Abstract
Objective
Insulin release from pancreatic islet β cells should be tightly controlled to avoid hypoglycemia and insulin resistance. The cortical actin cytoskeleton is a gate for regulated exocytosis of insulin secretory granules (SGs) by restricting their mobility and access to the plasma membrane. Prior studies suggest that SGs interact with F-actin through their transmembrane cargo islet cell autoantigen 512 (Ica512) (also known as islet antigen 2/Ptprn). Here we investigated how Ica512 modulates SG trafficking and exocytosis.
Methods
Transcriptomic changes in Ica512−/− mouse islets were analyzed. Imaging as well as biophysical and biochemical methods were used to validate if and how the Ica512-regulated gene villin modulates insulin secretion in mouse islets and insulinoma cells.
Results
The F-actin modifier villin was consistently downregulated in Ica512−/− mouse islets and in Ica512-depleted insulinoma cells. Villin was enriched at the cell cortex of β cells and dispersed villin−/− islet cells were less round and less deformable. Basal mobility of SGs in villin-depleted cells was enhanced. Moreover, in cells depleted either of villin or Ica512 F-actin cages restraining cortical SGs were enlarged, basal secretion was increased while glucose-stimulated insulin release was blunted. The latter changes were reverted by overexpressing villin in Ica512-depleted cells, but not vice versa.
Conclusion
Our findings show that villin controls the size of the F-actin cages restricting SGs and, thus, regulates their dynamics and availability for exocytosis. Evidence that villin acts downstream of Ica512 also indicates that SGs directly influence the remodeling properties of the cortical actin cytoskeleton for tight control of insulin secretion.
Keywords: F-actin, Granules, Ica512, Insulin, Secretion, Villin
Abbreviations: D, diffusion coefficient; EGFP, enhanced green fluorescent protein; Ica512, islet cell autoantigen; IPGTT, intraperitoneal glucose tolerance test; IVGTT, intravenous glucose tolerance test; OGTT, oral glucose tolerance test; RT-DC, real-time deformability cytometry; SE, standard error; SG, secretory granules; TIRFM, total internal reflection fluorescence microscopy
Highlights
-
•
Ica512-depletion reduces the genetic expression of the F-actin modifier villin.
-
•
Villin-depletion enhances basal insulin granule mobility and exocytosis.
-
•
Villin regulates the size of actin cages restraining insulin granules.
-
•
Villin acts downstream of insulin granule cargo Ica512.
-
•
The Ica512-villin genetic link enables granules to control cytoskeleton plasticity.
1. Introduction
Regulated exocytosis is a fundamental cellular process that involves the release of stored secretory cargos from membranous vesicles into the extracellular space. In pancreatic islet β cells, newly synthesized insulin-containing secretory granules (SGs) are formed at the trans-Golgi network and are transported to the cell cortex for preferential glucose-stimulated exocytosis relative to older SGs [1], [2]. The initial phase of vesicle transport is microtubule-dependent, while both microtubules and the cortical F-actin network regulate the motility and access of SGs to the plasma membrane and their release in response to stimuli only. The exocytosis of SGs is facilitated by drugs, which depolymerize microfilaments [3], [4], [5], [6], and is inhibited by drugs, which promote polymerization [3], [7], although high concentrations of F-actin disrupting drugs can also inhibit exocytosis [4]. Hence, the actin cytoskeleton has a complex role in regulating insulin release, but its precise molecular links with SGs remain undefined. Importantly, altered insulin secretion causes type 2 diabetes. Thus, improving our understanding of how the cytoskeleton controls insulin secretion could enable us to identify additional approaches to treat this disease.
We previously reported that the islet cell autoantigen 512 (Ica512; also known as Ia-2 and Ptprn) tethers insulin SGs to actin microfilaments via its association to the adapter protein β2-syntrophin [8], [9], [10]. Ica512 is an atypical member of the receptor protein-tyrosine phosphatase family with a single cytosolic PTP domain, which lacks protein phosphatase activity. It is mostly expressed in neuroendocrine cells, where it is selectively enriched in the SG membrane. Genetic deletion of Ica512 in mice is associated with mild glucose intolerance and decreased glucose-responsive insulin secretion [9], [10], [11], [12]. To gain further insight into how Ica512 regulates insulin secretion, we anaylzed the gene expression profile of Ica512-depleted mouse islets. We show that Ica512 depletion leads to downregulation of the F-actin modifier villin in β cells, thereby increasing the size of actin cages surrounding cortical SGs and thus their motility and exocytosis in basal conditions, while reducing glucose-stimulated insulin release.
2. Materials and methods
2.1. Culture of mouse islets and insulinoma MIN6 and INS-1 cells
The whole body knockout mice Ica512 [13] and villin [14] were generated as previously described. Pancreatic islets were isolated from 8 to 17-week-old Ica512−/− mice and 8 to 44-week-old villin−/− mice and wild type littermates and were cultured for 24 h before subsequent experiments. All animal protocols were approved by the institutional animal care and use committee and all experiments were performed in accordance with relevant guidelines and regulations. Mouse MIN6 and rat INS-1 insulinoma cells were kind gifts from Dr. Jun-ichi Miyazaki (Osaka University, Japan) and C. Wollheim (University of Geneva, Switzerland), respectively, and were grown in six-well plates as previously described [15], [16].
2.2. Transcriptomic profiling of mouse islets
Total RNA was isolated from the islets of 12-week-old wild-type and Ica512−/− mice (7 mice/group) using RNeasy (Qiagen, Hilden, Germany). For microarray analysis, 350 ng of islet RNA was amplified with the Illumina® Total Prep RNA Amplification Kit (Ambion, Inc., Austin, Tx) and cRNA was labeled with biotin-UTP. Then, 700 ng of labeled-cRNAs in 15 μL for each hybridization was dispensed on Sentrix MouseRef-8v2 Expression BeadChips (Illumina Inc., San Diego, CA). After hybridization (16 h, 58 °C), the arrays were washed according to the manufacturer's instructions (Illumina Inc.). The arrays were stained with streptavidin–cyanine-3, and scanned with the BeadArray Reader for quantification. For transcriptomic profiling using Agilent chips, total RNA from islets of 12-week-old wild-type and Ica512−/− mice (7 mice/group) was isolated as described above. Cyanine-3-labeled cRNA was prepared and hybridized onto 4 × 44K Whole Mouse Genome microarrays (AMADID 14868) from 0.6 μg of total RNA using the One-Color Microarray-Based Gene Expression Analysis v5.5 protocol (Agilent, Santa Clara, CA). Slides were scanned on an Agilent DNA Microarray Scanner (G2505C), and the data were extracted using Agilent Feature Extraction Software (version 10.0). Data analysis was done with Agilent GeneSpring software (version 11.0) with scale to median normalization of all samples and no baseline transformation. For strand-specific RNA sequencing, the library was prepared as previously described [17]. Sample libraries were pooled for 75-bp single end sequencing on an Illumina HiSeq 2000 (Illumina Inc.), resulting in approximately 30 million reads per sample. Alignment of the reads to the mm9 transcriptome was performed with pBWA [18]. Tests for differential gene expression were performed with DESeq [19]. P values for the statistical significance of the fold change were adjusted for multiple testing using the Benjamini–Hochberg method to control the false discovery rate [20].
2.3. cDNA constructs and siRNA oligonucleotides
The plasmid pEGFP-N1 was used to induce the expression of enhanced green fluorescent protein (EGFP; Clontech, Foster City, CA). The plasmids used to induce the expression of human Ica512-GFP and insulin-SNAP have been described elsewhere [21], [22]. The cDNA of mouse villin (IMAGE: 4236751) was cloned as an EcorI-AgeI insert into pEGFP-N1 using the oligonucleotides indicated in the supplementary material. The synthetic small interfering RNA (siRNA) oligonucleotides targeting mouse and rat Ica512 as well as mouse and rat villin (see Supplementary Table 1) were purchased from Riboxx (Radebeul, Germany) using the Elbashir algorithm [23].
2.4. Glucose and insulin tolerance tests
Oral, intraperitoneal, and intravenous glucose tolerance tests (OGTT, IPGTT, and IVGTT) were done on C57BL/6 wild-type and villin−/− mice after an overnight fast. Glucose (1 g/kg) was given orally, intraperitoneally, or intravenously at 0 min. For the insulin tolerance test, mice were fasted for 4 h before an injection of human regular insulin (0.75 U/kg). Tail blood glucose levels were measured at the indicated times with the Bayer Elite glucometer (Bayer, Leverkusen, Germany).
2.5. Transfection of islets and insulinoma cells, and measurement of insulin secretion
Silencing of villin in mouse islets was performed 1 day after isolation using 100 nM of siRNA and DOTAP as the transfection reagent (Roche, Basel, Switzerland). After 2 days, the islets were harvested for western blotting or incubated in resting buffer with 5.5 mM glucose for 1 h and then either kept at rest or stimulated with 16.7 mM glucose for 1.5 h, and insulin secretion was measured using a radioimmunoassay (Merck Millipore, Billerica, MA).
For RNAi-mediated knockdown of Ica512 or villin, insulinoma cells were transfected 1 day after seeding with the respective siRNAs (Ica512 siRNA, 50 nM; villin siRNA, 75 nM; scramble siRNA, 50–75 nM) using Dharmafect reagent (2.5 μL/well) (GE Dharmacon, Lafayette, CO). Three days later, cells were harvested for RNA extraction, western blotting, confocal imaging, or measurement of glucose-stimulated insulin secretion. For measurement of glucose-stimulated insulin secretion, cells were transferred to growth media containing 2.8 mM glucose for 1 h, then for 1 h in resting buffer also with 2.8 mM glucose, and then kept at rest or stimulated with 25 mM glucose for 1.5 h. Insulin content and release were measured using the AlphaLisa kit (Thermo Fisher, Waltham, MA).
For single or combined expression of Ica512-GFP, villin-GFP, INS-SNAP and pEGFPN.1, insulinoma cells were transfected with the corresponding plasmid(s) (1.5 μg plasmid/well) by electroporation as previously described [24], [25]. On day 1 post-transfection, some cells were re-treated with Ica512, villin, or scramble siRNAs, as described above.
2.6. Real-time PCR
RNA was isolated from control and villin-depleted MIN6 cells using the RNAeasy kit (Qiagen). Mouse insulin1 mRNA expression was quantified by RT-PCR using specific oligonucleotides (Supplementary Table 1).
2.7. Cell extracts and western blotting
Isolated mouse islets, MIN6 and INS-1 cells were washed with ice-cold PBS and extracted in lysis buffer (10 mM Tris–HCl, pH 8.0, 140 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1% phosphatase inhibitors [Calbiochem/Merck Millipore], and 1% protease inhibitor mixture [Sigma, St. Louis, MO]) at 4 °C. Western blotting was performed as previously described [26] using the following primary antibodies: mouse monoclonal anti-Ica512 HM1 [26] and anti-γ-tubulin (Sigma), rabbit polyclonal anti-villin (Cell Signaling Technology, Danvers, MA), and goat polyclonal anti-GFP (Clontech). Chemiluminescence was developed and quantified as previously described [9].
2.8. Immunocytochemistry and confocal microscopy
Immunocytochemistry on pancreas tissue sections of Ica512−/− and villin−/− mice and control littermates was performed as previously described [27]. Transfected or control INS-1 cells were immunolabeled as previously described [21]. In some instances, cells were incubated in resting buffer with 2.8 mM glucose or stimulated with 25 mM glucose and 55 mM KCl for 1.5 h before being fixed. Tissue sections and cultured cells were labeled using the following primary and secondary antibodies: guinea pig anti-insulin (Abcam, Cambridge, UK), rabbit anti-villin (Cell Signaling Technology), goat anti-rabbit Alexa568-conjugated IgG and goat anti-guinea pig Alexa488-conjugated IgG (Thermo Fisher). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Sigma), and coverslips were mounted with Mowiol (Calbiochem/Merck Millipore). Images of 0.5-μm optical tissue sections were acquired at room temperature with an inverted confocal microscope (Zeiss Axiovert 200M) equipped with a Plan-Apochromat × 63 oil objective, numerical aperture 1.4, a Zeiss LSM510 scan head with photomultiplier tubes, and Zeiss LSM 510 AIM software version 4 (Zeiss, Göttingen, Germany).
2.9. Total internal reflection fluorescence microscopy (TIRFM)
Prior to imaging, cells grown in an open chamber were incubated in resting media and transferred onto a thermostat-controlled (37 °C) stage. Insulin-SNAP+ SGs were labeled as previously described [22] with the SNAP substrate TMR-Star (New England Biolabs, Ipswich, MA). The SGs were visualized in cells cotransfected with either EGFP or villin-GFP after treatment with the Ica512, villin, or scramble siRNAs. Videos were acquired with a Nikon microscope (Nikon, Tokyo, Japan) equiped with Andor DU-897 camera (Andor, Belfast, Rochester, NY). Pixel binning was set to 1×, multiplier to 300, read out speed to 10 MHz, and the excitation wavelength at 561 nm was set to a power of 2%. Images were collected using NIS-AR4.11.00 software (Nikon) at 30 frames/s with an exposure time of 10 ms/frame giving a total recording time of 1 min (2000 frames) for each movie. Automated image analysis was performed with MotionTracking/Kalaimoscope software (Transinsight GmbH, Dresden, Germany), as previously described [2], [9]. The processive movement was defined as a motion in the same direction for at least four successive sequence frames and the percentage of processive excursions within total distance was calculated as previously described [22]. Other parameters of SG dynamics, including cumulative diffusion, mean square displacement, component analysis, and track maximum displacement were calculated as previously described [2]. The density of cortical SGs was calculated using ≥16 movies of resting or stimulated GFP+ control and villin-depleted cells and of villin-depleted cells co-expressing villin-GFP, by using the cytosolic GFP signal to calculate the cell surface area. To estimate the size of actin cages, we analyzed the mean square displacement of the SG center within the cortical region as this approach has been shown to provide values for actin cage size that are in good agreement with those theoretically predicted [28], [29]. Since the mean square displacement was measured to the saturation value, SGs could visit all available positions within the actin “cage”. Accordingly, the 2D projected area of the actin cage equaled the sum of the area covered by the displacement of the SG center plus the area covered by a SG according to the following formula:
Where Scage is the characteristic size of the actin cage, SSG is the size of a SG of 125 nm radius [30], is the saturation value of the SG center mean square displacement. Then .
2.10. Real-time deformability cytometry
Cell mechanical characterization was performed using real-time deformability cytometry (RT-DC) as previously described [31]. Pancreatic islets were isolated from 8 to 20-week-old villin−/− mice and wild-type littermates as previously described [13]. Approximately 200 islets per condition and per assay were enzmatically dispersed into single cell suspensions using accutase, centrifuged at 2000× g for 2 min and gently resuspended in PBS containing 0.5% (w/v) methylcellulose. For drug treatment, islets were treated with latrunculin A at 0.2 μM or DMSO (0.1%, v/v) 15 min before the RT-DC assay. Data analysis was performed as previously described [31]. One-dimensional linear mixed models were used to compare the size and mechanical properties independently. The experimental situation was assumed to be described by one fixed and one random effect and took into account the variation of three biological replicates.
2.11. Statistics
Statistical analyses were performed using t-tests in Microsoft Excel 12.3.6, while the ProbStudent- and Kolmogorov–Smirnov tests are included in the MotionTracking/Kalaimoscope software. The ProbStudent-t test was applied to null-hypothesis that difference between glucose disposal curves in villin+/+ and villin−/- mice equals zero. Briefly, we calculated the difference between corresponding points of the curves, then we took mean and SEM of differences and calculate p_value as incomplete Beta-function I((n − 1)/2, 0.5. (n − 1)/(n − 1 + (mean/SEM) ˆ 2), where n is number of points in curves [32]. For 12–14 weeks OGTT the following values were found: 1.366666667, 2.633333333, 0.766666667, 0.666666667, 0.966666667, 1.5. Therefore, the mean = 1.31667, the SEM 0.295365, n = 6, and the p-value = 0.00665419. For 40-40 weeks IPGTT the following values were found: 0.166666667, 3.033333333, 3.333333333, 1.633333333, 1.733333333, 2.933333333. Therefore, the mean = 2.13889, the SEM = 0.488794 (n = 6), and the p-value = 0.00718262. Since p-values are small (<<0.05), the null-hypothesis that glucose disposal curves in villin+/+ and villin−/− mice have no differences was rejected. Results are presented as the mean ± standard error (SE) unless otherwise stated. Values of p < 0.05 were considered statistically significant. Bars show standard deviations from at least three independent experiments unless otherwise stated. Histograms were prepared using Microsoft Excel (Microsoft Corp., Redmond, WA).
3. Results
3.1. Ica512 regulates the expression of villin in pancreatic β cells
The gene expression profiles of islets isolated from Ica512−/− mice and control littermates were compared. The gene ontology analysis indicated that villin was the most consistently downregulated gene in Ica512−/− mouse islets, with a mean decreased expression of ∼2 fold among the three transcriptomic profiling methods (Table 1).
Table 1.
Transcriptomic profiling of mouse islets from Ica512−/− and control littermates.
| Gene name | Probe ID | Illumina |
Agilent |
RNA sequencing |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log2 fold change | p-val | Adj p-val | Log2 fold change | p-val | Adj p-val | Log2 fold change | p-val | Adj p-val | ||
| Ica512/Ptprn | ILMN_1245627 | −2.98 | 3.20E−06 | 2.30E−02 | −2.19 | 0.0000 | 0.0000 | −4.36 | 4.16E−119 | 9.40E−115 |
| Vil1 | ILMN_2518406 | −1.04 | 1.90E−05 | 6.20E−02 | −1.32 | 0.0000 | 0.0000 | −0.66 | 8.27E−05 | 0.05 |
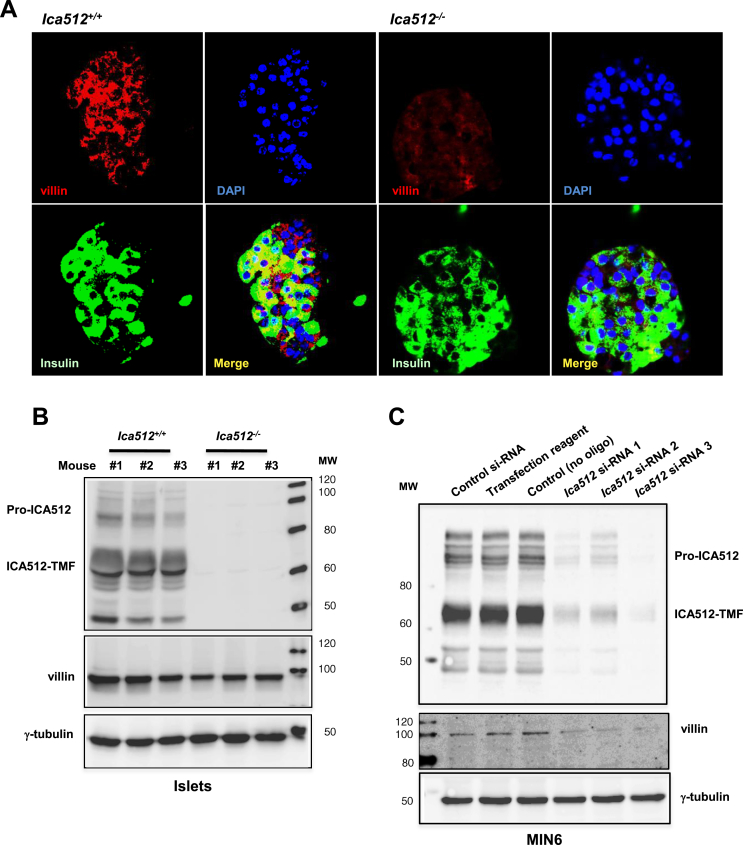
The genetic interaction between villin and Ica512 was verified by confocal imaging of mouse Ica512−/− and Ica512+/+ pancreatic cryosections immunostained for villin and insulin (Figure 1A). These analyses showed that villin is enriched in pancreatic islet cells, including insulin+ β cells, compared with pancreatic acinar cells and corroborated its downregulation in Ica512−/− islets in situ. Western blotting analysis also showed that its protein expression was ∼2-fold lower in Ica512−/− islets than in Ica512+/+ islets (Figure 1B and Supplementary Figure 1A), consistent with the transcriptomics data.
Figure 1.
Villin expression is reduced in Ica512−/−mice and Ica512-depleted MIN6 cells. (A) Confocal images of mouse pancreatic cryosections from Ica512+/+ and Ica512−/− mice were co-stained for villin (red) and insulin (green). The nuclei were stained with DAPI (blue). (B) Immunoblotting for Ica512, villin, and γ-tubulin using extracts of islets isolated from Ica512+/+ or Ica512−/− mice (in triplicate). (C) Immunoblotting for Ica512, villin, and γ-tubulin using extracts of control and Ica512-depleted MIN6 cells (in triplicate).
We then investigated whether acute depletion of Ica512 affected villin expression in mouse MIN6 and rat INS-1 insulinoma cells; the latter cell-type expresses more villin than the former cell-type (Supplementary Figure 1B). siRNA for Ica512 downregulated its protein expression by 90% ± 4.5% in MIN6 cells (Supplementary Figure 1C) and by 65% ± 10.4% in INS-1 cells (Supplementary Figure D,E), and that of villin by 52% ± 13.7% and 42% ± 9.6%, respectively (Figure 1C and Supplementary Figure 1F,G). Immunostaining of INS-1 cells showed that villin is enriched in the cell cortex, the typical location for an actin binding protein (Supplementary Figure 2A). Villin immunoreactivity was absent when the primary antibody was omitted and was sensitive to RNAi-mediated knockdown of villin (Supplementary Figure 2A,C). Accordingly, the islets from villin−/− mice were not immunoreactive for villin (Supplementary Figure 2B). Notably, villin immunoreactivity was enhanced in cells stimulated with 25 mM glucose and 55 mM KCl for 2 h (Supplementary Figure 2A), which promotes membrane depolarization and thus calcium influx. Because villin is a globular protein with an autoinhibited configuration that is released upon calcium binding [33], this observation suggests that villin changes its conformation in conditions that stimulate insulin release from β cells.
Taken together, these data indicate that the genetic association of villin and Ica512 is conserved in mouse and rat. The acute downregulation of villin after silencing Ica512 in insulinoma cells suggest that decreased expression of villin in Ica512−/− mouse islets is unlikely to be the result of a developmental adaptive process.
3.2. Role of villin in glucose homeostasis
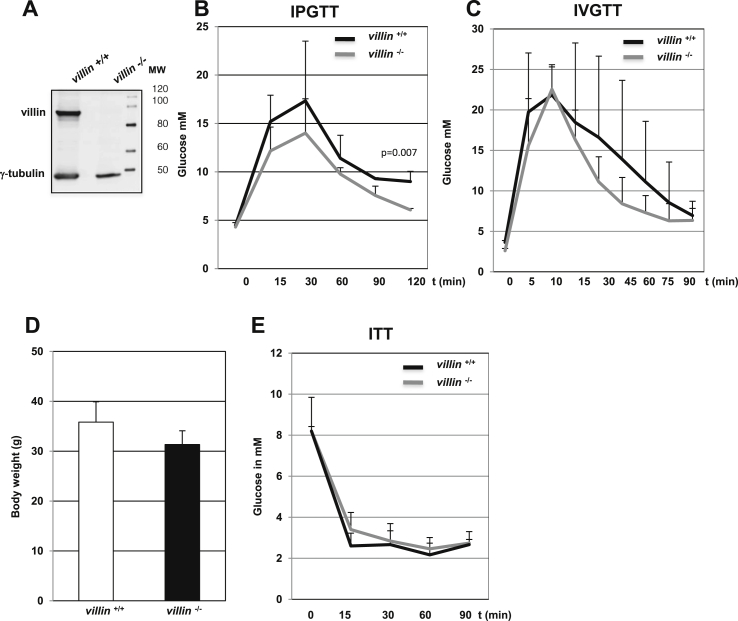
To gain insight into the role of villin in glucose homeostasis, we performed glucose tolerance tests in villin−/− and villin+/+ mice [14]. Glucose concentrations in 12–14-week-old villin−/− mice were lower (p-value of ProbStudent-test = 0.006, see Materials and Methods for details) than in villin+/+ mice in the OGTT (Supplementary Figure 3A) but not in the IPGTT (p-value of ProbStudent-test = 0.110) (Supplementary Figure 3B). However, in 40–44-week-old villin−/− mice glucose concentrations were lower (p-value of ProbStudent-test = 0.007, see Materials and Methods for details) than in villin+/+ mice also in the IPGTT (Figure 2B). This mild phenotype could reflect a modestly reduced glucose uptake by villin−/− intestine cells. Conversely, the IVGTT profiles of youger and older villin−/− and villin+/+ mice were comparable (Figure 2C and Supplementary Figure 3C). The body weight and insulin sensitivity of villin−/− and villin+/+ mice were similar (Figure 2D,E).
Figure 2.
Glucose tolerance and insulin sensitivity of villin−/−mice. (A) Immunoblotting for villin and γ-tubulin using extracts of islets isolated from villin+/+ and villin−/− mice. (B,C) Intraperitoneal (B) and intravenous (C) glucose tolerance tests in 40–44-week-old villin+/+ and villin−/− mice. (D) Body weight of 44-week-old villin+/+ and villin−/− mice. (E) Insulin tolerance tests in 44-week-old villin+/+ and villin−/− mice.
3.3. Villin regulates the cytoskeleton of islet cells
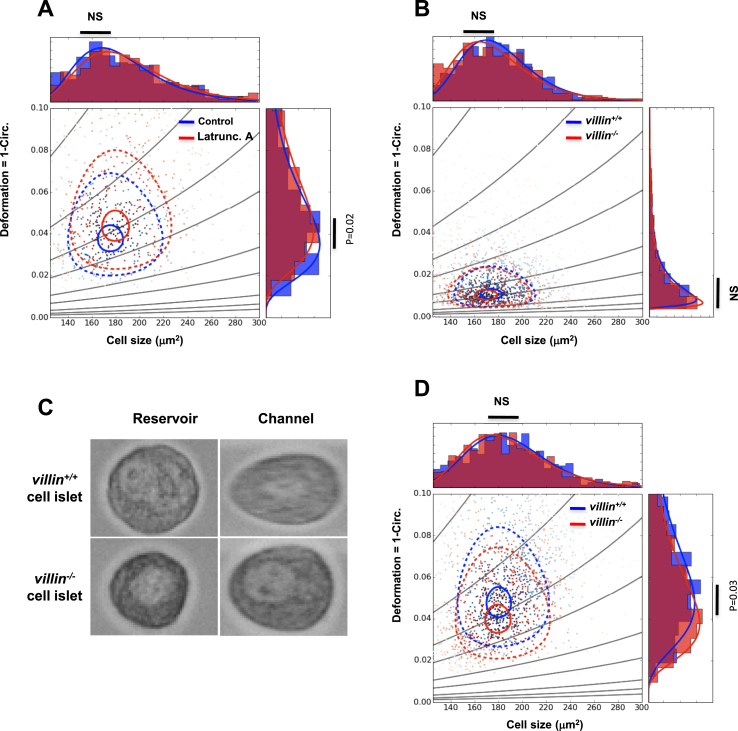
Next, we investigated whether villin regulates the cytoskeleton of islet β cells. To achieve this, we compared the mechanical properties of villin−/− and villin+/+ dispersed islet cells by RT-DC. RT-DC allows researchers to quantify cell deformability by measuring the deviation from circularity of single cells prior and after their flow from the reservoir into a channel of variable size within a microfluidic chamber [31]. The suitability of this assay was verified by measuring the deformation of villin+/+ islet cells treated with the F-actin depolymerizing agent latrunculin A or DMSO as a control. As expected, latrunculin A-treated islet cells were more deformed (i.e. less stiff) than untreated cells (Figure 3A). The size and deformation of villin−/− and villin+/+ islet cells in the reservoir were comparable (Figure 3B,C). When exposed to the flow, however, the deformation of villin−/− islet cells, but not their size, was reduced compared with that of villin+/+ islet cells (Figure 3C,D). The greater stiffness of villin−/− islet cells supports the role of villin in the regulation of the actin cytoskeleton in these cells.
Figure 3.
Deformability of villin−/−islets. (A) Deformability of mouse islets treated with or without latrunculin A (200 nM). (B) Deformation of villin+/+ and villin−/− islets in the reservoir. (C) Images of single villin+/+ and villin−/− islet cells in the reservoir or in the constricted channel of the microfluidic chamber. (D) Scatterplot of villin+/+ and villin−/− islet cell deformation versus cell size. Contour lines representing 95% and 50% of the maximum cell density are shown as solid and dashed lines, respectively. The size distribution and deformability distribution are shown in the top and right panels, respectively, in A, B, and D.
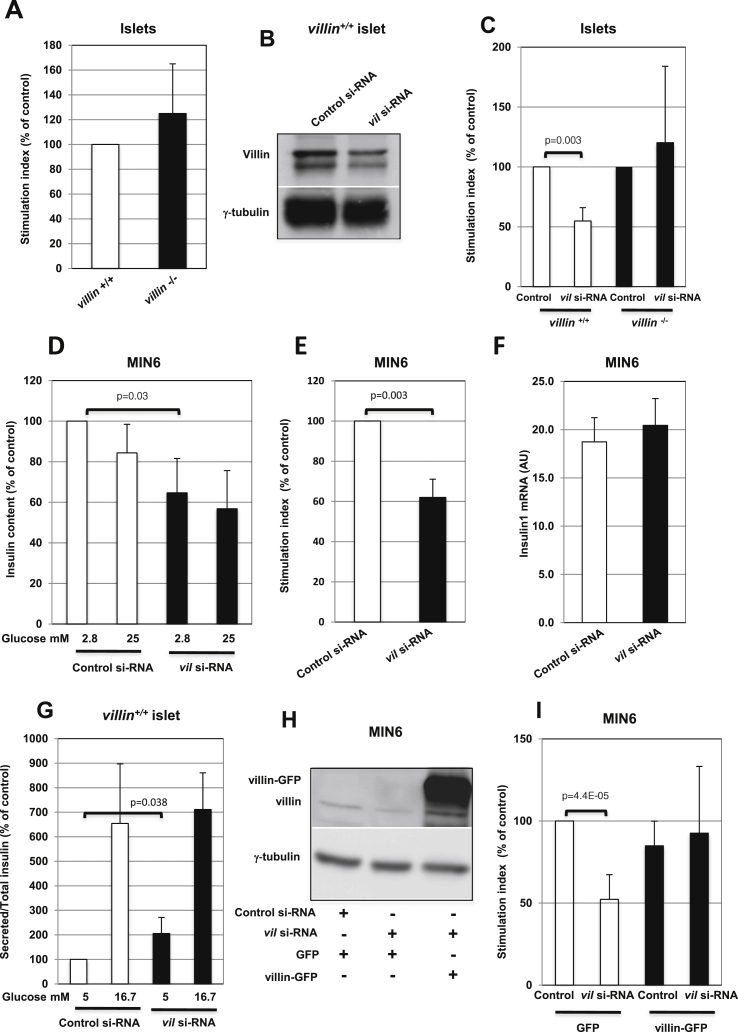
3.4. Acute depletion of villin impairs insulin release
Because villin is genetically linked to Ica512, which regulates SG exocytosis, we investigated whether villin affects insulin release. Intriguingly, isolated villin−/− and villin+/+ islets were comparable in terms of insulin content (Supplementary Figure 4A), basal insulin secretion (Supplementary Figure 4B), and glucose-stimulated insulin secretion (Figure 4A). However, acute downregulation of villin expression by 60% in villin+/+ islets (Figure 4B) reduced the insulin stimulation index (Figure 4C) but not insulin content (Supplementary Figure 4C). The reduction in glucose-stimulated insulin release in villin-depleted islets was not due to the concomitant downregulation of off-target genes, because this deficit was not observed in villin−/− islets exposed to the same treatment (Figure 4C). Intestinal epithelial cells compensate for the lack of villin by functional redundancy with other actin binding proteins, especially plastin 1 and espin [34], [35]. Likewise, we found that plastin was upregulated in villin−/− islets (Supplementary Figure 5A), but not in villin-depleted MIN6 or INS-1 cells (Supplementary Figure 5B and C). The insulin content (Figure 4D) as well as the levels of the SG cargo Ica512 (Supplementary Figure 4D) were reduced in villin-depleted MIN6 cells, which is compatible with a reduction of the SG stores. Moreover, stimulated insulin secretion (Figure 4E and Supplementary Figure 4E) was reduced in villin-depleted MIN6 cells while insulin1 mRNA expression was unchanged (Figure 4F). Insulin content (Supplementary Figure 4F) and secretion (Supplementary Figure 4G,H) were also reduced in villin-depleted INS-1 cells. The reduced stimulation index in villin-depleted insulinoma cells was driven by the increased basal insulin release (Figure 4G, Supplementary Figure 4E,H), in line with the data mentioned above suggesting an increased consumption of the SG stores. Notably, expression of a villin-GFP chimera resistant to silencing in MIN6 cells (Figure 4H) restored the stimulation index, confirming that villin regulates insulin secretion (Figure 4I).
Figure 4.
Insulin secretion is decreased in villin−/−islets and villin-depleted MIN6 cells. (A) Insulin stimulation index of villin+/+ and villin−/− islets. (B) Immunoblots for villin and γ-tubulin in extracts of villin+/+ islets treated with control or villin siRNA oligonucleotides. (C) Insulin stimulation index of villin+/+ and villin−/− islets treated with control or villin siRNA oligonucleotides. (D–F) Insulin content at 2.8 and 25 mM glucose (D), stimulation index (E), and insulin-1 mRNA expression (F) of control or villin-depleted MIN6 cells. (G) Basal and glucose-stimulated insulin secretion in villin+/+ islets treated with control or villin siRNA oligonucleotides. (H) Immunoblotting for GFP and γ-tubulin in extracts of MIN6 cells transfected with GFP or villin-GFP and treated with control or villin siRNA oligonucleotides. (I) Insulin stimulation index of MIN6 cells transfected with GFP or villin-GFP and treated with control or villin siRNA oligonucleotides.
3.5. Villin regulates insulin granule dynamics
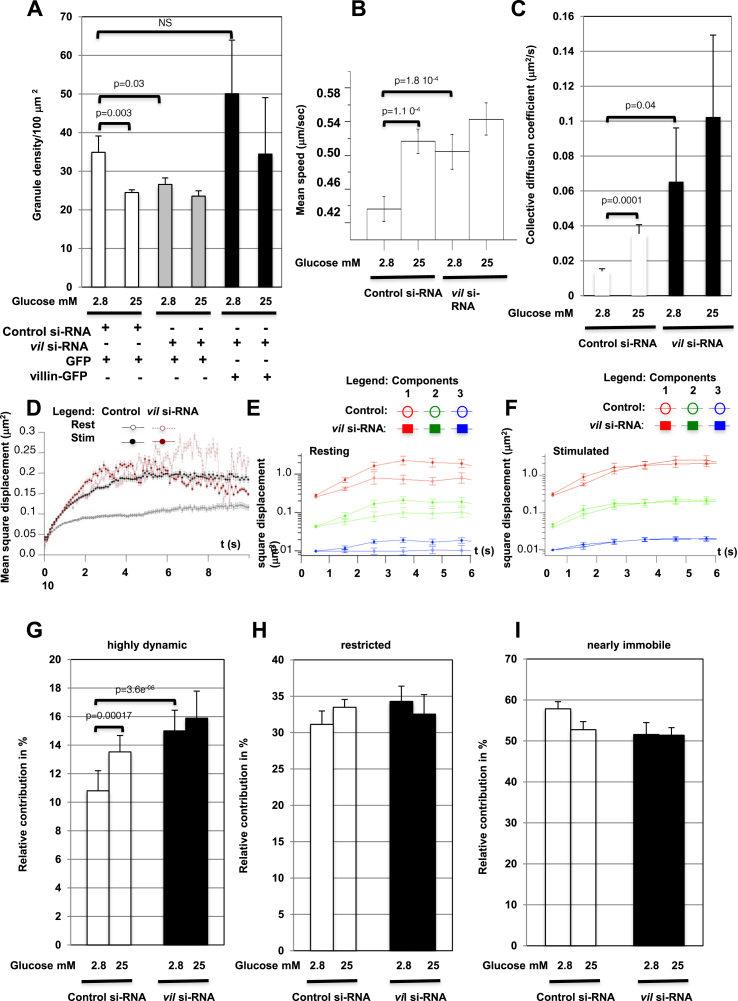
F-actin restrains SGs, partly via interactions between the actin binding complex β2-syntrophin/utrophin with Ica512 [9], [10]. Therefore, we analyzed whether villin also regulates SG dynamics by using TIRFM in combination with MotionTracking/Kalaimoscope software [2], [22] to automatically count cortical insulin SGs labeled with the fluorescent reporter Ins-SNAPTMR-Star and track their motion in living MIN6 cells co-transfected with GFP. As expected, stimulation of control GFP+ MIN6 cells with 25 mM glucose, which promotes insulin release, reduced the density of cortical Ins-SNAPTMR-Star+ SGs from 0.349 ± 0.042/μm2 to 0.245 ± 0.007/μm2 (Figure 5A). The density of Ins-SNAPTMR-Star+ SGs was already reduced to 0.266 ± 0.017/μm2 in villin-depleted GFP+ MIN6 cells kept at rest with 2.8 mM glucose, and it was not decreased by glucose stimulation. Overexpression of villin-GFP restored the density of Ins-SNAPTMR-Star+ SGs to 0.501 ± 0.014/μm2 in resting villin-depleted MIN6 cells.
Figure 5.
SG dynamics in villin-depleted MIN6. (A) Density of SGs in resting or glucose-stimulated MIN6 cells transfected with GFP or villin-GFP and treated with control or villin siRNA oligonucleotides. (B–D) Mean speed of processive SGs (B), collective diffusion coefficient (C), and mean square displacement of SGs (D) in resting or glucose-stimulated MIN6 cells transfected with GFP and treated with control or villin siRNA oligonucleotides. (E,F) Deconvolution in three dynamic components of the mean square displacement of SGs in resting (E) or glucose-stimulated (F) MIN6 cells transfected with GFP and treated with control or villin siRNA oligonucleotides. Highly dynamic SGs: red line; restricted SGs: green line; nearly immobile SGs: blue line. (G–I) Percentage of highly dynamic (G), restricted (H), and nearly immobile (I) SGs relative to the total number of tracked SGs in resting or glucose-stimulated MIN6 cells transfected with GFP and treated with control or villin siRNA oligonucleotides.
Glucose stimulation of control GFP+ MIN6 cells increased the mean speed of processively moving Ins-SNAPTMR-Star+ SGs from 0.43 ± 0.014 μm/s to 0.52 ± 0.015 μm/s (Figure 5B) and their track maximum displacement from 0.43 ± 0.015 μm to 0.51 ± 0.015 μm (Supplementary Figure 6A). Conversely, in resting villin-depleted GFP+ MIN6 cells, the processive movement of Ins-SNAPTMR-Star+ SGs displayed a mean speed of 0.50 ± 0.017 μm/s and track maximum displacement of 0.50 ± 0.016 μm. Both variables were unaffected by glucose stimulation with values of 0.54 ± 0.016 μm/s and 0.51 ± 0.017 μm, respectively. In line with these findings, glucose stimulation increased the collective diffusion coefficient (D) of Ins-SNAPTMR-Star+ SGs in GFP+ MIN6 cells but not in villin-depleted GFP+ MIN6 cells (Figure 5C). Analysis of the mean square displacement (MSD) provided additional evidence for the increased mobility of SGs in villin-depleted MIN6 cells prior to glucose stimulation (Figure 5D). In control GFP+ MIN6 cells, the MSD of Ins-SNAPTMR-Star+ SGs increased linearly for ∼2 s before reaching a plateau – a motion that is characteristic of objects restricted within F-actin cages. Accordingly, glucose stimulation, which induces the remodeling of microfilaments, increased the MSD of Ins-SNAPTMR-Star+ SGs for an extended period of time. Conversely, the MSD of SGs in villin-depleted cells did not vary between the resting and stimulated conditions. The increasing noise of the tracks over time can be explained by the fewer SGs with long trajectories (Supplementary Figure 6B). This effect was more pronounced in villin-depleted cells compared to control cells, presumably because the efficiency of the knockdown varied among cells.
Deconvolution of the MSD allowed us to classify the SGs into three dynamic components according to the previously defined D [13] as highly dynamic (D = ∼10−2 μm2/s), restricted (D = ∼10−3 μm2/s), and nearly immobile (D = ∼10−4 μm2/s) (Figure 5E,F). This analysis revealed that the D of all three dynamic components was higher in resting, but not in stimulated, villin-depleted cells than in the corresponding control cells. Glucose stimulation increased the fraction of highly dynamic SGs from 10.8% ± 1.4% to 13.5% ± 1.1% (Figure 5G). In resting villin-depleted cells, highly dynamic SGs accounted for 15.0% ± 1.44% of the total number of SGs, and this pool was not increased by stimulation. The contribution of the other two dynamic components did not differ between control and villin-depleted cells at rest or after stimulation (Figure 5H,I).
3.6. Villin acts downstream of Ica512 in regulating SG dynamics and exocytosis
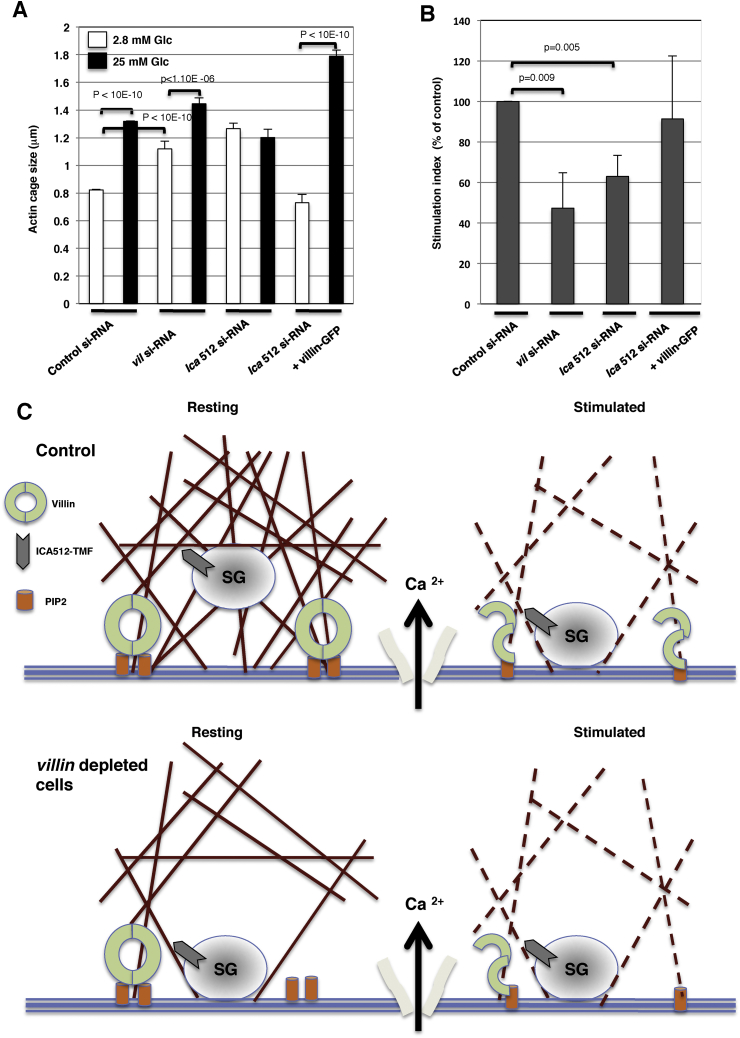
Finally, we investigated the interplay between villin and Ica512 in regulating SGs dynamics and exocytosis. To achieve this, we first estimated the size of the actin cages encasing cortical SGs based on the amplitude of the D of highly dynamic SGs. In control cells, the mean size of the actin cages increased in MIN6 cells from 0.82 μm at rest to 1.32 μm upon stimulation. The actin cages were enlarged in villin-depleted cells at rest to a mean size of 1.12 μm, which increased to 1.45 μm upon stimulation (Figure 6A). Enlarged actin cages were also present in resting Ica512-depleted cells (mean size 1.26 μm), but their size was not increased by stimulation (mean size 1.20 μm). These phenotypic changes were rescued by concomitant expression of villlin-GFP, which reduced the size of the actin cages at rest and induced greater remodeling of actin cages upon stimulation. The changes in the sizes of actin cages in resting cells and after remodeling upon stimulation were correlated with changes in the insulin stimulation index. Thus, larger cages in resting villin- or Ica512-depleted cells were coupled with a blunted stimulation index, presumably due to increased basal insulin release and thus exhaustion of SG stores, whereas expression of villin-GFP in Ica512-depleted cells normalized both the size of actin cages and the effects of glucose stimulation (Figure 6B). Taken together, these data indicate that villin regulates the cortical actin cytoskeleton downstream of Ica512 (Figure 6C).
Figure 6.
Altered actin cage size in villin- and Ica512-depleted MIN6 cells. (A,B) Actin cage size (A) and insulin stimulation index (B) in resting or glucose-stimulated MIN6 cells transfected with GFP or villin-GFP and treated with either control, villin, or Ica512 siRNA oligonucleotides. (C) Remodeling of SG cages in villin+/+ and villin-depleted cells.
4. Discussion
In this study, we have shown that Ica512 regulates the expression of villin in mouse β cells. Villin belongs to the gelsolin protein family and is expressed in a few restricted tissues, being a major constituent of the actin bundles in the microvilli of intestinal and kidney epithelial cells, where it is involved in the absorptive and secretory function of epithelial cells by modulating F-actin polymerization/depolymerization. However, it is also expressed in cells lacking microvilli, such as interstinal crypt and M cells [reviewed in 33]. Pancreatic β cells have microvilli-like structures, which are poorly defined in terms of their function, but these structures are enriched with glucose transporter 2, by about 6-fold relative to other domains of the plasma membrane [36]. A recent proteomics study revealed that villin and its paralog gelsolin were upregulated in mouse islets stimulated in vitro with 16.7 mM glucose for 24 h, [37].
Here, we confirmed the expression of villin in pancreatic islets and verified its enrichment in the cellular cortex. Villin immunoreactivity was enhanced in insulinoma cells costimulated with high glucose and KCl to induce Ca2+ influx. Similar to gelsolin, villin is a globular protein that, in its autoinhibited configuration, bundles actin microfilaments [38]. This arrangement can be released via a “hinge mechanism” following an increase in the intracellular Ca2+ concentration. The resulting conformational change exposes the F-actin binding site of villin and regulates its actin-severing activity. To verify that villin controls the islet cell cytoskeleton, we compared the mechanical properties between villin−/− and villin+/+ islets using high-throughput RT-DC. This is the first time RT-DC has been used for the analysis of islet cells, and allowed us to measure the changes in the shape and deformability of these cells. The evidence that villin−/− islet cells are less deformable than wild-type cells validates the role of villin in regulation of the islet cell cytoskeleton and agrees with the previous observation of the reduced migration of villin−/− epithelial cells [39]. This is in line with studies using differential centrifugation on extracts from villin−/− and villin+/+ intestinal cells and confirmed the important role of villin is establishing the balance between polymerization and severing on actin filaments [40].
Although the mechanical properties of villin−/− islet cells are clearly altered compared with wild-type islet cells, there are few systemic metabolic changes in villin−/− mice. Specifically, glucose homeostasis of villin−/− mice was modestly improved compared with control littermates, conceivably due to a slight reduction in intestinal glucose absorption. This mild phenotype can be explained by the compensatory mechanism of plastin in villin−/− intestinal cells [34]. Indeed, glucose tolerance was improved in villin−/−, plastin−/−, and espin−/− mice [35].
The wide tissue distribution of villin precluded the use of villin−/− mice to assess its specific role in β cells. Therefore, we assessed glucose-stimulated insulin secretion using isolated villin−/− islets in vitro and also used isolated villin+/+ islets and insulinoma cells in which villin expression had been acutely downregulated by RNAi. The insulin stimulation index of villin−/− islets was normal, possibly due to compensatory upregulation of plastin, but the insulin stimulation index was reduced in villin-depleted islets and insulinoma cells. Off-target effects were ruled out because the villin siRNAs did not affect insulin secretion in villin−/− islets and because the insulin stimulation index of insulinoma cells was rescued by concominant expression of RNAi-resistant villin-GFP. The decreased insulin stimulation index of villin-depleted β cells was driven by an increase in basal insulin secretion and a decrease in glucose-stimulated insulin release. Villin is not the first actin-severing protein to be implicated in insulin secretion. Studies in two MIN6 cell clones, which differed markedly in their secretory responses, showed that its paralogue gelsolin promotes insulin release via its Ca2+-dependent F-actin severing and remodeling activity upon glucose stimulation [41]. Gelsolin was also shown to modulate insulin secretion via Ca2+-regulated binding to syntaxin 4 [42] and to promote β cell survival [43]. Similarly, the Ca2+-activated actin severing activity of scinderin was reported to promote regulated exocytosis of SGs in chromaffin cells [44].
“Leaky” basal insulin secretion in villin-depleted β cells could explain the lower density of SGs near the plasma membrane, a finding that was correlated with the greater mobility of SGs in conditions in which actin bundling is conceivably reduced. The processive motion of SGs was 21% faster, and the collective diffusion of SGs was 2.5 times greater in stimulated control cells than in the corresponding unstimulated cells. These findings are consistent with the notion that stimulation extends the excursions of SGs, enabling them to move to areas not previously visited in the last few hundred milliseconds before exocytosis [45, reviewed in 46]. The processive motion and collective diffusion of SGs were 16% and 4.5 times greater, respectively, in resting villin-depleted cells than in resting control cells, but they were not increased further by glucose stimulation. Based on the MSD, we calculated that in resting control cells, SGs are confined within an area with a mean size of 0.82 μm, comparable to the estimated size of actin cages surrounding SGs in chromaffin cells [28], [47]. Villin-depletion was associated with a significant enlargement of the actin cages in resting cells, similar to the effects of glucose stimulation did in control cells. A comparable increase in the size of actin cages was observed in resting Ica512-depleted cells, in which villin expression was also downregulated. Notably, this phenotype was rescued by villin-GFP overexpression. In other words, the actin cages in resting Ica512-depleted, villin-GFP+ cells were similar in size to those in resting control cells. Moreover, the stimulation-induced expansion of actin cages was restored in villin-GFP+ cells, conceivably due to villin Ca2+-dependent actin-severing activity. These changes were accompanied by restoration of the insulin stimulation index relative to that in villin- or Ica512-depleted cells. Taken together, these findings suggest that villin acts downstream of Ica512 in regulating the mobility and access to the plasma membrane of SGs. However, since stimulation could promote the further enlargement of actin cages in villin-depleted, but not in Ica512-depleted cells, it seems likely that villin is not the only factor through which Ica512 regulates SG dynamics. One such factor could be β2-syntrophin, an adapter protein involved in the tethering of SGs to F-actin through Ica512 [10]. These considerations are in line with previous studies suggesting that neither Ica512/Ia-2 nor its paralogue Ia-2β/phogrin is indispensable for SG exocytosis but “that their absence facilitates the secretory response to the triggering Ca2+ signal, perhaps by suppressing the interaction with proteins [8], [48] that impede rather than promote granule access to the exocytotic sites” [49].
The signaling pathway for regulation of villin expression by Ica512 remains undefined. Intriguingly, villin expression is reduced in intestinal epithelial cells of Jak3−/− mice [50]. In rat β cells, cleavage of Ica512 intracellular domain upon SG exocytosis generates a cytosolic fragment that retrogradely enhances Jak/Stat activity in order to upregulate the transcription of SG cargoes, and thereby replenish SG stores [13], [26]. Hence, in the future it will be interesting to establish whether Ica512 regulates villin expression through Stat signaling.
5. Conclusions
Our data indicate that villin is key for tight control of insulin SG mobility and exocytosis and that its expression is reduced upon deletion of the SG cargo protein Ica512. The genetic link between Ica512 and villin may therefore enable β cells to adjust the dimension of their F-actin cytoskeleton and its plasticity to the size of the SG stores.
Authors' contributions
H.M. and M. S. designed the research and wrote the manuscript; H.M. performed most of the experimental work; B.M., P.H., A. I., and Y. K., contributed to the analysis of TIRF microscopic data; D. S. and T.H. performed transcriptomic analyses. M. H., O. O., and J.G. performed the RT-DC experiment; A. S. and C. M. provided technical assistance; J. D. and M. M.-H. contributed to data interpretation and modeling.
Acknowledgments
We thank Andreas Dahl (TU Dresden) and Julia Jarrells (MPI-CBG, Dresden) for transcriptomic analysis, Anne Friederich for pancreas cryosectioning, Katja Pfriem for administrative assistance, Sylvie Robine (Institut Curie, Paris, France) for providing the villin−/− mice, Nicholas Smith (Auckland, NZ) for professional copy-editing. This work was supported by funds provided to M.S. by Boeringher Ingelheim, the German Center for Diabetes Research (DZD e.V. - 82DZD00101) and the German Network of Diabetes Competence (01GI1102), which are financed by the German Ministry for Education and Research, and by the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 155005 (IMIDIA), the resources of which are composed of financial contributions from the European Union's Seventh Framework Program (FP7/2007-2013) and the kind contributions from EFPIA (European Federation of Pharmaceutical Industries and Associations) companies.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.05.015.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Figure S1: Villin expression is reduced in Ica512-depleted INS-1 and MIN6 cells. (A) Quantification of villin expression in extracts from islets isolated from Ica512+/+ or Ica512−/− mice as illustrated in Figure 1B. (B) Immunoblotting for villin and γ-tubulin in extracts of INS-1 and MIN6 cells. (C) Efficiency of Ica512 silencing in MIN6 cells treated with control or Ica512 siRNA oligonucleotides as illustrated in Figure 1C. (D) Immunoblotting for Ica512, villin, and γ-tubulin in extracts of INS-1 cells treated with control or Ica512 siRNA oligonucleotides. (E) Quantification of Ica512 expression as in D. (F,G) Densitometric analysis of villin content following the silencing of Ica512 in MIN6 (F) or in INS-1 (G) cells. The quantifications were performed from at least 3 immunoblots.
Figure S2: Villin immunoreactivity in INS-1 cells and mouse islets. (A) Confocal images for villin (red) and insulin (green) in INS-1 cells treated with control or villin siRNA oligonucleotides at rest (2.8 mM glucose) or after stimulation with 25 mM glucose and 55 KCl for 1.5 h. The nuclei were stained with DAPI (blue). (B) Confocal images of mouse pancreatic cryosections from villin+/+ and villin−/− mice co-stained for villin (red) and insulin (green). The nuclei were stained with DAPI (blue). (C) Immunblotting for villin and and γ-tubulin in extracts of INS-1 cells treated with control or villin siRNA oligonucleotides.
Figure S3: Glucose tolerance of 12–14-week-old villin−/− mice. (A–C) Oral (A), intraperitoneal (B), and intravenous (C) glucose tolerance tests in 12–14-week-old villin+/+ and villin−/− mice.
Figure S4: Insulin content and secretion in villin-depleted mouse islets and insulinoma cells. (A,B) Insulin content and basal insulin secretion in islets isolated from villin+/+ and villin−/− mice. (C) Insulin content in islets isolated from villin+/+ and villin−/− mice and treated with control or villin siRNA oligonucleotides. (D) Immunoblotting for Ica512 in MIN6 cells treated with control or villin siRNA oligonucleotides. (E) Insulin secretion in resting or glucose-stimulated MIN6 cells treated with control or villin siRNA oligonucleotides. (F,G) Insulin content (F) and stimulation index (G) in INS-1 cells treated with control or villin siRNA oligonucleotides. (H) Basal or stimulated insulin secretion in INS-1 cells treated with control or villin siRNA oligonucleotides.
Figure S5: Plastin expression in villin−/− islets and villin-depleted insulinoma cells. (A–C) Immunoblotting for villin, plastin, and γ-tubulin using extracts of islets isolated from villin+/+ and villin−/− mice (A) or from MIN6 cells (B) and INS-1 cells (C) treated with control or villin siRNA oligonucleotides.
Figure S6: Mean track displacement in villin-depleted MIN6 cells. (A,B) Track maximum displacement of SGs (A) and mean track number/movie (B) in resting or glucose-stimulated MIN6 cells treated with control or villin siRNA oligonucleotides.
References
- 1.Sando H., Borg J., Steiner D.F. Studies on the secretion of newly synthesized proinsulin and insulin from isolated rat islets of Langerhan. Journal of Clinical Investigation. 1972;51:1476–1485. doi: 10.1172/JCI106944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoboth P., Müller A., Ivanova A., Mziaut H., Dehghany J., Sönmez A. Aged insulin granules display reduced microtubule-dependent mobility and are disposed within actin-positive multigranular bodies. Proceedings of the National Academy of Sciences United States of America. 2015;112:667–676. doi: 10.1073/pnas.1409542112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lelkes P.I., Friedman J.E., Rosenheck K., Oplatka A. Destabilization of actin filaments as a requirement for the secretion of catecholamines from permeabilized chromaffin cells. FEBS Letters. 1986;208:357–363. doi: 10.1016/0014-5793(86)81049-3. [DOI] [PubMed] [Google Scholar]
- 4.Muallem S., Kwiatkowska K., Xu X., Yin H.L. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. Journal of Cell Biology. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borovikov Y.S., Norman J.C., Price L.S., Weeds A., Koffer A. Secretion from permeabilised mast cells is enhanced by addition of gelsolin: contrasting effects of endogenous gelsolin. Journal of Cell Science. 1995;108:657–666. doi: 10.1242/jcs.108.2.657. [DOI] [PubMed] [Google Scholar]
- 6.Henquin J.C., Mourad N.I., Nenquin M. Disruption and stabilization of β-cell actin microfilaments differently influence insulin secretion triggered by intracellular Ca2+ mobilization or store-operated Ca2+ FEBS Letters. 2012;586:89–95. doi: 10.1016/j.febslet.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Castellino F., Heuser J., Marchetti S., Bruno B., Luini A. Glucocorticoid stabilization of actin filaments: a possible mechanism for inhibition of corticotropin release. Proceedings of the National Academy of Sciences United States of America. 1992;89:3775–3779. doi: 10.1073/pnas.89.9.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ort T., Voronov S., Guo J., Zawalich K., Froehner S.C., Zawalich W. Dephosphorylation of beta2-syntrophin and Ca2+/mu-calpain-mediated cleavage of Ica512 upon stimulation of insulin secretion. EMBO Journal. 2001;20:4013–4023. doi: 10.1093/emboj/20.15.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trajkovski M., Mziaut H., Schubert S., Kalaidzidis Y., Altkrüger A., Solimena M. Regulation of insulin granule turnover in pancreatic beta-cells by cleaved ICA512. Journal of Biological Chemistry. 2008;283:33719–33729. doi: 10.1074/jbc.M804928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schubert S., Knoch K.P., Ouwendijk J., Mohammed S., Bodrov Y., Jäger M. β2-Syntrophin is a Cdk5 substrate that restrains the motility of insulin secretory granules. PLoS One. 2010;5:e12929. doi: 10.1371/journal.pone.0012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeki K., Zhu M., Kubosaki A., Xie J., Lan M.S., Notkins A.L. Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes. 2002;51:1842–1850. doi: 10.2337/diabetes.51.6.1842. [DOI] [PubMed] [Google Scholar]
- 12.Kubosaki A., Gross S., Miura J., Saeki K., Zhu M., Nakamura S. Targeted disruption of the IA-2beta gene causes glucose intolerance and impairs insulin secretion but does not prevent the development of diabetes in NOD mice. Diabetes. 2004;53:1684–1691. doi: 10.2337/diabetes.53.7.1684. [DOI] [PubMed] [Google Scholar]
- 13.Mziaut H., Trajkovski M., Kersting S., Ehninger A., Altkrüger A., Lemaitre R.P. Synergy of glucose and growth hormone signalling in islet cells through ICA512 and STAT5. Nature Cell Biology. 2006;8:435–445. doi: 10.1038/ncb1395. [DOI] [PubMed] [Google Scholar]
- 14.Ferrary E., Cohen-Tannoudji M., Pehau-Arnaudet G., Lapillonne A., Athman R., Ruiz T. In vivo, villin is required for Ca(2+)-dependent F-actin disruption in intestinal brush borders. Journal of Cell Biology. 1999;146:819–830. doi: 10.1083/jcb.146.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazaki J., Araki K., Yamato E., Ikegami H., Asano T., Shibasaki Y. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 16.Asfari M., Janjic D., Meda P., Li G., Halban P.A., Wollheim C.B. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 17.Behrendt R., Schumann T., Gerbaulet A., Nguyen L.A., Schubert N., Alexopoulou D. Mouse SAMHD1 has antiretroviral activity and suppresses a spontaneous cell-intrinsic antiviral response. Cell Reports. 2013;4:689–696. doi: 10.1016/j.celrep.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters D., Luo X., Qiu K., Liang P. Speeding up large-scale next generation sequencing data analysis with pBWA. Journal of Biocomputing. 2012;1:1. [Google Scholar]
- 19.Anders S., Huber W. Differential expression analysis for sequence count data. Genome Biology. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B. 1995;57:289–300. [Google Scholar]
- 21.Trajkovski M., Mziaut H., Altkrüger A., Ouwendijk J., Knoch K.P., Müller S. Nuclear translocation of an ICA512 cytosolic fragment couples granule exocytosis and insulin expression in {beta}-cells. Journal of Cell Biology. 2004;167:1063–1074. doi: 10.1083/jcb.200408172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanova A., Kalaidzidis Y., Dirkx R., Sarov M., Gerlach M., Schroth-Diez B. Age-dependent labeling and imaging of insulin secretory granules. Diabetes. 2013;62:3687–3696. doi: 10.2337/db12-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 24.Knoch K.P., Bergert H., Borgonovo B., Saeger H.D., Altkrüger A., Verkade P. Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nature Cell Biology. 2004;6:207–214. doi: 10.1038/ncb1099. [DOI] [PubMed] [Google Scholar]
- 25.Hermel J.M., Dirkx R., Jr., Solimena M. Post-translational modifications of ICA512, a receptor tyrosine phosphatase-like protein of secretory granules. European Journal of Neuroscience. 1999;11:2609–2620. doi: 10.1046/j.1460-9568.1999.00677.x. [DOI] [PubMed] [Google Scholar]
- 26.Mziaut H., Kersting S., Knoch K.P., Fan W.H., Trajkovski M., Erdmann K. ICA512 signaling enhances pancreatic beta-cell proliferation by regulating cyclins D through STATs. Proceedings of the National Academy of Sciences United States of America. 2008;105:674–679. doi: 10.1073/pnas.0710931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solimena M., Dirkx R., Jr., Hermel J.M., Pleasic-Williams S., Shapiro J.A., Caron L. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granule. EMBO Journal. 1996;15:2102–2114. [PMC free article] [PubMed] [Google Scholar]
- 28.Giner D., López I., Villanueva J., Torres V., Viniegra S., Gutiérrez L.M. Vesicle movements are governed by the size and dynamics of F-actin cytoskeletal structures in bovine chromaffin cells. Neuroscience. 2007;146:659–669. doi: 10.1016/j.neuroscience.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Wong I.Y., Gardel M.L., Reichman D.R., Weeks E.R., Valentine M.T., Bausch A.R. Anomalous diffusion probes microstructure dynamics of entangled F-actin networks. Physical Reviews Letters. 2004;92:178101–178104. doi: 10.1103/PhysRevLett.92.178101. [DOI] [PubMed] [Google Scholar]
- 30.Fava E., Dehghany J., Ouwendijk J., Müller A., Niederlein A., Verkade P. Novel standards in the measurement of rat insulin granules combining electron microscopy, high-content image analysis and in silico modelling. Diabetologia. 2012;55:1013–1023. doi: 10.1007/s00125-011-2438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otto O., Rosendahl P., Mietke A., Golfier S., Herold C., Klaue D. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nature Methods. 2015;12:199–202. doi: 10.1038/nmeth.3281. [DOI] [PubMed] [Google Scholar]
- 32.Press W.H., Teukolsky S.A., Vetterling W.T., Flannery B.P. Cambridge University Press; 2002. Numerical recipes in C. The art of scientific computing. chapter 6.4 (pp. 226–229) and chapter 14 (pp.609–655) [Google Scholar]
- 33.Khurana S., George S.P. Regulation of cell structure and function by actin-binding proteins: villin's perspective. FEBS Letters. 2008;582:2128–2139. doi: 10.1016/j.febslet.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm-Günter E.M.S., Revenu S., Ramos S., Hurbain I., Smyth N., Ferrary E. Plastin 1 binds to keratin and is required for terminal web assembly in the intestinal. Molecular Biology of the Cell. 2009;20:2549–2562. doi: 10.1091/mbc.E08-10-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Revenu C., Ubelmann F., Hurbain I., El-Marjou F., Dingli F., Loew D. A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Molecular Biology of the Cell. 2012;23:324–336. doi: 10.1091/mbc.E11-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orci L., Thorens B., Ravazzola M., Lodish H.F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989;245:295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- 37.Waanders L.F., Chwalek K., Monetti M., Kumar C., Lammert E., Mann M. Quantitative proteomic analysis of single pancreatic islets. Proceedings of the National Academy of Sciences United States of America. 2009;106:18902–18907. doi: 10.1073/pnas.0908351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hesterberg L.K., Weber K. Demonstration of three distinct calcium-binding sites in villin, a modulator of actin assembly. Journal of Biological Chemistry. 1983;258:365–369. [PubMed] [Google Scholar]
- 39.Ubelmann F., Chamaillard M., El-Marjou F., Simon A., Netter J., Vignjevic D. Enterocyte loss of polarity and gut wound healing rely upon the F-actin-severing function of villin. PNAS. 2013;110:1380–1389. doi: 10.1073/pnas.1218446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lhocine N., Arena E.T., Bomme P., Ubelmann F., Prévost M.C., Robine S. Apical invasion of intestinal epithelial cells by Salmonella typhimurium requires villin to remodel the brush border actin cytoskeleton. Cell Host Microbe. 2015;17:164–177. doi: 10.1016/j.chom.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomas A., Yermen B., Min L., Pessin J.E., Halban P.A. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. Journal of Cell Science. 2006;119:2156–2167. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- 42.Kalwat M.A., Wiseman D.A., Luo W., Wang Z., Thurmond D.C. Gelsolin associates with the N terminus of syntaxin 4 to regulate insulin granule exocytosis. Molecular Endocrinology. 2012;26:128–141. doi: 10.1210/me.2011-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yermen B., Tomas A., Halban P.A. Pro-survival role of gelsolin in mouse beta-cells. Diabetes. 2007;56:80–87. doi: 10.2337/db06-0769. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L., Marcu M.G., Nau-Staudt K., Trifaró J.M. Histamine-evoked chromaffin cell scinderin redistribution, F-actin disassembly, and secretion: in the absence of cortical F-actin disassembly, an increase in intracellular Ca2+ fails to trigger exocytosis. Journal of Neurochemistry. 1995;65:1297–1308. doi: 10.1046/j.1471-4159.1995.65031297.x. [DOI] [PubMed] [Google Scholar]
- 45.Degtyar V.E., Allersma M.W., Axelrod D., Holz R.W. Increased motion and travel, rather than stable docking, characterize the last moments before secretory granule fusion. Proceedings of the National Academy of Sciences United States of America. 2007;104:15929–15934. doi: 10.1073/pnas.0705406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holz R.W., Axelrod D. Secretory granule behaviour adjacent to the plasma membrane before and during exocytosis: total internal reflection fluorescence microscopy studies. Acta Physiologica (Oxford) 2008;192:303–307. doi: 10.1111/j.1748-1716.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 47.Torregrosa-Hetland C.J., Villanueva J., López-Font I., Garcia-Martinez V., Gil A., Gonzalez Vélez V. Association of SNAREs and calcium channels with the borders of cytoskeletal cages organizes the secretory machinery in chromaffin cells. Molecular and Cellular Neurobiology. 2010;30:1315–1319. doi: 10.1007/s10571-010-9565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y.F., Zhang H.L., Cai T., Harashima S., Notkins A.L. The IA-2 interactome. Diabetologia. 2005;48:2576–2581. doi: 10.1007/s00125-005-0037-y. [DOI] [PubMed] [Google Scholar]
- 49.Henquin J.C., Nenquin M., Szollosi A., Kubosaki A., Notkins A.L. Insulin secretion in islets from mice with a double knockout for the dense core vesicle proteins islet antigen-2 (IA-2) and IA-2beta. Journal of Endocrinology. 2008;196:573–581. doi: 10.1677/JOE-07-0496. [DOI] [PubMed] [Google Scholar]
- 50.Mishra J., Verma R.K., Alpini G., Meng F., Kumar N. Role of Janus kinase 3 in mucosal differentiation and predisposition to colitis. Journal of Biological Chemistry. 2013;288:31795–31806. doi: 10.1074/jbc.M113.504126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.