Abstract
Objective
The ventromedial hypothalamic nucleus (VMH) regulates energy balance and glucose homeostasis. Leptin and insulin exert metabolic effects via their cognate receptors expressed by the steroidogenic factor 1 (SF1) neurons within the VMH. However, detailed cellular mechanisms involved in the regulation of these neurons by leptin and insulin remain to be identified.
Methods
We utilized genetically-modified mouse models and performed patch-clamp electrophysiology experiments to resolve this issue.
Results
We identified distinct populations of leptin-activated and leptin-inhibited SF1 neurons. In contrast, insulin uniformly inhibited SF1 neurons. Notably, we found that leptin-activated, leptin-inhibited, and insulin-inhibited SF1 neurons are distinct subpopulations within the VMH. Leptin depolarization of SF1 neuron also required the PI3K p110β catalytic subunit. This effect was mediated by the putative transient receptor potential C (TRPC) channel. On the other hand, hyperpolarizing responses of SF1 neurons by leptin and insulin required either of the p110α or p110β catalytic subunits, and were mediated by the putative ATP-sensitive K+ (KATP) channel.
Conclusions
Our results demonstrate that specific PI3K catalytic subunits are responsible for the acute effects of leptin and insulin on VMH SF1 neurons, and provide insights into the cellular mechanisms of leptin and insulin action on VMH SF1 neurons that regulate energy balance and glucose homeostasis.
Keywords: Cellular mechanism, Conditional knockout mouse, Patch clamp technique, Functional heterogeneity, Homeostasis
Highlights
-
•
Leptin recruits p110β/TRPC channels to depolarize/activate SF1 neurons.
-
•
Leptin recruits p110α/p110β/KATP channels to hyperpolarize/inhibit SF1 neurons.
-
•
Insulin recruits p110α/p110β/KATP channels to hyperpolarize/inhibit SF1 neurons.
-
•
Acute leptin and insulin responses are segregated to distinct subsets of VMH SF1 neurons.
1. Introduction
The ventromedial nucleus of hypothalamus (VMH) has long been recognized as a brain site that regulates energy balance [1]. The expression of nuclear receptor, steroidogenic factor 1 (SF1), is highly restricted to the VMH and is critical for its development [2]. Notably, SF1-expressing neurons within VMH (VMH SF1 neurons) regulate energy balance and glucose homeostasis [3], [4].
SF1 neurons express both leptin receptors (LepRs) and insulin receptors (InsRs). Deficiency of LepRs or InsRs selectively in SF1 neurons results in opposite body weight phenotypes. In particular, SF1-specific LepR deficiency results in obesity when challenged with high fat diet (HFD) [5], [6]. Inversely, loss of InsR in SF1 neurons protects against HFD-induced obesity [7]. These results are somewhat paradoxical as both receptors are known to activate phosphatidylinositol-3-kinase (PI3K)-dependent intracellular signaling pathways [8], [9], [10]. These observations may be explained in part by the findings that leptin acutely activates (or depolarizes) while insulin acutely inhibits (or hyperpolarizes) SF1 neurons [5], [7]. Moreover, we cannot exclude the role of PI3K-dependent regulation of genomic pathways (e.g. FOXO1 signaling), which are similarly activated by leptin and insulin and may also play a determining role in chronic metabolic phenotypes [11]. Notably, the acute (and possibly chronic) effects of leptin and insulin are segregated to distinct subpopulations of VMH SF1 neurons [7], supporting functionally heterogeneous SF1 neurons, which independently may contribute to different facets of metabolism. However, detailed cellular mechanisms that underlie the distinct actions of leptin and insulin on VMH SF1 neurons still remain to be identified.
PI3K functions as heterodimers that consist of the 110 kDa catalytic subunits (p110α and p110β) and the 85 kDa regulatory subunits (p85α and p85β) [12]. Deletion of the regulatory PI3K subunits (p85α and p85β) in the arcuate pro-opiomelanocortin (POMC) neurons blunts the acute effects of both leptin and insulin [13]. Moreover, p110β isoform has a dominant role over p110α in mediating the acute effects of leptin and insulin in POMC neurons as well as in maintaining energy balance [14]. However, contribution of specific PI3K catalytic subunit isoforms to the acute effects of leptin and insulin on VMH SF1 neurons remains undefined. In this study, we characterized the acute effects of leptin and insulin on VMH SF1 neurons and identified the role of specific PI3K catalytic subunit isoforms (p110α and p110β) in this response.
2. Methods
2.1. Mice
Male (4–16 weeks old) pathogen-free SF1-cre mice [5] were crossed with either the Z/EG (GFP) reporter mice (Jackson Laboratory, # 003920) or the tdTomato reporter mice (Jackson Laboratory, #007908) to identify VMH SF1 neurons. For some experiments, SF1-cre::GFP or SF1-cre::tdTomato mice were crossed with either p110αflox/flox mice [15] or p110βflox/flox mice [16] to delete p110α or p110β specifically in SF1 neurons. All mice used in this study were housed in a light–dark (12 h on/off; lights on at 7:00 A.M.) and temperature-controlled environment with food and water available ad libitum in the University of Texas Southwestern Medical Center and Korea Advanced Institute of Science and Technology (KAIST) facilities. All experiments were performed in accordance with the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals, as well as with those established by the University of Texas and KAIST Institutional Animal Care and Use Committee.
2.2. Electrophysiology
Whole-cell patch-clamp recordings from SF1 neurons maintained in hypothalamic slice preparations and data analysis were performed as previously described [13], [17]. Briefly, 4- to 16-week-old male mice were deeply anesthetized with i.p. injection of 7% chloral hydrate or isoflurane inhalations and transcardially perfused with a modified ice-cold artificial CSF (ACSF) (described below), in which an equiosmolar amount of sucrose was substituted for NaCl. The mice were then decapitated, and the entire brain was removed and immediately submerged in ice-cold, carbogen-saturated (95% O2 and 5% CO2) ACSF (126 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 5 mM glucose). A brain block containing the hypothalamus was made. Coronal sections (250 μm) were cut with a Leica VT1000S or VT1200S Vibratome and then incubated in oxygenated ACSF at room temperature for at least 1 h before recording. Slices were transferred to the recording chamber and allowed to equilibrate for 10–20 min before recording. The slices were bathed in oxygenated ACSF (32°C–34 °C) at a flow rate of ∼2 ml/min. The pipette solution for whole-cell recording was modified to include an intracellular dye (Alexa Fluor 594 or Alexa Fluor 488) for whole-cell recording: 120 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 5 mM EGTA, 1 mM CaCl2, 1 mM MgCl2, and 2 mM MgATP, 0.03 mM Alexa Fluor 594 or Alexa Fluor 488 hydrazide dye, pH 7.3. Epifluorescence was briefly used to target fluorescent cells, at which time the light source was switched to infrared differential interference contrast imaging to obtain the whole cell recording (Zeiss Axioskop FS2 Plus equipped with a fixed stage and a QuantEM:512SC electron-multiplying charge-coupled device camera or Nikon Eclipse FN1 equipped with a fixed stage and an optiMOS scientific CMOS camera). Electrophysiological signals were recorded using an Axopatch 700B amplifier (Molecular Devices), low-pass filtered at 2–5 kHz, and analyzed offline on a PC with pCLAMP programs (Molecular Devices). Recording electrodes had resistances of 2.5–5 MΩ when filled with the K-gluconate internal solution. Input resistance was assessed by measuring voltage deflection at the end of the response to a hyperpolarizing rectangular current pulse steps (500 ms of −10 to −50 pA). For some experiments measuring current–voltage (I–V) relationships by applying ramp pulses in voltage clamp mode, the K-gluconate internal solution was replaced by Cs-glucoate and QX-314 (4 mM) was added. The extracellular solution for voltage clamp experiments contained 4-AP (5 mM), CdCl2 (100 μM), CsCl (1 mM), and TTX (1 μM). Membrane potential values were compensated to account for junction potential (−8 mV).
Leptin (100 nM; provided by A. F. Parlow, through the National Hormone and Peptide Program) and insulin (50 nM, Humulin-R 100 U/ml; Eli Lilly and Company) were added to the ACSF for specific experiments. Solutions containing leptin or insulin were typically perfused for 2–4 min. A drug effect was required to be associated temporally with peptide application, and the response had to be stable within a few minutes. A neuron was considered depolarized or hyperpolarized if a change in membrane potential was at least 2 mV in amplitude. After recording, slices were fixed with 4% formalin in PBS at 4 °C overnight. After washing in PBS, slices were mounted onto slides, covered in Vectashield (Vector Laboratories), and coverslipped to reduce photo-oxidation during visualization with fluorescent light. Cells were then visualized with ApoTome imaging system (Imager Z1; Zeiss) to identify post hoc the anatomical location of the recorded neuron.
2.3. Drugs
SKF96365 and 2-APB were obtained from Tocris. LY294002 and wortmannin were obtained from Calbiochem. Tolbutamide was obtained from Sigma. All solutions were made according to manufacturer's specifications. Stock solutions of SKF96365, 2-APB, LY294002, and wortmannin were made by dissolution in DMSO (Sigma). The concentration of DMSO in the external solution was <0.1%. Stock solutions of tolbutamide were made by dissolution in 100% ethanol, with the final ethanol concentration in ACSF less than 0.5%. Stock solutions of leptin were made by dissolution in D-PBS (Gibco).
2.4. Data analysis
Statistical data are expressed as mean ± s.e.m., where n represents the number of cells studied. The significance of differences between was evaluated using unpaired 2-tailed Student's t test with a confidence level of p < 0.05 (*) or p < 0.01 (**).
3. Results
3.1. Leptin either depolarizes or hyperpolarizes distinct subsets of VMH SF1 neurons
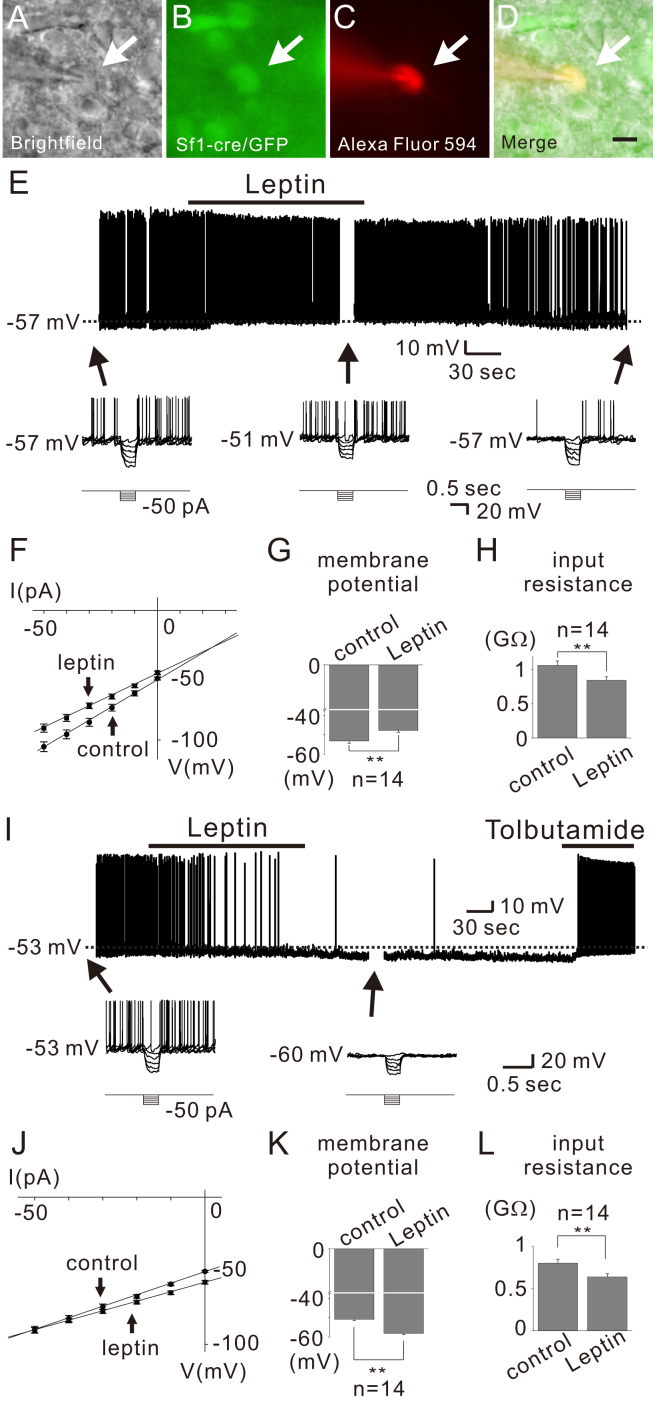
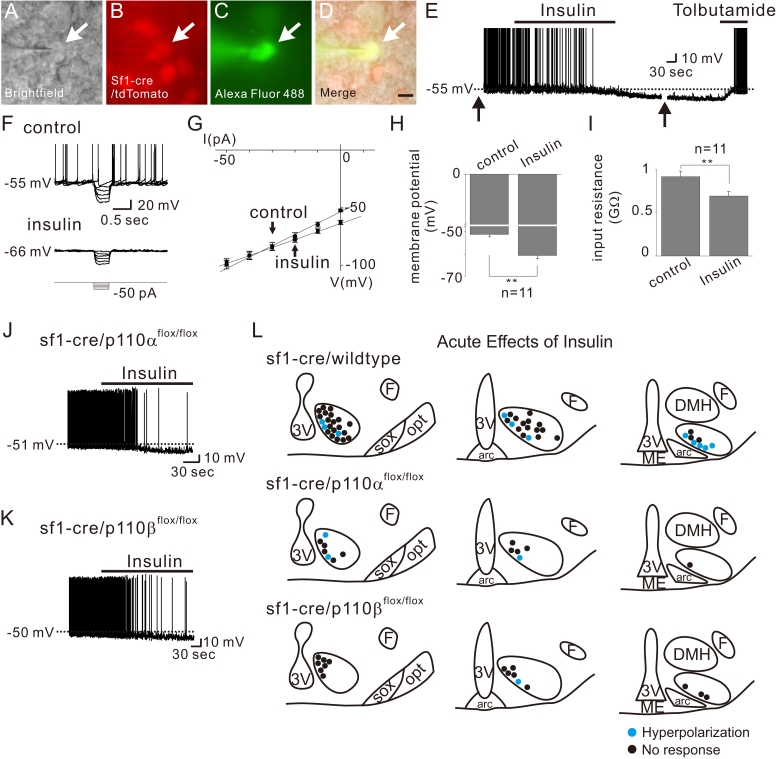
To identify the cellular mechanisms underlying the leptin-induced depolarization of VMH SF1 neurons, we targeted identified neurons from SF1-cre::green fluorescent protein (GFP) or SF1-cre::tdTomato mice and examined the acute responses to leptin application (Figure 1). Similar to previous reports [5], leptin (100 nM) reversibly depolarized a subset (14 of 80; 17.5%) of VMH SF1 neurons by 5.4 ± 0.3 mV (from −53.2 ± 1.1 mV to −47.8 ± 1.0 mV, n = 14) (Figure 1E, upper panel and Figure 1G). The leptin-induced depolarization was accompanied by ∼20% decrease of input resistance (from 1,063.1 ± 63.9 MΩ to 844.9 ± 50.0 MΩ, n = 14, Figure 1F and H) as measured by voltage responses to small hyperpolarizing current steps (Figure 1E, lower panels and 1F). The reversal potential was calculated to be −30.5 ± 1.7 mV (n = 14) (Figure 1F). These results suggested that leptin activates a putative non-selective (mixed) cation conductance to depolarize VMH SF1 neurons.
Figure 1.
VMH SF1 neurons are either depolarized or hyperpolarized by leptin. (A–D) Brightfield illumination (A), fluorescent (FITC) illumination (B), fluorescent (TRITC) illumination, and merged image of targeted VMH SF1 neuron (D) Arrows indicate the targeted cell. Scale bar = 10 μm. (E) Image demonstrates a leptin-induced depolarization of VMH SF1 neurons (upper panel). Dashed line indicates the resting membrane potential. Lower panel demonstrates voltage responses to hyperpolarizing current steps applied before, during, and after leptin application. (F) IV relationship demonstrates leptin-induced decrease in input resistance. (G–H) Effects of leptin on membrane potential and input resistance. Results are shown as mean ± SEM. ** indicates p < 0.01. (I) Image demonstrates a leptin-induced hyperpolarization of VMH SF1 neurons (upper panel). Lower panel demonstrates voltage responses to hyperpolarizing current steps in the course of recording at time points indicated with arrows. (J) IV relationship demonstrates leptin-induced decrease in input resistance. (K–L) Effects of leptin on membrane potential and input resistance. Results are shown as mean ± SEM. ** indicates p < 0.01.
We also found that bath application of leptin hyperpolarized a different subset (14 of 80; 17.5%) of SF1 neurons by −7.2 ± 0.5 mV (from −51.4 ± 0.7 mV to −58.7 ± 1.0 mV, n = 14) (Figure 1I, upper panel and Figure 1K). The leptin-induced hyperpolarization was accompanied by ∼20% decrease of input resistance (from 801.3 ± 45.7 MΩ to 637.4 ± 37.7 MΩ, n = 14, Figure 1J and L) as measured by voltage responses to small hyperpolarizing current steps (Figure 1I, lower panels and 1J). The reversal potential was calculated to be −90.4 ± 3.0 mV (n = 14) (Figure 1J). The leptin-induced hyperpolarization was fully reversed by tolbutamide, a specific blocker of ATP-sensitive K+ (KATP) channels (Figure 1I). These results suggested that leptin hyperpolarizes a subset of VMH SF1 neurons via activation of putative KATP conductance.
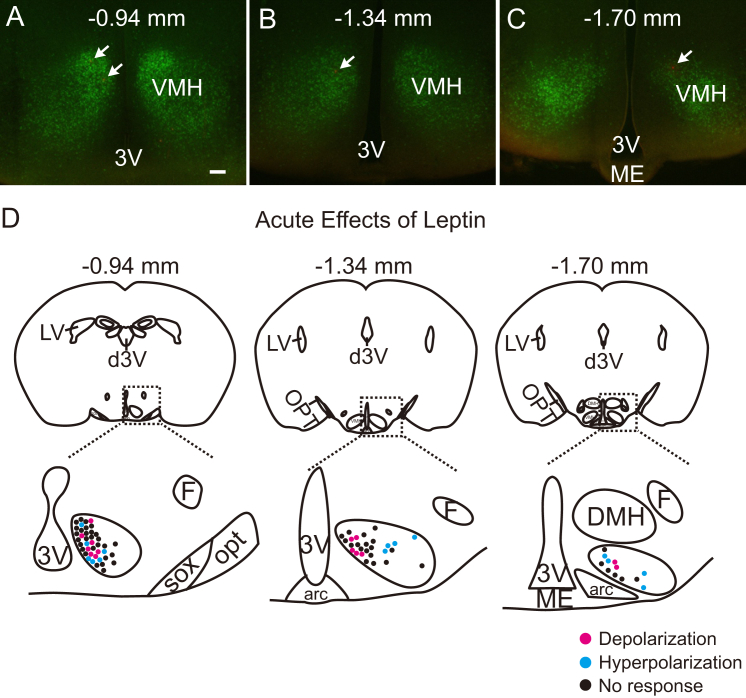
Following all recordings, the slices were fixed in order to identify the location of recorded SF1 neurons within the rostrocaudal and mediolateral axis of VMH (Figure 2A–C). Leptin-activated SF1 neurons were predominantly located within the dorsomedial subdivision of the VMH (dmVMH), as previously reported [5] (Figure 2D). In contrast, leptin-inhibited SF1 neurons were found to be scattered throughout the VMH (Figure 2D). These findings may explain the discrepancy between results from the current study and a previous study, which reported that 14 of 17 SF1 neurons are depolarized by leptin [5]. Based on our findings, we predict that these results reflect the fact that recordings were targeted in the dmVMH. Taken together, our results suggest that leptin-responsive SF1 neurons are heterogeneous in their location and acute responses to leptin (Table 1).
Figure 2.
Distribution of acute leptin effects on VMH SF1 neurons. (A–C) Images demonstrate coronal hypothalamic sections containing VMH. Arrows indicate recorded neurons marked with Alexa Fluor-594 dye. (D) Images show the location of leptin-activated (red) and leptin-inhibited (blue) SF1 neurons within the VMH. Note that leptin-activated cells are located in the dorsomedial subdivision of VMH while leptin-activated cells are scattered throughout the VMH. 3V = third ventricle, are = arcuate nucleus, d3V = dorsal third ventricle, DMH = dorsomedial nucleus of hypothalamus, F = fornix, LV = lateral ventricle, ME = median eminence, opt = optic tract, sox = supraoptic decussation.
Table 1.
Acute effects of leptin and insulin on VMH SF1 neurons and the role of specific p110 catalytic subunits.
| sf1-cre/wildtype (13.9 ± 0.4 pF, n = 130) |
sf1-cre/p110αflox/flox (11.8 ± 1.1 pF, n = 25) |
sf1-cre/p110βflox/flox (14.0 ± 0.7 pF, n = 19) |
sf1-cre/p110αflox/flox, p110βflox/flox (10.1 ± 0.5 pF, n = 20) |
|||||
|---|---|---|---|---|---|---|---|---|
| Leptin | Insulin | Leptin | Insulin | Leptin | Insulin | Leptin | Insulin | |
| Depolarized | 14 (17.5%) | 0 (0%) | 1 (5.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperpolarized | 14 (17.5%) | 11 (23.4%) | 3 (15.0%) | 3 (23.1%) | 2 (11.1%) | 1 (6.3%) | 0 (0%) | 0 (0%) |
| No response | 52 (65.0%) | 36 (76.6%) | 16 (80.0%) | 10 (76.9%) | 16 (88.9%) | 15 (93.9%) | 18 (100.0%) | 17 (100.0%) |
| Recorded | 80 | 47 | 20 | 13 | 18 | 16 | 18 | 17 |
3.2. Leptin activates TRPC channels to depolarize VMH SF1 neurons
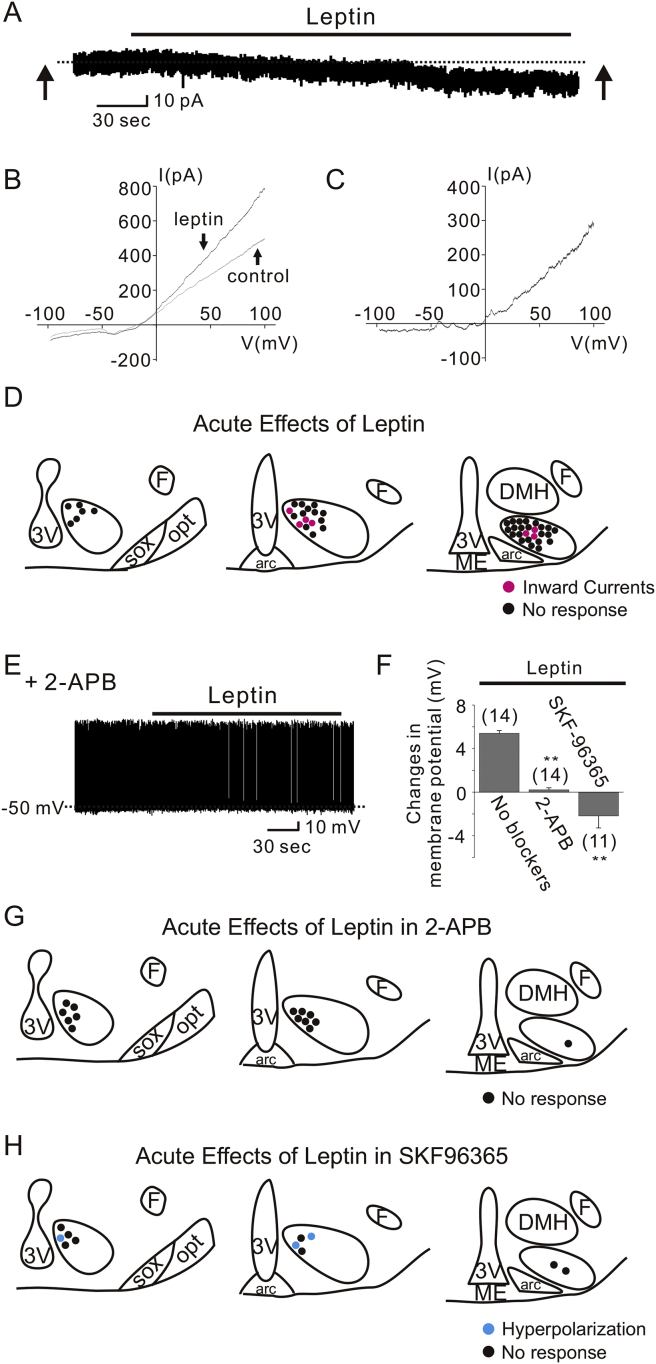
The leptin-induced depolarization in arcuate POMC neurons and LepR-expressing neurons of ventral premammillary nucleus (PMV) occurred via the activation of transient receptor potential C (TRPC) channels [17], [18], [19]. To determine if a similar mechanism exists in VMH SF1 neurons, we recorded leptin-activated currents in voltage clamp mode (Figure 3A). To better isolate leptin-activated conductance, Cs+ was used as the primary cation in the recording pipette, and the K+ channel blocker 4-AP (5 mM), the Ca2+ channel blocker CdCl2 (100 μM), the Ih blocker CsCl (1 mM), the Na channel blocker TTX (1 μM) were added to the external solution. At a holding potential of −60 mV, application of leptin induced inward currents of −15.3 ± 4.0 pA in 7 of 46 neurons tested (Figure 3A). Voltage ramp pulses (from −100 mV to +100 mV in 2 s, 100 mV/s) were applied before and during leptin application to determine current–voltage (IV) relationships of leptin-induced currents (Figure 3B, averages of 7 traces). The subtracted current revealed a net outward-rectifying current, which is consistent with the activation of TRPC conductance [18], [19] (Figure 3C, average of 7 subtracted traces). The remaining neurons were unaffected by leptin in this recording condition (−0.5 ± 0.6 pA, n = 39). It should be noted that the percentage of leptin-responsive neurons (15.2%) are similar to those depolarized by leptin in the current clamp mode (17.5%). Analogous to leptin-activation, SF1 neurons that displayed a leptin-induced inward current were predominantly located within dmVMH (Figure 3D). Also, leptin failed to induce any outward currents, likely due to the presence of Cs+ in the pipette solution, which blocks potassium conductances.
Figure 3.
TRPC channels underlie the leptin-induced activation of VMH SF1 neurons. (A) Leptin application produced an inward current in SF1 neurons. (B) Current–voltage relationships were examined by applying voltage ramps (−100 to +100 mV in 2 s, 100 mV/S) before and during leptin application (indicated by arrows) in the same cell from (A). Traces are averages of 7 current responses. (C) The I–V relationship obtained from subtracting the control current from the leptin-induced current in (B). (D) Images show the location of recordings and acute effects of leptin. (E) Leptin did not affect SF1 neuron membrane potential when pretreated with 2-APB. (F) Histogram shows membrane potential changes of SF1 neurons with or without TRPC channel blockers. (G–H) Drawings at three rostrocaudal levels of the mouse hypothalamus demonstrate that leptin-induced depolarization of VMH SF1 neurons are not observed when pretreated with 2-APB (G) or SKF96365 (H). Note that the leptin-induced hyperpolarization was not affected by the pretreatment with SKF96365. Numbers in parentheses indicate number of cells tested. Results are shown as mean ± SEM. ** indicates p < 0.01.
We also performed a series of experiments using pharmacological inhibitors of TRPC channels. We found that pretreatment of cells with 2-APB completely blocked leptin-induced depolarization of SF1 neurons (0.2 ± 0.2 mV, n = 14; Figure 3E–G). Similarly, leptin failed to depolarize cells that were pretreated with SKF96365. Notably, the net effect of leptin in the presence of SKF96365 was hyperpolarizing (Figure 3F), as leptin hyperpolarized 3 of 11 cells (−7.7 ± 1.2 mV, n = 3; Figure 3H). The remaining cells were unchanged in response to leptin (−0.1 ± 0.4 mV, n = 8; Figure 3H). These results suggest that pharmacological antagonists of TRPC channels selectively abolish leptin-induced depolarization (Figure 3E–H); however, the leptin-induced hyperpolarization is not affected by TRPC channel blockade.
3.3. Leptin-induced depolarization specifically requires the p110β isoform
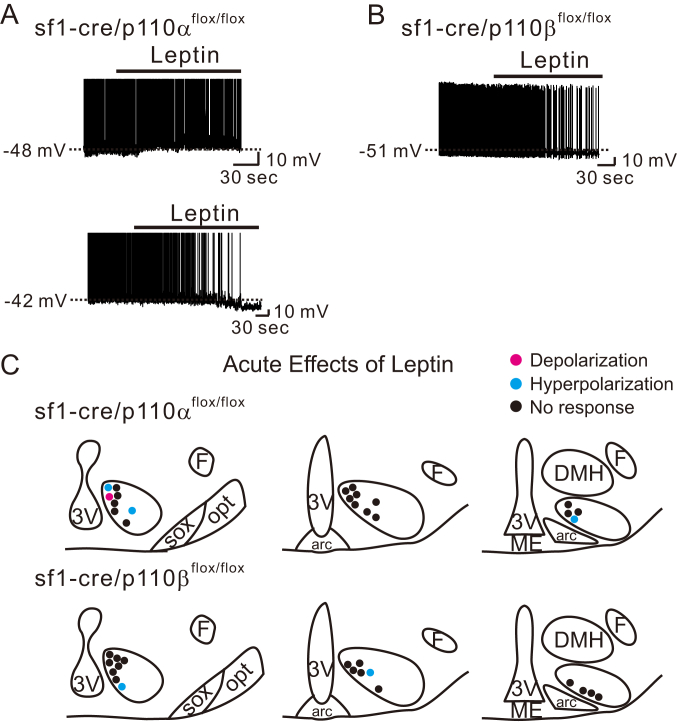
The role of p110α isoform in VMH SF1 neurons in regulating in vivo energy balance has been assessed [15]. However, we currently do not know the roles of specific p110 isoforms in mediating the acute effects of leptin on SF1 neurons. Therefore, we assessed cellular responses in mice in which VMH SF1 neurons were deficient for either the p110α or p110β isoforms (sf1-cre::p110αflox/flox or sf1-cre::p110βflox/flox, respectively). Both types of leptin-induced acute responses (depolarization or hyperpolarization) were present in VMH SF1 neurons deficient for p110α (Figure 4A and C, upper panels). In contrast, leptin-induced depolarization was absent in VMH SF1 neurons deficient for p110β subunits (Figure 4B and C, lower panels). We did observe that VMH SF1 neurons deficient for p110β subunits were hyperpolarized in response to leptin. Taken together, these data suggest that the p110β isoform of PI3K catalytic subunit is specifically required for leptin-induced acute activation of VMH SF1 neurons. In contrast, deficiency of either p110α or p110β isoform alone is not sufficient to abolish the leptin-induced acute inhibition. The acute effects of leptin on p110α- or p110β-deficient VMH SF1 neurons are summarized in Table 1.
Figure 4.
Leptin specifically recruits p110β to depolarize VMH SF1 neurons. (A) Traces demonstrate acute effects of leptin are not affected by SF1 neuron-specific deletion of p110α. (B) Traces demonstrate acute activation of leptin is specifically abolished by SF1 neuron-specific deletion of p110β. (C) Images show the location of recordings and acute effects of leptin.
3.4. Insulin hyperpolarizes a subset of VMH SF1 neurons via p110α/p110β-dependent activation of KATP channels
Similar to previous reports [7] we found that insulin hyperpolarized a subset (11 of 47; 23.4%) of SF1 neurons by −9.4 ± 1.2 mV (from −52.6 ± 1.1 mV to −62.0 ± 2.0 mV, n = 11) (Figure 5E and H). The insulin-induced hyperpolarization was accompanied by ∼25% decrease of input resistance (from 915.5 ± 63.5 MΩ to 692.1 ± 51.2 MΩ, n = 11, Figure 5G and I) as measured by voltage responses to small hyperpolarizing current steps (Figure 5F and G). The reversal potential was calculated to be −90.4 ± 3.1 mV (n = 11) (Figure 5G). The insulin-induced hyperpolarization was also reversed by tolbutamide (Figure 5E), supporting a role for KATP channels in the acute insulin-induced hyperpolarization of VMH SF1 neurons.
Figure 5.
Insulin hyperpolarizes a subset of SF1 neurons by p110α- or p110β-independent activation of KATP channels. (A–D) Brightfield illumination (A), fluorescent (FITC) illumination (B), fluorescent (TRITC) illumination, and merged image of targeted VMH SF1 neuron (D) Arrows indicate the targeted cell. Scale bar = 10 μm. (E) Image demonstrates an insulin-induced hyperpolarization of VMH SF1 neurons. Dashed line indicates the resting membrane potential. Arrows indicate interruption to apply current step pulses. (F–G) Insulin-induced decrease in input resistance as determined by small hyperpolarizing steps. (H–I) Effects of insulin on membrane potential and input resistance. Results are shown as mean ± SEM. ** indicates p < 0.01. (J–K) Traces demonstrate acute inhibitory effects of insulin are not affected by SF1 neuron-specific deletion of p110α or p110β. (L) Images show the location of recordings and acute effects of insulin.
The in vivo metabolic effects of insulin receptors expressed by VMH SF1 neurons are dependent on the activity of PI3Ks [7]. However, it's currently unknown whether specific PI3K catalytic subunit isoforms are involved in the acute effects of insulin. To determine which specific p110 isoforms are responsible for the acute effects of insulin, we assessed cellular responses of VMH SF1 neurons deficient for either p110α or p110β isoforms (sf1-cre::p110αflox/flox or sf1-cre::p110βflox/flox, respectively). We found that neither p110α deletion nor p110β deletion affected the insulin-induced acute hyperpolarization of VMH SF1 neurons (Figure 5J to 5L; Table 1).
3.5. Acute inhibition of SF1 neurons by leptin and insulin is blocked by the deletion of both p110α and p110β isoforms
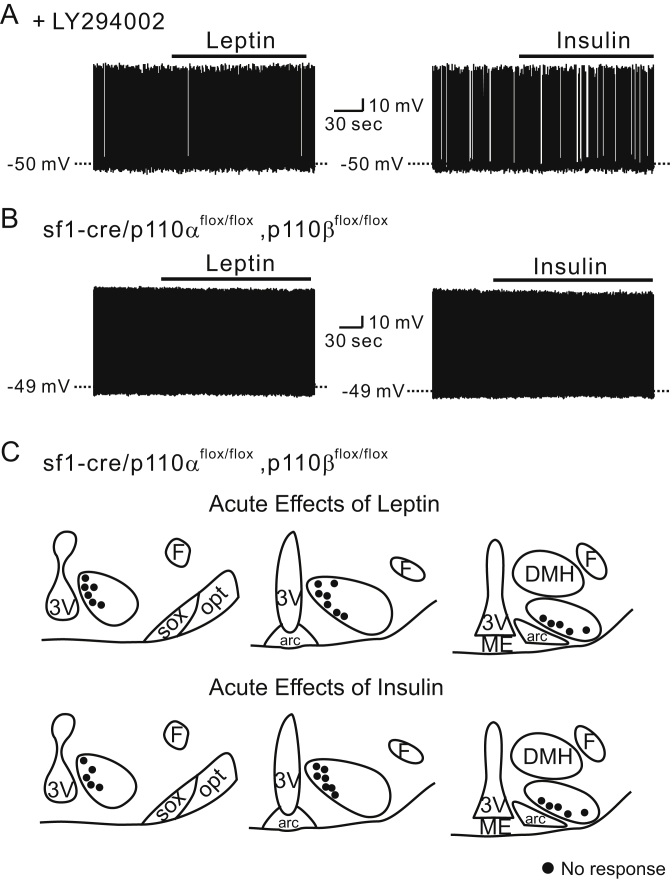
Subsequently, we tested if pretreatment with pharmacological inhibitors of PI3K (10 μM LY294002 or 100 nM wortmannin) could block the acute effects of leptin and insulin. To this end, the cells were pretreated with 10 μM LY294002 for 10 min before applications of leptin or insulin. In this condition, neither leptin nor insulin affected the membrane potential of SF1 neurons (−0.4 ± 0.2 mV, n = 9, for leptin and −0.4 ± 0.2 mV, n = 9, for insulin, Figure 6A). We found similar effects with 100 nM wortmannin pretreatment (−0.4 ± 0.1 mV, n = 8, for leptin and −0.3 ± 0.1 mV, n = 9, for insulin). In agreement with these data, both leptin and insulin failed to alter the membrane potential of VMH SF1 neurons deficient for both p110α and p110β (sf1-cre::p110αflox/flox, p110βflox/flox; Figure 6B and C). Taken together, the acute depolarizing and hyperpolarizing effects of leptin and insulin on VMH SF1 neurons are dependent on PI3K activity. Our findings suggest that the acute depolarization by leptin is mediated by p110β while the acute hyperpolarization of membrane potential by leptin or insulin requires the presence of either p110α or p110β (Table 1).
Figure 6.
Acute effects of leptin and insulin on VMH SF1 neurons require PI3K activity. (A) Traces demonstrate the absence of acute leptin and insulin effects when the cells are pretreated with LY294002. (B) Traces demonstrate the absence of acute leptin and insulin effects when both p110α and p110β are specifically deleted in SF1 neurons. (C) Images show the location of recordings and acute effects of leptin and insulin.
3.6. Acute effects of leptin and insulin are anatomically segregated to distinct SF1 neuronal populations
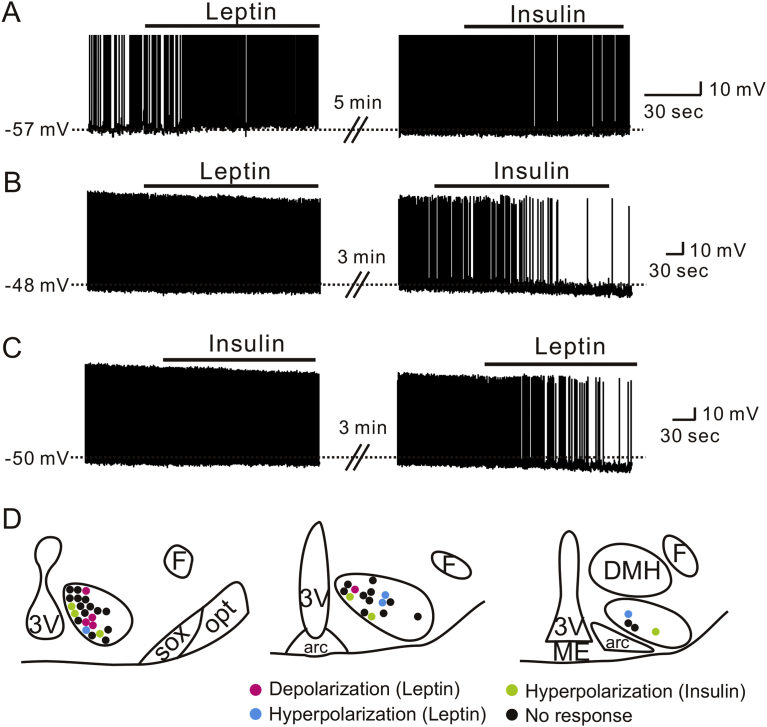
SF1 neuron-specific deletions of LepRs and InsRs have opposite effects on body weight regulation [5], [6], [7]. In addition, previous findings suggested that LepR-expressing and InsR-expressing SF1 neurons are segregated within the VMH [7]. We performed sequential applications of leptin and insulin to VMH SF1 neurons and characterized the acute responses in detail. In particular, leptin-activated SF1 neurons (5.4 ± 0.3 mV, n = 3) did not respond to subsequent insulin application (−0.3 ± 0.2 mV, n = 3, Figure 7A). In addition, a subset of SF1 neurons that did not respond to leptin application (−0.5 ± 0.4 mV, n = 6) was hyperpolarized by insulin (−7.7 ± 1.4 mV, n = 6, Figure 7B). Moreover, a group of SF1 neurons that did not respond to insulin (−0.3 ± 0.2 mV, n = 6) were either hyperpolarized (−6.5 ± 1.2 mV, n = 4, Figure 7C) or depolarized (6 mV and 7 mV, trace not shown) by subsequent leptin application. The leptin- and insulin-induced acute hyperpolarization was not readily reversible so that we could not perform dual application experiments when we observed hyperpolarization in the first application. We further analyzed the sub-nuclear locations of each response within VMH and found the distribution of acute leptin and insulin responses are similar to those described in Figure 2, Figure 5L (Figure 7D). Therefore, our findings suggest that VMH SF1 neurons are functionally heterogeneous in their response to leptin and insulin so that LepR-expressing SF1 neurons protect against HFD-induced obesity while InsR-expressing SF1 neurons promote obesity in HFD.
Figure 7.
Acute effects of leptin and insulin on SF1 neurons segregate to distinct subpopulations of VMH SF1 neurons. (A) Traces demonstrate a neuron that is acutely activated by leptin, but is not affected by insulin. (B) Traces demonstrate a neuron that is not affected by leptin, but is acutely inhibited by insulin. (C) Traces demonstrate a neuron that is not affected by insulin, but is acutely inhibited by leptin. (D) Images show the location of recordings and acute effects of leptin and insulin.
4. Discussion
In the current study, we characterized the acute effects of leptin and insulin on VMH SF1 neurons and delineated the involvement of specific p110 isoforms. Briefly, leptin either depolarized or hyperpolarized SF1 neurons, while insulin consistently hyperpolarized a different subpopulation of SF1 neurons. The acute effects of leptin and insulin were dependent on PI3K activity, as their effects were completely abolished by the pretreatment with PI3K inhibitors or the deletion of both p110α and p110β isoforms. Interestingly, we found that the leptin-induced depolarization, which was mediated by TRPC channel activation, was solely dependent upon the PI3K p110β catalytic subunit. In contrast, the leptin- and insulin-induced hyperpolarization, which involved KATP channel activation, was dependent on the presence of either p110α or p110β. Our electrophysiological data provide novel mechanistic insight into the cell biology that underlies functional heterogeneity of VMH SF1 neurons, which may underlie the differential effects on body weight regulation observed in SF1 neuron-specific deletion of LepR or InsR [5], [6], [7].
Insulin-induced acute inhibition was previously demonstrated in arcuate POMC neurons, arcuate NPY/AgRP neurons and VMH SF1 neurons [7], [13], [20], [21], [22]. These acute effects of insulin were shown to be mediated by a PI3K-dependent activation of KATP channels. Deletion of InsRs specifically in SF1 neurons ameliorated the obese phenotype associated with HFD challenge [7], which suggests that InsRs expressed by SF1 neurons promote obesity in HFD. As it was previously demonstrated that VMH and SF1 neurons send glutamatergic projections to arcuate POMC neurons [7], [23], the acute inhibition of SF1 neurons by insulin could result in the suppression of an anorexigenic pathway from SF1 neuron to POMC neuron.
Acute leptin effects on neuronal activity have previously been described in several distinct brain nuclei [24]. For instance, leptin depolarizes arcuate POMC neurons and PMV LepR neurons via PI3K-dependent activation of TRPC channels [13], [19], [25]. By contrast, neurons within the lateral hypothalamic area (LHA), dorsal motor nucleus of vagus nerve (DMV), and nucleus tractus solitarius (NTS) are hyperpolarized by leptin via the activation of KATP channels [26], [27], [28]. Acute and chronic effects of leptin are mediated at least in part by the signaling pathways downstream of PI3K in hypothalamus [13], [14], [15]. Specifically, the deletion of both p85α and p85β regulatory subunits abolished acute leptin effects in arcuate POMC neurons [13]. In addition, the p110β catalytic subunits were shown to have dominant role over p110α isoforms to mediate the acute effects of leptin and insulin in arcuate POMC neurons [14]. However, the involvement of specific PI3K catalytic subunit isoforms in leptin-induced acute activation or inhibition has not yet been described in VMH SF1 neurons. In this study, we demonstrated that leptin depolarizes a subpopulation of SF1 neurons within the dmVMH and hyperpolarizes another subpopulation of SF1 neurons that are scattered throughout the VMH. It is of particular interest to note that leptin specifically recruits the p110β isoform to open TRPC channels and depolarize the membrane potential, while the leptin-induced activation of KATP channels and resulting hyperpolarization requires either the p110α or p110β isoforms. These results highlight the existence of distinct coupling between specific p110 isoforms and ion channels within discrete subdivisions of VMH.
It is notable that only p110β was found to mediate leptin-induced acute activation of SF1 neurons. Currently, we have not assessed in vivo metabolic effects in mice that lack p110β specifically in SF1 neurons. Nonetheless, we predict that p110β deletion in SF1 neurons, in contrast to p110α deletion, may significantly affect homeostatic regulation of blood glucose levels. This is because p110β mediates leptin-induced acute regulation of SF1 neuronal activity, which may be important for appropriately responding to changes in glucose level. It should be noted that SF1 neurons lacking p110β subunits have cell capacitance comparable to that of wildtype SF1 neurons. In this respect, leptin-activated SF1 neurons are confined to dmVMH, which was recently highlighted as a key brain area that mediates counter-regulatory response (CRR) to hypoglycemia [29], [30]. In support of these data, a Cre-dependent viral tracing study demonstrated that SF1 neurons within the VMH project to the dorsal vagal complex (DVC), which is one of the autonomic centers that regulate blood glucose levels [31]. However, two studies have reported distinct roles of glutamatergic neurotransmission from SF1 neurons in CRR [32], [33]. Additional studies are warranted to define the role of specific subpopulations of SF1 neurons in maintaining glucose homeostasis.
It was previously demonstrated that deletion of LepRs in VMH SF1 neurons led to obesity both in normal chow diet and HFD [5], [6]. The increased weight gain was largely dependent upon decreased energy expenditure independent of caloric intake [5]. Similarly, deletion of p110α in SF1 neurons resulted in impaired thermogenesis concomitant with obesity when exposed to HFD, independent of effects on food intake or glucose homeostasis [15]. These findings suggest that LepR/p110α signaling in SF1 neurons may protect against HFD-induced obesity via increasing energy expenditure. It was previously predicted that leptin-induced activation of SF1 neurons is the cellular correlates for this effect [5], however we found that p110α deletion in SF1 neurons does not prevent leptin-induced activation of SF1 neurons. Thus, our data suggest that chronic and/or genomic events downstream of LepR/p110α signaling (possibly independent of PI3K), rather than the leptin-induced acute activation, may be more important for protection against HFD-induced obesity. In support of this idea, we found that p110α-deficient SF1 neurons have smaller cell capacitance, indicative of a smaller cell size (Table 1). This is consistent with previous reports that demonstrated that overexpression of PTEN or constitutively-active p110α leads to cardiac hypertrophy [34], [35]. Also, the overexpression of dominant-negative p110α results in smaller heart [35]. Thus, although p110α does not mediate acute activation of SF1 neurons by leptin, chronic genomic pathways downstream of LepR/p110α activation may contribute to the effects of leptin.
LepRs and InsRs share multiple intracellular signaling molecules that control genomic pathways [36]. FOXO1 is a transcription factor that may regulate leptin and insulin activity in brain [37], [38]. Interestingly, deletion of FOXO1 specifically in SF1 neurons (SF1-cre::Foxo1flox/flox) resulted in a lean body weight phenotype due to increased energy expenditure [39]. SF1-cre::Foxo1flox/flox mice showed increased leptin-induced anorexia and increased insulin sensitivity, suggesting that deletion of FOXO1 increased the responsiveness of SF1 neurons to both leptin and insulin [39]. Protein-tyrosine phosphatase 1B (PTP1B) is also an important negative regulator of leptin and insulin effects [40], [41]. In a recent study, PTP1B deletion in SF1 neurons (SF1-cre::Ptpn1flox/flox) resulted in an obese phenotype in HFD-fed female mice, which was associated with decreased energy expenditure and impaired sympathetic output [42]. They also found that SF1 neurons showed increased sensitivity to both leptin and insulin treatments in SF1-cre::Ptpn1flox/flox mouse model [42]. As SF1 neuron-specific deletions of LepRs and InsRs generate opposite phenotypes, results obtained from SF1-cre::Foxo1flox/flox and SF1-cre::Ptpn1flox/flox mouse models are intriguing and suggest that either influence of increased leptin sensitivity (e.g. in SF1-cre::Foxo1flox/flox) or influence of increased insulin sensitivity (e.g. in SF1-cre::Ptpn1flox/flox) predominates to determine adiposity phenotypes. Although it is currently unclear why the influence of leptin or insulin effects on SF1 neurons is dominant over the other when common signaling molecules are engineered, localization of leptin and insulin signaling to distinct SF1 neurons of VMH may explain these findings.
5. Conclusions
The VMH occupies a large area within the mediobasal hypothalamus. While SF1 neurons within the VMH express the transcription factor SF1 and lack of SF1 results in obesity, these findings do not necessarily mean that all SF1 neurons are functionally homogeneous. In fact, it was previously shown that SF1 is expressed transiently by the developing hypothalamus including the ventrolateral subdivision of VMH (vlVMH). This expression is absent in vlVMH of adult mice [43]. These findings suggest that all VMH neurons require SF1 expression during development, but SF1 may be dispensable in adult vlVMH neurons. It was previously demonstrated that LepRs and InsRs have distinct metabolic function [5], [6], [7]. In the current study, we provided further evidence that SF1 neurons are heterogeneous in their acute response to leptin and insulin. We also demonstrated specificity in receptor-enzyme-target ion channel. Therefore, identification of more specific markers of subpopulations of SF1 cells will allow the functional characterization of distinct neuronal populations within the VMH.
Acknowledgments
This work was supported by the Korean Ministry of Health and Welfare (HI14C1946 to J.-W.S.), the Korean Ministry of Science, ICT & Future Planning (NRF-2015M3A9E7029177 to J.-W.S.; NRF-2014K1A3A1A19066980, NRF-2016R1C1B3012748 to K.W.K.), KAIST Future Systems Health Care Project (to J.-W.S.); NIH R01 DK100699 (to K.W.W.); NIH R01 DK100659, R37 DK053301, P01 DK088761 (to J.K.E.).
Contributor Information
Jong-Woo Sohn, Email: jwsohn@kaist.ac.kr.
Kevin W. Williams, Email: Kevin.Williams@UTSouthwestern.edu.
Conflict of interest
None declared.
References
- 1.Hetherington A.W., Ranson S.W. Hypothalamic lesions and adiposity in the rat. Anatomical Record. 1940;78(2):149–172. [Google Scholar]
- 2.Ikeda Y., Luo X., Abbud R., Nilson J.H., Parker K.L. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Molecular Endocrinology. 1995;9(4):478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- 3.Kim K.W., Zhao L., Donato J., Jr., Kohno D., Xu Y., Elias C.F. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10673–10678. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majdic G., Young M., Gomez-Sanchez E., Anderson P., Szczepaniak L.S., Dobbins R.L. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143(2):607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon H., Zigman J.M., Ye C., Lee C.E., McGovern R.A., Tang V. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Bingham N.C., Anderson K.K., Reuter A.L., Stallings N.R., Parker K.L. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149(5):2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klockener T., Hess S., Belgardt B.F., Paeger L., Verhagen L.A., Husch A. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nature Neuroscience. 2011;14(7):911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu A.W., Kaelin C.B., Takeda K., Akira S., Schwartz M.W., Barsh G.S. PI3K integrates the action of insulin and leptin on hypothalamic neurons. Journal of Clinical Investigation. 2005;115(4):951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niswender K.D., Schwartz M.W. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Frontiers in Neuroendocrinology. 2003;24(1):1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- 10.Plum L., Schubert M., Bruning J.C. The role of insulin receptor signaling in the brain. Trends in Endocrinology and Metabolism. 2005;16(2):59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda M., Jones J.E., Olson D., Hill J., Lee C.E., Gautron L. Monitoring FoxO1 localization in chemically identified neurons. Journal of Neuroscience. 2008;28(50):13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantley L.C. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 13.Hill J.W., Williams K.W., Ye C., Luo J., Balthasar N., Coppari R. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. Journal of Clinical Investigation. 2008;118(5):1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Qassab H., Smith M.A., Irvine E.E., Guillermet-Guibert J., Claret M., Choudhury A.I. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metabolism. 2009;10(5):343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y., Hill J.W., Fukuda M., Gautron L., Sohn J.W., Kim K.W. PI3K Signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metabolism. 2010;12(1):88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utermark T., Rao T., Cheng H., Wang Q., Lee S.H., Wang Z.C. The p110alpha and p110beta isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes & Development. 2012;26(14):1573–1586. doi: 10.1101/gad.191973.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohn J.W., Xu Y., Jones J.E., Wickman K., Williams K.W., Elmquist J.K. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71(3):488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu J., Fang Y., Ronnekleiv O.K., Kelly M.J. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. Journal of Neuroscience. 2010;30(4):1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams K.W., Sohn J.W., Donato J., Jr., Lee C.E., Zhao J.J., Elmquist J.K. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. Journal of Neuroscience. 2011;31(37):13147–13156. doi: 10.1523/JNEUROSCI.2602-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill J.W., Elias C.F., Fukuda M., Williams K.W., Berglund E.D., Holland W.L. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metabolism. 2010;11(4):286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konner A.C., Janoschek R., Plum L., Jordan S.D., Rother E., Ma X. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metabolism. 2007;5(6):438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Plum L., Ma X., Hampel B., Balthasar N., Coppari R., Munzberg H. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. Journal of Clinical Investigation. 2006;116(7):1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sternson S.M., Shepherd G.M., Friedman J.M. Topographic mapping of VMH--> arcuate nucleus microcircuits and their reorganization by fasting. Nature Neuroscience. 2005;8(10):1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 24.Sohn J.W. Ion channels in the central regulation of energy and glucose homeostasis. Frontiers in Neuroscience. 2013;7:85. doi: 10.3389/fnins.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leshan R.L., Louis G.W., Jo Y.H., Rhodes C.J., Munzberg H., Myers M.G., Jr. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. Journal of Neuroscience. 2009;29(10):3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui H., Sohn J.W., Gautron L., Funahashi H., Williams K.W., Elmquist J.K. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. Journal of Comparative Neurology. 2012;520(18):4168–4183. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams K.W., Smith B.N. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. Journal of Physiology. 2006;573(2):395–412. doi: 10.1113/jphysiol.2006.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams K.W., Zsombok A., Smith B.N. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology. 2007;148(4):1868–1881. doi: 10.1210/en.2006-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garfield A.S., Shah B.P., Madara J.C., Burke L.K., Patterson C.M., Flak J. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metabolism. 2014;20(6):1030–1037. doi: 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flak J.N., Patterson C.M., Garfield A.S., D'Agostino G., Goforth P.B., Sutton A.K. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nature Neuroscience. 2014;17(12):1744–1750. doi: 10.1038/nn.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindberg D., Chen P., Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. Journal of Comparative Neurology. 2013;521(14):3167–3190. doi: 10.1002/cne.23338. [DOI] [PubMed] [Google Scholar]
- 32.Tong Q., Ye C., McCrimmon R.J., Dhillon H., Choi B., Kramer M.D. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metabolism. 2007;5(5):383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung C.C., Krause W.C., Edwards R.H., Yang C.F., Shah N.M., Hnasko T.S. Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output. Molecular Metabolism. 2015;4(11):857–866. doi: 10.1016/j.molmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crackower M.A., Oudit G.Y., Kozieradzki I., Sarao R., Sun H., Sasaki T. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110(6):737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 35.Shioi T., Kang P.M., Douglas P.S., Hampe J., Yballe C.M., Lawitts J. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO Journal. 2000;19(11):2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams K.W., Scott M.M., Elmquist J.K. Modulation of the central melanocortin system by leptin, insulin, and serotonin: co-ordinated actions in a dispersed neuronal network. European Journal of Pharmacology. 2011;660(1):2–12. doi: 10.1016/j.ejphar.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitamura T., Feng Y., Kitamura Y.I., Chua S.C., Jr., Xu A.W., Barsh G.S. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nature Medicine. 2006;12(5):534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 38.Nakae J., Biggs W.H., 3rd, Kitamura T., Cavenee W.K., Wright C.V., Arden K.C. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nature Genetics. 2002;32(2):245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 39.Kim K.W., Donato J., Jr., Berglund E.D., Choi Y.H., Kohno D., Elias C.F. FOXO1 in the ventromedial hypothalamus regulates energy balance. Journal of Clinical Investigation. 2012;122(7):2578–2589. doi: 10.1172/JCI62848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng A., Uetani N., Simoncic P.D., Chaubey V.P., Lee-Loy A., McGlade C.J. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Developmental Cell. 2002;2(4):497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 41.Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A.L. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 42.Chiappini F., Catalano K.J., Lee J., Peroni O.D., Lynch J., Dhaneshwar A.S. Ventromedial hypothalamus-specific Ptpn1 deletion exacerbates diet-induced obesity in female mice. Journal of Clinical Investigation. 2014;124(9):3781–3792. doi: 10.1172/JCI68585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung C.C., Kurrasch D.M., Liang J.K., Ingraham H.A. Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. Journal of Comparative Neurology. 2013;521(6):1268–1288. doi: 10.1002/cne.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]