, 7 (47%) showed light-evoked IPSC. D, Cumulative food intake was increased by 3 h optical stimulation of axonal terminals of DMH GABAergic neurons at PVN. Optical stimulation started at the onset of dark phase. Optical stimulation group, n = 3, non-optical stimulation group, n = 4. *p < 0.05 by two-way ANOVA followed by Sidak multiple range test.
4. Discussion
The present study indicates that DMH GABAergic neurons are activated by lowering glucose, a peripheral orexigenic signal, and inhibited by leptin, a peripheral anorexigenic signal, and that once activated they promote food intake via inhibitory synaptic transmission to the neurons of PVN.
Although orexigenic function of DMH has long been suggested, definitive role of DMH in the regulation of feeding has remained unclear. This is partly due to that the neuron subpopulation in the DMH that regulates feeding is less specified, in contrast to the ARC where orexigenic NPY/AgRP and anorexigenic POMC/CART neurons have been well established. The present study employed the optogenetics to selectively activate DMH GABAergic neurons, and demonstrated that their activation promotes feeding behavior. Furthermore, DMH GABAergic neurons substantially projected and exerted inhibitory synaptic transmission to the neurons of PVN, the integrative center of feeding. Moreover, DMH GABAergic neurons were directly regulated by leptin and lowering glucose, the factors reflecting systemic energy states and implicated in physiological regulation of feeding. These results suggest that the projection of DMH GABAergic neurons to the PVN serves as a pathway to promote food intake under physiological conditions. This finding reinforces that DMH plays a role in promoting feeding.
The present study indicated that leptin inhibits orexigenic GABAergic neurons in DMH. This fits with previous report that leptin action on GABAergic neurons prevents obesity, as evidenced by the study on leptin receptor-deficient GABAergic neurons [21]. In contrast, it was reported that leptin activates DMH neurons expressing LepR, NPY, galanin or PrRP, and thereby stimulates thermogenesis by brown adipose tissue [9], [20], [25], [26]. Additionally, leptin activates PrRP neurons to suppress food intake [9]. Thus, it is suggested that in DMH leptin inhibits GABAergic neurons to suppress energy intake and activates another subpopulations of neurons to promote energy expenditure and/or suppress energy intake, all contributing to reduction of body weight. The opposing effects of leptin on different subpopulations of neurons have been well known in ARC where leptin inhibits NPY/AgRP neurons and activates POMC neurons.
The present study showed that DMH GABAergic neurons project to PVN, being consistent with previous reports that DMH neurons, including GABAergic neurons [27], project to PVN [28], [29]. Although the target neuron in PVN remains to be identified, the neurons expressing oxytocin, CRH, nesfatin-1 and/or MC3/4 [30], potent anorectic neuropeptides and receptor, are the candidates.
The DMH GABAergic projection to PVN may regulate not only feeding but also other functions, in considering diverse functions of DMH and PVN. SCN, the master clock governing circadian rhythm, projects to DMH [31]. PVN is part of a multi-synaptic pathway that is responsible for the circadian regulation of several functions including glucocorticoid and melatonin release [32] and feeding behavior [33], [34]. These findings suggest a possible functional connection between DMH and PVN for circadian regulation. The DMH GABAergic projection to PVN, found in the present study, could serve as a neuro-circuit that relays DMH to PVN for circadian regulation.
We found that light activation of the presynaptic terminal of DMH ChRFRR-C167-expressing GABAergic neurons evoked IPSC onto PVN neurons. Open and close time constants of ChRFR-C167A are slower than those of Channelrhodopsin2 under blue light exposure [22]. Therefore, we expected that photo-activation of ChRFRR-C167 would evoke IPSCs continually and hence increase both amplitude and frequency of total IPSC for a certain period, unlike channelrhodopsin2 that evokes single large IPSC immediately after photo-activation. However, our recording showed that IPSC frequency was not changed, although the cumulative distribution of IPSC amplitude was increased. The unchanged IPSC frequency could be due to possible additional neuronal circuits. DMH GABAergic neurons may project to not only PVN neurons but also GABAergic interneurons around PVN or presynaptic terminals of GABAergic neurons onto PVN neurons. Light-evoked GABA release from presynapses of DMH GABAergic neurons could not only directly suppress PVN neurons but also inhibit GABAergic interneurons and/or presynaptic terminals that secondarily suppress PVN neurons. The net effects of these distinct neuro-circuits could result in little change in IPSC frequency.
Our findings suggested that DMH GABAergic neurons project axonal terminals to 47% of PVN neurons (Figure 3C), similarly to ARC AgRP neurons that project to 49% of PVN neurons [35]. However, activation of DMH GABAergic neurons took more than 2 h to increase food intake, whereas activation of AgRP neurons immediately increased food intake [35]. Although the mechanisms underlying the different time course of feeding behavior following DMH GABAergic and ARC. AgRP neuron activation remain to be elucidated, we can speculate a few explanations for the delayed effect of the DMH GABAergic neuron activation on food intake. First, AgRP neurons release AgRP and NPY, well-established potent orexigenic neuropeptides. On the other hand, it is still unclear what neuropeptide(s) DMH GABAergic neurons can release. Secondly, DMH GABAergic neurons were found to project to not only PVN but other feeding regulatory nuclei including ARC, VMH, LH, and DMNV (data not shown), in consistent with previous reports [17], [36]. Some of these projections could inhibit orexigenic neurons and thereby cancel out the effect of the PVN neuron inhibition that increases food intake, which may take place for the initial few hours. In contrast, AgRP neurons project to the nuclei where anorexigenic neurons dominate [37], [38]. It was also observed that the light administration on DMH and that on PVN increased food intake in slightly different time courses. This could be due to that the former activates additional subpopulations of DMH GABAergic neurons.
5. Conclusion
DMH GABAergic neurons are regulated by metabolic signals leptin and glucose and, once activated, promote food intake via inhibitory synaptic transmission to PVN.
Acknowledgments
We thank Dr. Hiromu Yawo and Dr. Toru Ishizuka at Tohoku Univ. for discussion and technical advices on optogenetics. A part of this work was supported by Grant-in-Aid for Challenging Exploratory Research (26670453) from JSPS, Strategic Research Program for Brain Sciences (10036069) by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), MEXT-Supported Programs for Strategic Research Foundation at Private Universities 2011–2015 and 2013–2017, Grant-in-Aid from Health Labor Sciences Research Grants from the Ministry of Health, Labor, and Welfare, Japan, grant for Joint Researches in National Institute for Physiological Sciences, and grant from Japan Diabetes Foundation to TY. This study was subsidized by JKA through its promotion funds from KEIRIN RACE to TY. JMU Graduate Student Research Award to ZO.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Bellinger L.L., Evans J.F., Gietzen D.W. Dorsomedial hypothalamic lesions alter intake of an imbalanced amino acid diet in rats. The Journal of Nutrition. 1998;128(7):1213–1217. doi: 10.1093/jn/128.7.1213. [DOI] [PubMed] [Google Scholar]
- 2.Bernardis L.L., Bellinger L.L. Effect of palatable diet on growth, caloric intake and endocrine-metabolic profile in weanling rats with dorsomedial hypothalamic lesions. Appetite. 1986;7(3):219–230. doi: 10.1016/s0195-6663(86)80027-7. [DOI] [PubMed] [Google Scholar]
- 3.Bernardis L.L., Bellinger L.L. Brown (BAT) and white (WAT) adipose tissue in high-fat junk food (HFJF) and chow-fed rats with dorsomedial hypothalamic lesions (DMNL rats) Behavioural Brain Research. 1991;43(2):191–195. doi: 10.1016/s0166-4328(05)80070-1. [DOI] [PubMed] [Google Scholar]
- 4.Mieda M., Williams S.C., Richardson J.A., Tanaka K., Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(32):12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooley J.J., Schomer A., Saper C.B. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nature Neuroscience. 2006;9(3):398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 6.Landry G.J., Kent B.A., Patton D.F., Jaholkowski M., Marchant E.G., Mistlberger R.E. Evidence for time-of-day dependent effect of neurotoxic dorsomedial hypothalamic lesions on food anticipatory circadian rhythms in rats. PLoS One. 2011;6(9):e24187. doi: 10.1371/journal.pone.0024187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi S., Kim Y.J., Zheng F. Dorsomedial hypothalamic NPY and energy balance control. Neuropeptides. 2012;46(6):309–314. doi: 10.1016/j.npep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias C.F., Lee C.E., Kelly J.F., Ahima R.S., Kuhar M., Saper C.B. Characterization of CART neurons in the rat and human hypothalamus. The Journal of Comparative Neurology. 2001;432(1):1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- 9.Dodd G.T., Worth A.A., Nunn N., Korpal A.K., Bechtold D.A., Allison M.B. The thermogenic effect of leptin is dependent on a distinct population of prolactin-releasing peptide neurons in the dorsomedial hypothalamus. Cell Metabolism. 2014;20(4):639–649. doi: 10.1016/j.cmet.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmquist J.K., Bjørbaek C., Ahima R.S., Flier J.S., Saper C.B. Distributions of leptin receptor mRNA isoforms in the rat brain. The Journal of Comparative Neurology. 1998;395(4):535–547. [PubMed] [Google Scholar]
- 11.Liu H., Kishi T., Roseberry A., Cai X., Lee C., Montez J. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. Journal of Neuroscience. 2003;23(18):7143. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen P., Williams S.M., Grove K.L., Smith M.S. Melanocortin 4 receptor-mediated hyperphagia and activation of neuropeptide Y expression in the dorsomedial hypothalamus during lactation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(22):5091–5100. doi: 10.1523/JNEUROSCI.0588-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fetissov S.O., Kopp J., Hökfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides. 2004;38(4):175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Bellinger L.L., Bernardis L.L. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiology & Behavior. 2002;76(3):431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- 15.Bi S. Role of dorsomedial hypothalamic neuropeptide Y in energy homeostasis. Peptides. 2007;28(2):352–356. doi: 10.1016/j.peptides.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Draper S., Kirigiti M., Glavas M., Grayson B., Chong C.N.A., Jiang B. Differential gene expression between neuropeptide Y expressing neurons of the dorsomedial nucleus of the hypothalamus and the arcuate nucleus: microarray analysis study. Brain Research. 2010;1350:139–150. doi: 10.1016/j.brainres.2010.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L., Scott K.A., Hyun J., Tamashiro K.L., Tray N., Moran T.H. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(1):179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng F., Kim Y.J., Chao P.-T., Bi S. Overexpression of neuropeptide Y in the dorsomedial hypothalamus causes hyperphagia and obesity in rats. Obesity. 2013;21(6):1086–1092. doi: 10.1002/oby.20467. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.J., Bi S. Knockdown of neuropeptide Y in the dorsomedial hypothalamus reverses high fat diet-induced obesity and impaired glucose tolerance in rats. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology: AJPREGU. 2015;310(2):R134–R144. doi: 10.1152/ajpregu.00174.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.J., Verma S., Simonds S.E., Kirigiti M.A., Kievit P., Lindsley S.R. Leptin stimulates neuropeptide Y and cocaine amphetamine-regulated transcript coexpressing neuronal activity in the dorsomedial hypothalamus in diet-induced obese mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013;33(38):15306–15317. doi: 10.1523/JNEUROSCI.0837-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vong L., Ye C., Yang Z., Choi B., Chua S., Jr., Lowell B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hososhima S., Sakai S., Ishizuka T., Yawo H. Kinetic evaluation of photosensitivity in bi-stable variants of chimeric channelrhodopsins. PLoS One. 2015;10(3):e0119558. doi: 10.1371/journal.pone.0119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suyama S., Kodaira-Hirano M., Otgon-Uul Z., Ueta Y., Nakata M., Yada T. Fasted/fed states regulate postsynaptic hub protein DYNLL2 and glutamatergic transmission in oxytocin neurons in the hypothalamic paraventricular nucleus. Neuropeptides. 2015 doi: 10.1016/j.npep.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Katsurada K., Maejima Y., Nakata M., Kodaira M., Suyama S., Iwasaki Y. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochemical and Biophysical Research Communications. 2014;451(2):276–281. doi: 10.1016/j.bbrc.2014.07.116. [DOI] [PubMed] [Google Scholar]
- 25.Enriori P.J., Sinnayah P., Simonds S.E., Garcia Rudaz C., Cowley M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(34):12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laque A., Zhang Y., Gettys S., Nguyen T.-A., Bui K., Morrison C.D. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. American Journal of Physiology- Endocrinology and Metabolism. 2013;304(9):E999–E1011. doi: 10.1152/ajpendo.00643.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudaba C., Szabó K., Tasker J.G. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. The Journal of Neuroscience. 1996 doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elmquist J.K., Ahima R.S., Elias C.F., Flier J.S., Saper C.B. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(2):741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kataoka N., Hioki H., Kaneko T., Nakamura K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metabolism. 2014;20(2):346–358. doi: 10.1016/j.cmet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Wilson J.L., Enriori P.J. A talk between fat tissue, gut, pancreas and brain to control body weight. Molecular and Cellular Endocrinology. 2015;418(Pt 2):108–119. doi: 10.1016/j.mce.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Thompson R. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Research Reviews. 1998;27(2):89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 32.Moore R.Y. Neural control of the pineal gland. Behavioural Brain Research. 1996;73(1–2):125–130. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- 33.Nakata M., Gantulga D., Santoso P., Zhang B., Masuda C., Mori M. Paraventricular NUCB2/nesfatin-1 supports oxytocin and vasopressin neurons to control feeding behavior and fluid balance in male mice. Endocrinology. 2016;157(6):2322–2332. doi: 10.1210/en.2015-2082. [DOI] [PubMed] [Google Scholar]
- 34.Sedbazar U., Maejima Y., Nakata M., Mori M., Yada T. Paraventricular NUCB2/nesfatin-1 rises in synchrony with feeding suppression during early light phase in rats. Biochemical and Biophysical Research Communications. 2013;434(3):434–438. doi: 10.1016/j.bbrc.2013.03.090. [DOI] [PubMed] [Google Scholar]
- 35.Atasoy D., Betley J.N., Su H.H., Sternson S.M. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S.J., Kirigiti M., Lindsley S.R., Loche A., Madden C.J., Morrison S.F. Efferent projections of neuropeptide Y-expressing neurons of the dorsomedial hypothalamus in chronic hyperphagic models. The Journal of Comparative Neurology. 2013;521(8):1891–1914. doi: 10.1002/cne.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betley J.N., Cao Z.F.H., Ritola K.D., Sternson S.M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D., He X., Zhao Z., Feng Q., Lin R., Sun Y. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Frontiers in Neuroanatomy. 2015;9 doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]