Abstract
Objective
In this study, we analyzed the fatty acid profile of brains and plasma from male and female mice fed chow or a western-style high fat diet (WD) for 16 weeks to determine if males and females process fatty acids differently. Based on the differences in fatty acids observed in vivo, we performed in vitro experiments on N43 hypothalamic neuronal cells to begin to elucidate how the fatty acid milieu may impact brain inflammation.
Methods
Using a comprehensive mass spectrometry fatty acid analysis, which includes a profile for 52 different fatty acid isomers, we assayed the plasma and brain fatty acid composition of age-matched male and female mice maintained on chow or a WD. Additionally, using the same techniques, we determined the fatty acid composition of N43 hypothalamic cells following exposure to palmitic and linoleic acid, alone or in combination.
Results
Our data demonstrate there is a sexual dimorphism in brain fatty acid content both following the consumption of the chow diet, as well as the WD, with males having an increased percentage of saturated fatty acids and reductions in ω6-polyunsaturated fatty acids when compared to females. Interestingly, we did not observe a sexual dimorphism in fatty acid content in the plasma of the same mice. Furthermore, exposure of N43 cells to the ω6-PUFA linoleic acid, which is higher in female brains when compared to males, reduces palmitic acid-induced inflammation.
Conclusions
Our data suggest male and female brains, and not plasma, differ in their fatty acid profile. This is the first time, to our knowledge, lipidomic analyses has been used to directly test the hypothesis there is a sexual dimorphism in brain and plasma fatty acid composition following consumption of the chow diet, as well as following exposure to the WD.
Keywords: Obesity, N43, Palmitic acid, Linoleic acid, Central nervous system, Western diet, ω6-fatty acids
Abbreviations: AA, arachidonic acid; ACC, acetyl-CoA carboxylase; B2m, beta-2 microglobulin; BBB, blood brain barrier; BSA, bovine serum albumin; C, Chow diet; CNS, central nervous system; DHA, docosahexaenoic acid; F, female; FAs, fatty acids; FABP, fatty acid binding protein; FAT/CD36, fatty acid transporter; FATP1, fatty acid transport protein 1; FAS, fatty acid synthase; FFAs, free fatty acids; IL6, interleukin 6; LA, linoleic acid; M, male; MCD, malonyl-CoA decarboxylase; MSFD2a, membrane protein major facilitator super family domain containing 2a; MUFAs, monounsaturated fatty acids; NF-κB, Nuclear Factor-κ Beta; PA, palmitic acid; PUFAs, polyunsaturated fatty acids; SatFAs, saturated fatty acids; TFAs, total fatty acids; TNFα, Tumor Necrosis Factor α; UnsatFAs, unsaturated fatty acids; WD, western diet; WT, wild-type
Highlights
-
•
There is a sexual dimorphism in brain lipid composition.
-
•
Brains of male mice following high fat diet exposure are enriched in saturated fatty acids.
-
•
Male brains are depleted of polyunsaturated fatty acids following high fat diet exposure.
-
•
Linoleic acid inhibits palmitic acid-induced inflammation in hypothalamic cells.
1. Introduction
In the central nervous system, lipids represent more than 50% of the brain's dry weight, making the brain second only to adipose tissue with respect to its lipid content [1]. Fatty acids (FAs), the principal constituents of brain lipids [2], can be synthesized by de-novo lipogenesis in brain cells. Essential FAs however are the exception, and must be provided through the diet. FAs are taken up from circulating blood into the brain through the blood brain barrier (BBB) [3], [4].
FAs are key components of cellular membranes and precursors for biosynthesis of phospholipids and sphingolipids [4]. FAs play essential roles in signaling and influencing neuronal function [4]. The biological properties of lipid bi-layers depend on their FA composition; correspondingly, FA concentrations are tightly regulated [5]. Additionally, FAs provide an important energy source for cells via mitochondrial β-oxidation and through the generation of metabolic intermediates [4], [6].
Brain lipids are rich in polyunsaturated fatty acids (PUFAs) containing double bonds at the ω3 and ω6 position, such as arachidonic (AA; 20:4ω6) and docosahexaenoic (DHA; 22:6ω3) fatty acids [1]. In contrast with other organs or plasma, AA and DHA precursors, which are the essential FAs linoleic (18:2ω6) and α-linolenic (18:3ω3) acids, represent less than 1% of the lipid content of the brain [7]. As previously indicated, essential PUFAs cannot be synthesized by the brain and are derived from the diet, and they are critical mediators of normal brain development and function [8]. Importantly, decrements in dietary consumption of ω3-PUFAs and alterations in lipid metabolism are implicated in neuropsychiatric diseases including neurodegenerative diseases [9], [10] such as cognitive decline, Alzheimer's disease and neuroinflammation [11], [12], [13]. High concentrations of dietary saturated long chain fatty acids (SatFAs) have also been associated with neurological dysfunction [5]. Unfortunately, diets consumed by westernized civilizations are typically rich in SatFAs and are low in PUFAs [14]. Consumption of westernized diets is associated with the development of obesity, cognitive dysfunction, as well as cancer.
Research has focused on understanding lipid and FA metabolism; however, due to the complexity with hundreds of different enzymes, activators, and substrates leading to tens of thousands of different lipid species, a full understanding FA metabolism remains elusive. Not only is little known about the impact of dietary FA consumption on brain lipid uptake and assimilation, but even less is known if there is a sexual dimorphism in lipid processing. What is known is that the prevalence of many diseases associated with the metabolic syndrome and cognitive function differs based on sex [7], [15], [16]. Therefore, it is important to begin to address if the brain varies with respect to uptake and response to dietary FAs.
Recently, we reported males and females differ with respect to their metabolic response to a high fat, westernized diet [17], [18]. We further demonstrated that the SatFA content of the brain differs between the sexes, with increased concentrations of SatFAs in the brains of male mice when compared to female mice [17]. Here, we sought to extend our initial findings, and determine in a more comprehensive FA profile analysis of brain and plasma, if there are other sexually dimorphic differences in FAs. To this end, we analyzed age-matched wild-type (WT) male and female mice fed a low fat (chow) or western-style high fat diet (42% of the calories derived from fat, WD) for 16 weeks. Importantly, this type of diet was chosen because it is similar in nutrient composition to the human diet, which based on the latest statistics published by the National Institute of Health, contains approximately 35–40% of the calories derived from SatFas as well as is high in simple sugars [21]. Our data demonstrate there is a sexual dimorphism in brain-FA content both following consumption of the low fat/chow diet as well as following consumption of the WD. Specifically, we found males have an increased percentage of SatFAs and reductions in ω6-PUFAs when compared to females. To begin to elucidate how the FA milieu may impact brain inflammation, in vitro experiments were performed and our findings suggest ω6-PUFA linoleic acid (18:2ω6, LA), which is higher in female brains, has an anti-inflammatory role and reduces palmitic acid (16:0, PA)-induced inflammation.
2. Methods
2.1. Animals and body weight
Animal care and procedures were approved by the University of Texas Southwestern Medical Center. All the methods were in accordance with the approved guidelines. C57BL/6 mice, purchased from the Jackson Laboratory (The Jackson Laboratory, Bar Harbor, MA, USA) were housed in groups of two to five per cage, in a temperature-controlled environment at 22°C–24 °C using a 12-hour light/dark cycle. Mice were fed standard chow (#2916, Harlan-Teklad, Madison, WI), or exposed to 42% WD (#88137, Harlan Teklad) at 8 weeks of age with free access to water. Animals were food restricted for 3 h before sacrifice, which occurred 3 h following the onset of the light phase.
2.2. Tissues collection
Brains were collected from mice following cervical dislocation, weighed and quickly frozen in liquid nitrogen and stored at −80 °C. Blood was collected from anesthetized mice through eye bleeding and centrifuged twice at 8,000 rpm at 4 °C to collect plasma. Plasma aliquots were stored at −80 °C.
2.3. Fatty acid analysis
52 different FA isomers were analyzed using pentafluorobenzoyl bromide by GC-ECNI-MS as previously described [19], [20]. Half of each of the brains was homogenized while still frozen in 5 ml of methanol. To avoid lipase activity, which might overestimate the free fatty acid (FFA) fraction, these homogenates were kept on dry ice at all times and processed immediately after homogenization. For the quantification of the total fatty acids (TFAs), samples were hydrolyzed and extracted as previously described [17]. For the FFA quantification samples were directly extracted from the homogenized brain solution by liquid–liquid extraction, using a 1:1:2 mixture of MeOH:PBS:isooctane under acidified conditions (25 mM HCL) as described in [20]. For the plasma analysis, 10 ul samples were assayed for the quantification of both TFA and FFA (separately), following the same extraction protocols used for brains. In the analysis of FFAs, no fatty acyl-CoAs or other mono-acyl lipids are measured [19].
2.4. Cell culture
N43 cells were purchased from CELLutions Biosystem Inc. (Cedarlane, Burlington, NC, USA) and maintained in HyClone™ DMEM medium (Thermo Scientific, Waltham, MA) containing 10% fetal bovine serum (Gemini, West Sacramento, CA) 100 units/mL penicillin G sodium and 100 μg/mL streptomycin sulfate and 100 mg/L sodium pyruvate (Thermo Scientific). Cells were grown for 24 h before treatments with medium containing 2% fetal bovine serum. Cells were pre-treated for 1 h with 30 μM LA (Sigma, St-Louis, MO) conjugated with fatty acid free bovine serum albumin (BSA) (MP Biomedicals, LLC, Solon, OH) and then treated for 5 h with 30 μM LA or 100 μM PA (Matreya, Pleasant Gap, PA) conjugated with BSA alone or in combination.
2.5. qPCR
For the analysis of gene expression in the cell culture experiments, cells were washed twice with ice-cold PBS and lysed in 1 ml of TRIzol® (Ambion, Life Technologies). RNA was extracted from cells using RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Total RNA (1 μg) was reverse-transcribed using the SuperScript® III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Assay-on-demand kits with TaqMan® Universal Master Mix II (Applied Biosystems, Foster City, CA, USA) were used according to manufacturer's protocol and analyzed with the ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Relative mRNA expression levels for interleukin 6 (IL6, Mm00446190_m1) and Tumor Necrosis Factor α (ΤNFα, Mm00443260_g1) were assessed. Values were normalized to beta-2 microglobulin (B2m, Mm00437762_m1). The ΔΔCT method was used for relative quantification analysis.
2.6. Statistical analysis
FA data are presented as mean ± SEM. Statistical analyses were performed with GraphPad Prism software (GraphPad Software Inc.) and Microsoft Office 2010 Excel. Comparisons between 2 conditions were made using the unpaired 2-tailed Student t test. One-way ANOVA was used for comparison of more than 2 groups. p < 0.05 was considered to be statistically significant.
3. Results
3.1. Sexual dimorphism in brain total fatty acid composition
We evaluated the brain FA composition using a comprehensive mass spectrometry FA analysis, which includes a profile for 52 different FA isomers. Two different treatments for each sample were performed to evaluate the different FA pools: (1) total free fatty acids (FFAs) and (2) the pool of free and esterified FAs combined, which is hereafter defined as total FAs (TFAs). Due to the limited amount of sample, it was not possible to isolate brain-specific regions, thus we analyzed the whole brain.
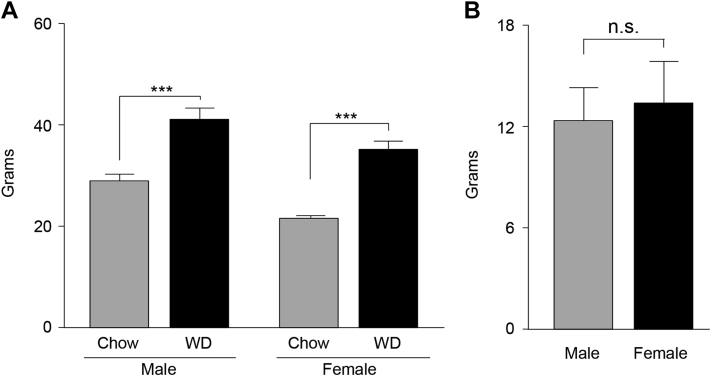
We directly compared age-matched WT male and female animals fed either chow or WD for 16 weeks (Table 1). Admittedly, this diet is both high in saturated fatty acids and in simple sugars. Importantly, this type of diet was chosen because it is similar in nutrient composition to the human diet, which based on the latest statistics published by the National Institute of Health, contains approximately 35–40% of the calories derived from Sat Fas as well as is high in simple sugars [21]. The body weights of the mice at the time of sacrifice are reported in Table 2. Importantly, animals of both sexes gained similar amounts of weight following exposure to the WD (Figure 1). Additionally, the dry weights of the brains did not differ between groups (Table 2).
Table 1.
Composition of the two diets, chow and western high fat diet (WD) showing the amounts relative amounts of FAs and the calories from fat. Information obtained from the commercial companies.
| Chow | WD | |
|---|---|---|
| Kcal/g food | 3.0 | 4.5 |
| % Kcal from fat | 12 | 42 |
| g fat/100 g food | 4 | 20.7 |
| Chow |
WD |
|||
|---|---|---|---|---|
| % total FA | g/100 g food | % total FA | g/100 g food | |
| SatFAs | 15 | 0.6 | 61.8 | 12.8 |
| MUFAs | 17.5 | 0.7 | 27.3 | 5.6 |
| PUFAs | 52.5 | 2.1 | 4.7 | 1 |
| Unknown | 15 | 0.6 | 6.2 | 1.3 |
| 10:0 | – | – | 2.6 | 0.5 |
| 12:0 | – | – | 3.3 | 0.7 |
| 14:0 | – | – | 10.6 | 2.2 |
| 16:0 | 12.5 | 0.5 | 28.9 | 6.0 |
| 16:1 | – | – | 1.5 | 0.3 |
| 18:0 | 2.5 | 0.1 | 12.5 | 2.6 |
| 18:1 | 17.5 | 0.7 | 20.9 | 4.3 |
| 18:1 (isomers) | – | – | 4 | 0.8 |
| 18:2 | 50 | 2 | 2.3 | 0.5 |
| 18:2 (isomers) | – | – | 1.3 | 0.3 |
| 18:3 | 2.5 | 0.1 | 0.7 | 0.1 |
Table 2.
Body weights of the mice at sacrifice (in grams), and dry weight of the brains (in grams) of the male and female mice fed chow and WD. Data represent averages (Av) and standard error of the mean (SEM) (n = 4).
| Chow M |
WD M |
Chow F |
WD F |
|||||
|---|---|---|---|---|---|---|---|---|
| Av | SEM | Av | SEM | Av | SEM | Av | SEM | |
| Body weight (g) | 27.3 | 1.1 | 41.2 | 2.5 | 21.5 | 0.6 | 34.9 | 1.9 |
| Brain dry weight (g) | 0.31 | 0.01 | 0.29 | 0.01 | 0.30 | 0.02 | 0.30 | 0.03 |
Figure 1.
Mice Body Weight: (A) Animals body weight at time of sacrifice. (B) Body-weight gain in male and female mice following WD exposure. All data are presented as mean ± SEM, and ***p < 0.001. n = 4/group.
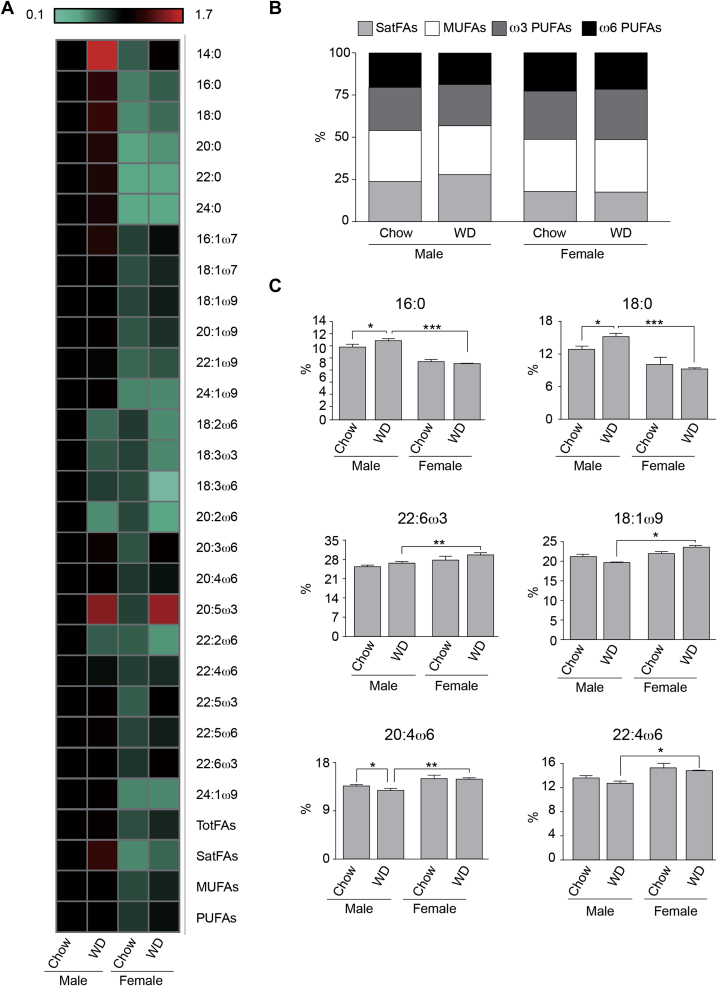
Our data suggests there is a sexual dimorphism in the absolute amounts of TFAs in the brains of male and female mice, which represents the sum of all FAs detected, with males having about 30% more TFAs by weight than females (Figure 2A and Table S2). These differences are mainly derived from significantly higher amounts of SatFAs in the male brains on chow and on the WD when compared to the females (Figure 2A and Figure S1), while the female brain is comprised of a higher percentage of PUFAs (Figure 2B).
Figure 2.
Brain Total Fatty Acid (TFA) composition: (A) Heat-map showing the concentration of the FAs in brains of male and female mice fed chow or WD. Levels expressed as a relative fold-change with the male chow condition used as the baseline. (B) Relative abundance (in %) of the different classes of TFAs in the brain of male and female mice fed chow or WD. (C) concentration levels (in ng of FA per mg of brain dry weight) of the eight most abundant fatty acids quantified in the brain. All data are presented as mean ± SEM, and *p < 0.05, **p < 0.01, ***p < 0.001. n = 4/group. Statistically significant differences in (B) found between M Chow and M WD SatFA* and ω6-PUFA***; between Male WH and Female WD SatFA*, ω3-PUFA*** and ω6-PUFA***.
Our data further indicate that 95% of brain TFA is composed of 8 FAs; two SatFAs, 16:0 and 18:0, three monounsaturated FAs (MUFAs), 18:1ω7, 18:1ω9, 20:1ω9, and three PUFAs, 20:4ω6, 22:4ω6 and 22:6ω3. Additionally, following consumption of the WD, differences between the sexes became even more evident, as the SatFAs were significantly increased, while ω6-PUFAs were significantly reduced in the male but not in the female brains (Figure 2A,B). Specifically, the two main SatFAs, palmitic (16:0) and stearic (18:0) acids, are statistically increased in WD-fed males, while they remain unchanged in females exposed to the same diet (Figure 2C), and these differences are independent of weight gain. Only three FAs are significantly increased in the females exposed to the WD (Figure 1A), the SatFA myristic acid (14:0) and the ω3-PUFAs 22:5ω3 and 20:5ω3, which are precursors of DHA. Importantly, DHA and its precursors have protective/anti-inflammatory properties, which influence production and maintenance of brain structures [22], [23], [24], [25].
Interestingly, in the female brain following WD consumption, there are significant increases in the PUFAs (22:6ω3 – DHA –, 18:1ω9 – oleic acid –, 20:4ω6 and 22:4ω6) when compared to the males (Figure 2C). DHA, as previously mentioned, is key for normal brain function and is required for fetal brain development, and regulates neuronal gene expression [26]. DHA has been demonstrated to provide an anti-inflammatory and protective role in the brain [22], [23], [24], [25], [27], [28]. Interestingly, some studies have reported in plasma from women, the levels of DHA are higher than in men [29], [30], which is consistent with the data we show here. Another PUFA significantly increased in the WD-female brains is oleic acid (18:1ω9), which is also broadly known for its anti-inflammatory role in the brain by preventing PA-induced mitochondrial dysfunction in neurons and inhibiting the activation of the pro-inflammatory Nuclear Factor-κ Beta (NF-κB) signaling pathways in neurons and astrocytes [31], [32]. The other PUFAs significantly higher in female brains following WD exposure are AA (20:4ω6) and adrenic acid (22:4ω6), and these FAs are generally known for their pro-inflammatory functions [11], [12]. Their pro-inflammatory effect is generally associated with their inhibitory role in the conversion of ω3-PUFAs into their long-chain forms caused by high intake of ω6-PUFAs, thereby decreasing the available amount of anti-inflammatory ω3-PUFAs such as α-linoleic acid (18:3ω3), eicosapentaenoic acid (20:5ω3) and DHA [33], [34]. Surprisingly, despite the increases in AA and adrenic acid, we observed in the female brains following exposure to the WD, the concentration of these anti-inflammatory ω3-PUFAs is either unchanged or increased following the WD exposure (Table S2), suggesting a disconnect with the previous reported literature. However, we have to underline that this is the first time this type of analysis has been performed in the brain of female animals, suggesting that this might be the effect of a sexually dimorphic concentration/activity of proteins involved in the metabolism of these specific lipids.
In summary, our results suggest that the brains of females, both under the control/chow fed and under WD fed conditions, are enriched in FAs known for their anti-inflammatory and neuroprotective effects when compared to the male brains on the same diets.
3.2. Fatty acid concentration in plasma
FAs are taken up from circulating blood into the central nervous system (CNS); however, there has been a considerable debate about how FAs are actually delivered into the brain. Currently, there are three proposed mechanisms: (1) direct diffusion of the circulating FFAs through the BBB; (2) cleavage of esterified-FAs from lipoproteins by the lipoprotein lipases followed by direct diffusion of cleaved-FAs through the BBB; and (3) translocation of lyso-phosphatidylcholine containing PUFAs mediated by the membrane protein major facilitator super family domain containing 2a (MSFD2a) protein located on the BBB [35]. Therefore, to begin to interrogate the brain lipid composition and to elucidate if it is dependent on the plasma FA concentration, we evaluated the plasma FFAs and TFAs lipid profile.
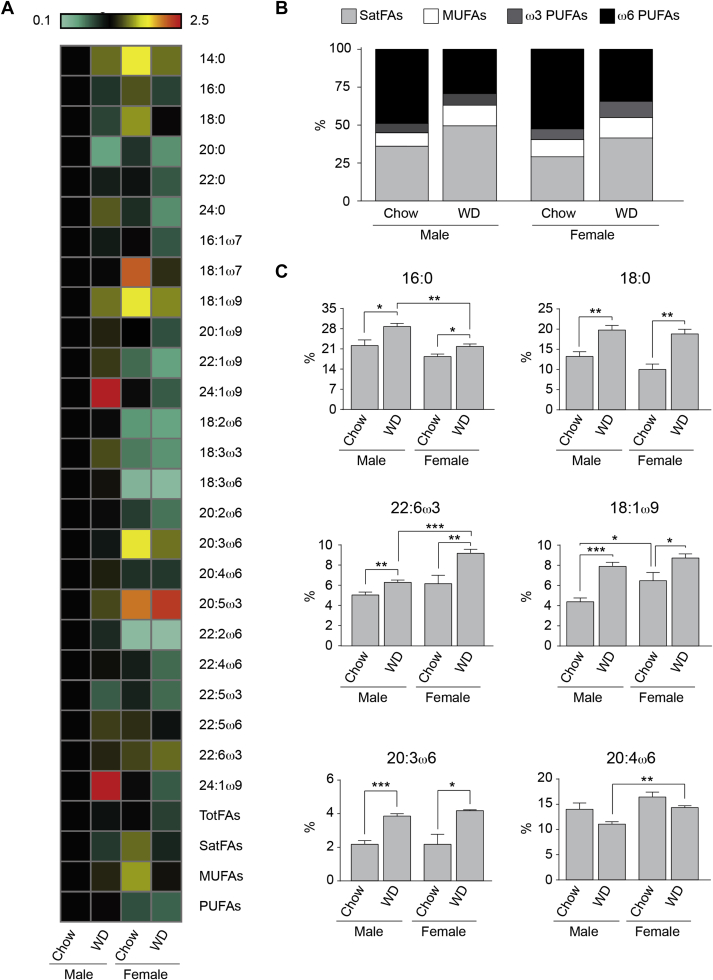
Our data demonstrate plasma FFAs are highly altered by the diet (Table S4). Specifically, there is an increase in the percentage of SatFAs and a reduction in the percentage of unsaturated fatty acids (UnsatFAs), both MUFAs and PUFAs, following chronic feeding with the WD in both males and females. Additionally, we found on the chow diet, there is a sexual dimorphism in different classes of FFAs with SatFAs being higher in males, while UnsatFAs were significantly higher in the females. These differences were lost following long-term WD exposure, suggesting there is no sexual dimorphism in plasma FAs concentrations following the WD. Females on a WD do have significantly higher levels of ω3-PUFAs, specifically 20:5ω3 and 22:6ω3, both known for their neuroprotective and anti-inflammatory function [28], [36].
Chronic exposure to the WD significantly altered TFAs composition in both the males and females (Table S3). The percentage of SatFAs is significantly higher in males following exposure to the WD when compared to females (Figure 3A,B). Additionally, for the PUFAs, even though the concentration is similar in both sexes on the chow diet, we found they were significantly reduced in the males fed the WD when compared to the females (Figure 3A, b). Importantly, these results do not match the variations in TFAs and thus we might speculate that mechanisms of diffusion and uptake of FAs from the plasma to the brain might not be the only mechanism contributing to CNS FA concentrations.
Figure 3.
Plasma Total Fatty Acid (TFA) composition: (A) Heat-map showing the concentration of the FAs in plasma of male and female mice fed chow or WD. Levels expressed as a relative fold-change with respect to the male chow concentration. (B) Relative abundance (in %) of the different classes of TFAs in the plasma of male and female mice fed chow or WD. (C) concentration levels (in ng of FA per μL of plasma) of the eight most abundant fatty acids quantified the plasma. All data are presented as mean ± SEM, and *p < 0.05, **p < 0.01, ***p < 0.001. n = 4/group. Statistically significant differences in (B) found between Male Chow and Male WD SatFA***, MUFA***, ω3-PUFA*** and ω6-PUFA***; between Female Chow and WD SatFA**, ω3-PUFA** and ω6-PUFA***; between Male WD and F WD SatFA**, ω3-PUFA*** and ω6-PUFA*.
The concentration of PA is significantly higher in males when compared to females, while the amount of oleic acid (18:1ω9) and DHA is significantly higher in females (Figure 3C), and these findings are consistent with data obtained in humans [30]. Estrogens have been linked to altering these FAs [29].
Our results suggest two different events might occur: 1) the BBB functions differently between male and females. In males, the uptake of plasma SatFAs in higher; whereas the uptake of PUFAs is higher in the females; and/or 2) there is a sexual dimorphism in CNS FA generation and metabolism. Additional studies are required to determine if the uptake and or processing of fatty acids differs by sex.
3.3. Linoleic acid reduces palmitic acid-induced inflammation in hypothalamic neurons
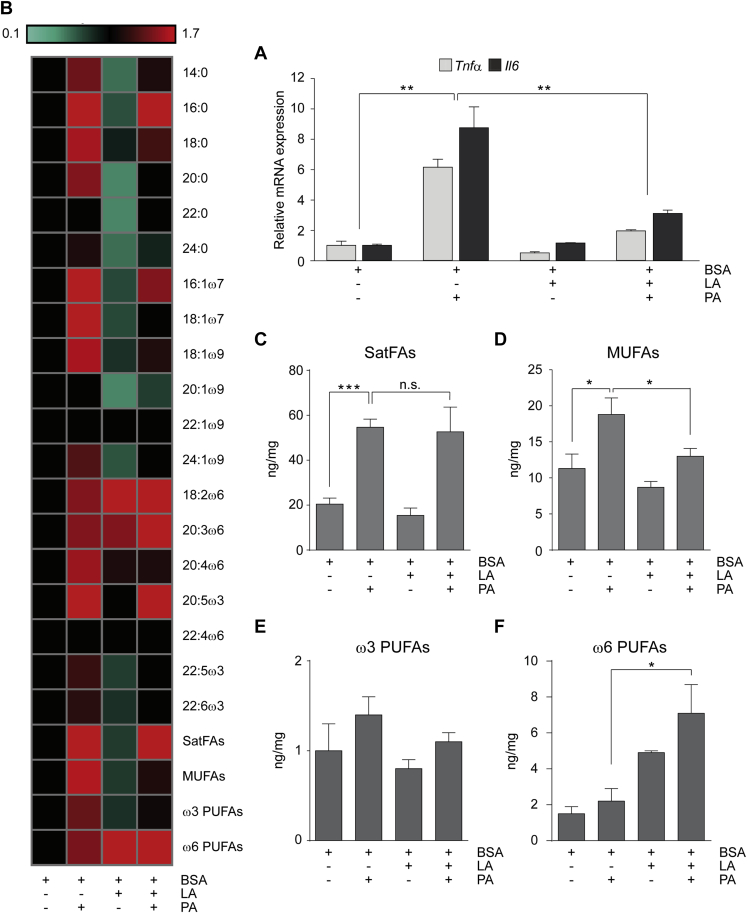
Previously, we reported males, and not females, exposed to the WD have a significant increase in markers of inflammation in the CNS [17]. Data presented here suggest that males and females differ with respect to their CNS FA composition. Therefore, to begin to understand how the CNS FA milieu may impact inflammation, we tested if the combination/ratio of differing FAs impacted inflammation. Specifically, we attempted to replicate the ratio of PA to LA found in the female brain in vitro to test the hypothesis that in the presence of LA, there is a reduction in the ability of PA to induce inflammation. To test this, we treated the N43 hypothalamic cell line with either PA alone or in combination with LA. Consistent with previous reports, treatment with PA increases the transcription of pro-inflammatory cytokines [17], while LA treatment alone does not (Figure 4A). Interestingly, pretreatment with LA followed by PA and LA co-treatment significantly reduces PA-induced inflammation (Figure 4A).
Figure 4.
The anti-inflammatory activity of LA correlates with increased ω6-PUFA concentration: N43 cells were pre-treated for 1 h with LA and then treated for 5 h as indicated. (A) mRNA levels of inflammatory markers in N43 cells following treatments. All data are presented as mean ± SEM, and *p < 0.05, *p < 0.01. n = 3. (B) Heat-map of the different FA isomers analyzed in N43 following treatments. (C–F) Graphs of the concentration (ng/mg) of the different classes of FAs in N43 cells following the aforementioned treatments. All data are presented as mean ± SEM, and *p < 0.05, ***p < 0.001. n.s. = not significant. n = 3.
In parallel, we evaluated the FAs profile of the cells to identify correlations between inflammation and FAs metabolism (Table S5). Interestingly, following the co-treatment, which significantly reduces pro-inflammatory markers, the FAs profile is significantly enriched in ω6-PUFAs, and specifically in 18:2ω6 and 20:3ω6, suggesting the anti-inflammatory effect of LA derives from the increase in this specific class of PUFAs (Figure 4 and Table S5). Importantly, these data correlate with the in vivo results, and further suggest ω6-PUFAs may mediate an anti-inflammatory/protective effect. Additionally, the increased concentration in ω6-PUFAs in female brains might explain the reduced inflammatory response that we see in female hypothalami following chronic exposure to the WD [17].
4. Discussion
In this study, we performed a comprehensive sexually dimorphic FA profile of the brains and plasma from age-matched male and female mice. We assayed the FA profile of the mice following consumption of either the standard chow diet or following exposure to a WD for 16 weeks. Our data demonstrate the TFAs composition of the brain is sexually dimorphic, with the male brains having 30% more FAs enriched in SatFAs and depleted in PUFAs, specifically ω6-PUFAs, when compared to female brains in the chow condition. These differences in SatFAs and ω6-PUFAs are even more pronounced following consumption of the WD and appear to be brain-specific, since the plasma did not replicate the sexual dimorphism observed in the brain. Our WD is high in both SatFAs as well as simple sugars, and where our assumptions are that the FAs in the diet are causing the sexually dimorphic FA differences observed, we cannot rule out that the simple sugars might also contribute to our findings.
We went on to demonstrate that the higher levels of SatFAs in combination with lower levels of ω6-PUFAs, observed in the brains of male mice exposed to the WD, cause hypothalamic inflammation both in vivo and in vitro [17], and we further demonstrate ω6-PUFAs have a protective effect in vitro, and reduce the pro-inflammatory impact of PA. Our findings suggest increased brain levels ω6-PUFAs may be protective against brain inflammation; whereas previously, it has been reported that ω6-PUFAs are pro-inflammatory in the periphery [37], [38], [39]. Consistent with the notion of an anti-inflammatory role of ω6-PUFAs in the brain, reduced concentrations of LA have been identified in brains of Alzheimer's disease patients, thus suggesting decreased amounts of LA in the brain might be detrimental [40]. Importantly, what is yet unknown is if changes in brain FA content are causative or a consequence of neuropsychiatric or neurodegenerative diseases.
The mechanisms responsible for sexual dimorphism observed for the differing FA concentrations in the brain are unknown. We speculate these differences may be caused by one of the following processes: (1) increased de-novo lipogenesis of SatFAs in the brain of male animals; (2) a slower degradation or turnover of FAs in the brain, which might involve PUFAs in the females or SatFAs in the males; and/or (3) an impaired mechanism of uptake of FAs from the periphery through the BBB, which differs by sex.
The de-novo FA biosynthesis pathway, which has been shown to occur in brain areas key in controlling energy balance such the hypothalamus [41], is comprised of three key enzymes: acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS) and malonyl-CoA decarboxylase (MCD) [42]. However, to date, whether these enzymes are differentially expressed in the brain of male and female rodents or humans is unknown and future studies are needed to address these questions. Unfortunately, the methodologies we used for our FA analysis did not allow us to also probe for FA-CoAs or any other esterified fatty acyl lipid (such as ethanolamines), which are also an important metabolically active pool of lipids and may also be sexually dimorphic. Interestingly, the expression of FA receptors and enzymes involved in FA synthesis and of FA uptake across plasma membranes appears to be sexually dimorphic [43], [44]. In skeletal muscle, proteins such as the fatty acid transporter FAT/CD36, fatty acid binding protein (FABP), and fatty acid transport protein 1 (FATP1), have been shown to be higher in women than in men [43]. Consistently, FABP protein levels have been shown to be higher in livers from female rats, indicating a sex difference in long chain FA utilization in hepatocytes [44]. Additional studies reported higher elongase 6 activity in the liver of female rats, which may contribute to the sexually dimorphic concentrations of SatFAs such as stearic and PA [45].
Another factor that might affect the FA brain concentration is the permeability of the BBB. Previous studies have reported sex differences in BBB integrity in response to different types of challenges [46], [47], [48]. As an example, the permeability of the BBB was increased in males, but not in young females following LPS exposure [48]. In the same study, the authors demonstrated that estrogens affect BBB integrity by regulating the expression of tight junction proteins [48]. Where this may be true, in our specific experimental settings, 17-β estradiol plasma levels can not explain the differences in brain FA concentration since they did not differ between the males and females [17]. In addition, it is known that, following WD exposure, the integrity of the BBB is compromised, suggesting this might affect brain FA concentrations [49], [50]. To date, no studies have determined if chronic WD exposure affects the BBB in female rodents or humans. Lastly, where we have focused on the sexual dimorphism in brain lipid composition, we would be remiss not to note that this may be due to differences in absorption or altered gut processing of ingested lipids. Understanding the genesis of the sexual dimorphism is an important next step.
5. Conclusions
Our study demonstrates that male and female brains of age-matched mice differ in their FA composition even when fed a regular/chow diet. Additionally, when chronically exposed to a WD, the sexual dimorphism continues to exist, specifically in the proportions of SatFAs as well as essential PUFAs. Our data suggest in male brains, there is a significant increase of SatFAs and reductions in ω6-PUFAs when compared to female brains. We found no sexual dimorphism in the plasma FA profile, perhaps suggesting a sexual dimorphism in the diffusion, processing, or uptake of plasma FAs into the brains. Our study provides new important insights into sex-specific differences in tissue lipid composition and confirms that basic research and clinical studies need to consider sex as an important variable to be included in analyses. Admittedly, our findings are descriptive, and future research will need to focus on the mechanisms associated with these sex-related differences in brain FA composition.
Author contributions statement
C.R.-N. and E.M. performed, analyzed the experiments and prepared the figures. C.R.-N. prepared the supplemental tables. All authors contributed in the writing of the manuscript and reviewed the final version of it.
Acknowledgments
Authors thank Aaron Frank for technical support. C.R.-N. is supported by NIH grant HL-20948 and Clayton Foundation for Research. E.M is supported by the initiation supporting program of the Pontificia Universidad Católica de Chile. D.J.C. was supported by NIH/NIDDK grant P01 088761-01 and the Klarman Foundation for Eating Disorders.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.06.014.
Contributor Information
Eugenia Morselli, Email: emorselli@bio.puc.cl.
Deborah J. Clegg, Email: Deborah.clegg@cshs.org.
Conflict of interests
The authors declare no conflict of interests.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Carrie I., Clement M., de Javel D., Frances H., Bourre J.M. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. Journal of Lipid Research. 2000;41(3):465–472. [PubMed] [Google Scholar]
- 2.Chang C.Y., Ke D.S., Chen J.Y. Essential fatty acids and human brain. Acta Neurologica Taiwanica. 2009;18(4):231–241. [PubMed] [Google Scholar]
- 3.Chen C.T., Green J.T., Orr S.K., Bazinet R.P. Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;79(3–5):85–91. doi: 10.1016/j.plefa.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Bazinet R.P., Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nature Reviews Neuroscience. 2014;15(12):771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 5.Hussain G., Schmitt F., Loeffler J.P., Gonzalez de Aguilar J.L. Fatting the brain: a brief of recent research. Frontiers in Cellular Neuroscience. 2013;7:144. doi: 10.3389/fncel.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C.T., Bazinet R.P. Beta-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2015;92:33–40. doi: 10.1016/j.plefa.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Martinez M., Mougan I. Fatty acid composition of human brain phospholipids during normal development. Journal of Neurochemistry. 1998;71(6):2528–2533. doi: 10.1046/j.1471-4159.1998.71062528.x. [DOI] [PubMed] [Google Scholar]
- 8.Luchtman D.W., Song C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology. 2013;64:550–565. doi: 10.1016/j.neuropharm.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Conquer J.A., Tierney M.C., Zecevic J., Bettger W.J., Fisher R.H. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35(12):1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer E.J., Bongard V., Beiser A.S., Lamon-Fava S., Robins S.J., Au R. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Archives of Neurology. 2006;63(11):1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 11.Calder P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. American Journal of Clinical Nutrition. 2006;83(6 Suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 12.Calder P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;75(3):197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Kiecolt-Glaser J.K., Belury M.A., Porter K., Beversdorf D.Q., Lemeshow S., Glaser R. Depressive symptoms, omega-6: omega-3 fatty acids, and inflammation in older adults. Psychosomatic Medicine. 2007;69(3):217–224. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. American Journal of Physiology – Renal Physiology. 2011;301(5):F919–F931. doi: 10.1152/ajprenal.00068.2011. [DOI] [PubMed] [Google Scholar]
- 15.Gillies G.E., McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological Reviews. 2010;62(2):155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biology of Sex Differences. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morselli E., Fuente-Martin E., Finan B., Kim M., Frank A., Garcia-Caceres C. Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Reports. 2014;9(2):633–645. doi: 10.1016/j.celrep.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morselli E., Frank A.P., Palmer B.F., Rodriguez-Navas C., Criollo A., Clegg D.J. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. International Journal of Obesity (London) 2016 Feb;40(2):206–920. doi: 10.1038/ijo.2015.114. Epub 2015 Jun 15. Review. PMID: 26073655. [DOI] [PubMed] [Google Scholar]
- 19.Quehenberger O., Armando A.M., Dennis E.A. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochimica et Biophysica Acta. 2011;1811(11):648–656. doi: 10.1016/j.bbalip.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H., Rodriguez-Navas C., Kollipara R.K., Kapur P., Pedrosa I., Brugarolas J. Unsaturated fatty acids stimulate tumor growth through stabilization of beta-catenin. Cell Reports. 2015;13(3):495–503. doi: 10.1016/j.celrep.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health N.I.H. 2015. Dietary guidelines for Americans 2015–2012 Eighth Edition. [Google Scholar]
- 22.Paterniti I., Impellizzeri D., Di Paola R., Esposito E., Gladman S., Yip P. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: in-vivo and in-vitro studies. Journal of Neuroinflammation. 2014;11:6. doi: 10.1186/1742-2094-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hjorth E., Zhu M., Toro V.C., Vedin I., Palmblad J., Cederholm T. Omega-3 fatty acids enhance phagocytosis of Alzheimer's disease-related amyloid-beta42 by human microglia and decrease inflammatory markers. Journal of Alzheimer's Disease. 2013;35(4):697–713. doi: 10.3233/JAD-130131. [DOI] [PubMed] [Google Scholar]
- 24.Labrousse V.F., Nadjar A., Joffre C., Costes L., Aubert A., Gregoire S. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS One. 2012;7(5):e36861. doi: 10.1371/journal.pone.0036861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michael-Titus A.T., Priestley J.V. Omega-3 fatty acids and traumatic neurological injury: from neuroprotection to neuroplasticity? Trends in Neurosciences. 2014;37(1):30–38. doi: 10.1016/j.tins.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Crawford M.A., Golfetto I., Ghebremeskel K., Min Y., Moodley T., Poston L. The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants. Lipids. 2003;38(4):303–315. doi: 10.1007/s11745-003-1065-1. [DOI] [PubMed] [Google Scholar]
- 27.Simopoulos A.P. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Molecular Neurobiology. 2011;44(2):203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 28.Dasilva G., Pazos M., Garcia-Egido E., Gallardo J.M., Rodriguez I., Cela R. Healthy effect of different proportions of marine omega-3 PUFAs EPA and DHA supplementation in Wistar rats: lipidomic biomarkers of oxidative stress and inflammation. Journal of Nutritional Biochemistry. 2014 May;406(12) doi: 10.1016/j.jnutbio.2015.07.007. Epub 2014 Mar 12. PMID: 24618987. [DOI] [PubMed] [Google Scholar]
- 29.Giltay E.J., Gooren L.J., Toorians A.W., Katan M.B., Zock P.L. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. American Journal of Clinical Nutrition. 2004;80(5):1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- 30.Kitson A.P., Stroud C.K., Stark K.D. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010;45(3):209–224. doi: 10.1007/s11745-010-3391-6. [DOI] [PubMed] [Google Scholar]
- 31.Kwon B., Lee H.K., Querfurth H.W. Oleate prevents palmitate-induced mitochondrial dysfunction, insulin resistance and inflammatory signaling in neuronal cells. Biochimica et Biophysica Acta. 2014;1843(7):1402–1413. doi: 10.1016/j.bbamcr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Gupta S., Knight A.G., Gupta S., Keller J.N., Bruce-Keller A.J. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. Journal of Neurochemistry. 2012;120(6):1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapoport S.I. Arachidonic acid and the brain. Journal of Nutrition. 2008;138(12):2515–2520. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyall S.C. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Frontiers in Aging Neuroscience. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Zvi A., Lacoste B., Kur E., Andreone B.J., Mayshar Y., Yan H. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orr S.K., Trepanier M.O., Bazinet R.P. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2013;88(1):97–103. doi: 10.1016/j.plefa.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Johnson G.H., Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. Journal of the Academy of Nutrition and Dietetics. 2012;112(7):1029–1041. doi: 10.1016/j.jand.2012.03.029. 1041 e1021–1015. [DOI] [PubMed] [Google Scholar]
- 38.Harris W.S. Linoleic acid and coronary heart disease. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;79(3–5):169–171. doi: 10.1016/j.plefa.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Farvid M.S., Ding M., Pan A., Sun Q., Chiuve S.E., Steffen L.M. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130(18):1568–1578. doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iuliano L., Pacelli A., Ciacciarelli M., Zerbinati C., Fagioli S., Piras F. Plasma fatty acid lipidomics in amnestic mild cognitive impairment and Alzheimer's disease. Journal of Alzheimer's Disease. 2013;36(3):545–553. doi: 10.3233/JAD-122224. [DOI] [PubMed] [Google Scholar]
- 41.Lopez M., Vidal-Puig A. Brain lipogenesis and regulation of energy metabolism. Current Opinion in Clinical Nutrition and Metabolic Care. 2008;11(4):483–490. doi: 10.1097/MCO.0b013e328302f3d8. [DOI] [PubMed] [Google Scholar]
- 42.Lopez M., Lelliott C.J., Vidal-Puig A. Hypothalamic fatty acid metabolism: a housekeeping pathway that regulates food intake. BioEssays. 2007;29(3):248–261. doi: 10.1002/bies.20539. [DOI] [PubMed] [Google Scholar]
- 43.Lundsgaard A.M., Kiens B. Gender differences in skeletal muscle substrate metabolism – molecular mechanisms and insulin sensitivity. Frontiers in Endocrinology (Lausanne) 2014;5:195. doi: 10.3389/fendo.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ockner R.K., Burnett D.A., Lysenko N., Manning J.A. Sex differences in long chain fatty acid utilization and fatty acid binding protein concentration in rat liver. Journal of Clinical Investigation. 1979;64(1):172–181. doi: 10.1172/JCI109437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marks K.A., Kitson A.P., Stark K.D. Hepatic and plasma sex differences in saturated and monounsaturated fatty acids are associated with differences in expression of elongase 6, but not stearoyl-CoA desaturase in Sprague–Dawley rats. Genes & Nutrition. 2013;8(3):317–327. doi: 10.1007/s12263-012-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oztas B., Camurcu S., Kaya M. Influence of sex on the blood brain barrier permeability during bicuculline-induced seizures. International Journal of Neuroscience. 1992;65(1–4):131–139. doi: 10.3109/00207459209003284. [DOI] [PubMed] [Google Scholar]
- 47.Diler A.S., Uzum G., Akgun Dar K., Aksu U., Atukeren P., Ziylan Y.Z. Sex differences in modulating blood brain barrier permeability by NO in pentylenetetrazol-induced epileptic seizures. Life Sciences. 2007;80(14):1274–1281. doi: 10.1016/j.lfs.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 48.Maggioli E., McArthur S., Mauro C., Kieswich J., Kusters D.H., Reutelingsperger C.P. Estrogen protects the blood-brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain, Behavior, and Immunity. 2016;51:212–222. doi: 10.1016/j.bbi.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Hsu T.M., Kanoski S.E. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Frontiers in Aging Neuroscience. 2014;6:88. doi: 10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pallebage-Gamarallage M., Lam V., Takechi R., Galloway S., Clark K., Mamo J. Restoration of dietary-fat induced blood-brain barrier dysfunction by anti-inflammatory lipid-modulating agents. Lipids in Health and Disease. 2012;11:117. doi: 10.1186/1476-511X-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.