Abstract
Background
Despite recent advances the pathogenesis of Crohn's disease remains incompletely understood. A variety of animal models have been utilized in an effort to provide further insights and develop more therapeutic options. In order to simulate, to an extent, the pathogenesis and the clinical course of the disease, TNBS induced colitis is often used. Various approaches for inducing TNBS -colitis have been described in the literature.
Methods/results
In this review, we sought to present the animal model of TNBS induced colitis and outline the pathogenesis, pathophysiology, clinical course and pathological characteristics of the model. Furthermore, we describe the differences among those protocols regarding types of animals and colitis induction.
Data sources
The MEDLINE database was thoroughly searched using the keywords: TNBS, colitis, Crohn's disease, animal model. Two investigators independently reviewed the abstracts and appropriate articles were included in this review. Additional articles were gathered and evaluated.
Conclusion
The aim of this study was to thoroughly present an updated review of the TNBS-induced colitis protocols that are implemented by researchers.
Keywords: TNBS, Colitis, Crohn's disease, Animal model
Highlights
-
•
We sought to present the animal model of TNBS induced colitis and outline the pathogenesis, pathophysiology, clinical course and pathological characteristics of the model.
-
•
Furthermore, we describe the differences among those protocols regarding types of animals and colitis induction.
-
•
The MEDLINE database was thoroughly searched using the keywords: TNBS, colitis, Crohn's disease, animal model.
-
•
Two investigators independently reviewed the abstracts and appropriate articles were included in this review.
-
•
The aim of this study was to thoroughly present an updated review of the TNBS-induced colitis protocols that are implemented by researchers.
1. Introduction
Crohn's disease is a chronic, relapsing immunologic disorder that primarily affects the gastrointestinal tract [1] The estimated incidence of the disease approximates 20.2 cases per 100.000 persons in Northern Europe and USA and it should be emphasized that incidence depends on the exact geographic region. In the modern era, intense research has provided insight on the pathogenesis of the disease and the current understanding relates Crohn's to a dysregulated immune response towards gut microbiota in genetically predisposed persons. Despite recent advances, the exact pathogenesis remains not well defined. Thus, Crohn's disease is still an incurable, life-long disease that warrants better understanding and more efficient treatment.
While clinical research findings are more easily extrapolated and integrated to clinical practice, basic research and especially suitable animal models have provided valuable insights to the molecular level and have allowed researchers to manipulate genetic factors in order to study their role. However, conventional animal models that are not so sophisticated, like the modern genetically engineered rodents that develop spontaneous disease, seem to still carry a significant role in studying the pathways that lead to overt Crohn's disease. Among them, hapten reagent 2,4,6-trinitrobenzene sulfonic acid (TNBS) induced colitis introduced in 1989 by Morris et al. bears a pivotal role especially in the pre-clinical testing of various chemical or natural compounds in terms of their anti-inflammatory and/or anti-oxidative effects. Briefly, TNBS colitis belongs to the group of chemically induced colitis animal models that includes among others DSS colitis, and oxazolone colitis [2], [3], [4], [5]. Those three are the most commonly utilized animal models of that category and have shown a significant consistency that reflects to their extensive use during the last decades.
Since the first report of the model in 1989, a plethora of variations and modifications in technical aspects of the main protocol have been described. Our aim was to search meticulously, identify and present all variations of TNBS-induced colitis and further discuss their impact. This review could be of significant benefit for the researcher who plans to perform an experiment involving TNBS–induced colitis as well as for the scientist who needs a critical appraisal of the different protocols utilized in literature.
1.1. Pathogenesis
The exact mechanisms that mediate pathogenesis in that particular model remain elusive. As it has already been emphasized TNBS colitis does not recapitulate the aetiopathogenesis of Crohn's disease. However, the relevance of the model to Crohn's disease in terms of pathogenesis is evident by the involvement of NOD2 (a key CD susceptibility gene) in the pathogenesis of TNBS colitis [6]. Specifically, the administration of plasmid carrying intact NOD2, but not plasmid carrying associated frame-shift-mutated NOD2, makes mice more resistant to this colitis [7].
1.2. Pathophysiology of TNBS-induced colitis
Based on the original report by Morris et al. [8] ethanol and TNBS (Trinitrobenzenesulfonic acid) at a dose of 100 mg/kg are co-administered intra-rectally to rats. Ethanol is used as a means to effectively disrupt intestinal barrier and enable the interaction of TNBS with colon tissue proteins [9], [10]. Trinitrobenzenesulfonic acid, a classical skin contactant serves as a hapten and as Little et al. suggested as early as 1966 [11], when coupled with proteins with high molecular weight can elicit significant immunologic responses by rendering those proteins immunogenic to the host immune system. A single administration of the combined substances leads to the development of an excessive cell mediated immune response reflected by acute Th1 inflammation [12].
The phenotype of Th1 inflammation includes a dense colonic tissue infiltration by CD4 T cells and the secretion of various potent pro-inflammatory cytokines [13]. The most characteristic cytokines in that network include TNF-a and IL-12 [14]. The clinical importance of anti-IL-12p40 mAbs in the treatment of colitis was suggested for the first time in 1995 after implementing a TNBS colitis model [9]. That particular study triggered physicians almost 10 years later to conduct a human trial of anti-IL-12p40 mAb therapy in patients with active disease [15]. Interestingly, while colitis developed in the TNBS model has been shown to be mediated and exacerbated by IL-12p70, this is not the case in other IBD models [16]. Likewise, while IL-23 receptor (IL-23R) is known as a major IBD susceptibility gene [17], [18] in the TNBS model the same receptor seems to exert a protective role [19].
Alternatively, IL-23 serves as the key factor of maintaining Th17 cells and inducing IL-17-producing innate lymphoid cells [20]. IL-17 receptor KO mice are shown to resist against TNBS colitis. Recent findings point towards a prominent role of Th17 cells in the pathogenesis of TNBS-induced colitis. Specifically, absence of the IL-17 receptor renders mice resistant to trinitrobenzene sulfonic acid (TNBS)-induced acute colitis. In an acute trinitrobenzenesulfonic acid model of colitis, Zhang et al. [4] showed that IL-17 protein levels are increased in wild-type mice treated with TNBS. Il-17R(−/−) mice that were treated with TNBS developed only mild inflammation with less infiltration from neutrophils, and a reduction in the amount of weight loss compared to wildtype mice. Measurement of IL-6 protein levels revealed a reduction in the amount of IL-6 produced in Il-17R(−/−) mice substantiating the lack of IL-17 signaling. These data reflect the importance of IL-17 signaling in the development of acute colitis in mice treated with TNBS. In the same molecular context, IL-23 a cytokine that is secreted by Th17 as well as mucosal T follicular helper cells (Tfh) seems to exert a significant protective role. In the histopathological level, the prominent feature of TNBS-colitis includes the development of a transmural inflammation that closely resembles the histopathological lesions that develop in human Crohn's disease.
1.3. Clinical course of TNBS-colitis
After the colitis induction the animals develop several manifestations of acute colitis. Those include inconsistent stool formation and occult or even bloody diarrhea. Intracolonic administration of TNBS/ethanol to rats induces a severe illness characterized by bloody diarrhea and a dramatic loss of body weight during the first week. Then the body weight increases but diarrhea still persists for about two weeks. Introduction of TNBS, for example, led to significant weight loss (mean 10% loss in body weight) and development of liquid bloody stools in all exposed animals, whereas control (saline) animals remained healthy and gained weight (mean 30% gain over 12 days). Body weight loss is caused in part by the marked effects of TNBS itself on the gut (diarrhea and perhaps reduced fluid absorption additionally), but systemic inflammatory response may also play a role. Further non-specific signs that point to a general deterioration include piloerection of fur and decreased movements of the animals. The onset and severity of the aforementioned symptoms is variable and depends on the specific animal species, strain and the dosing of ethanol and TNBS that are administered. The significance of assessing those symptoms is reflected by the development of multiple scores that quantify acute intestinal inflammation.
Among them, the DAI score has a prominent role in assessing the magnitude of inflammation. In a recent study, rats that were treated with TNBS showed a significant rapid deterioration in DAI score on day 2. During the time course, DAI progressively deteriorated, which evidently was terminal. In the same study intestinal symptoms consisted of a palpable abdominal mass, which was evident in 7 of 11 control animals. In all groups body weight deteriorated until day 3, after which weight began to increase, returning to baseline levels in all but TNBS-control animals. Such weight regain is artifactual because of the clear bowel obstruction evident in this group. Mortality in the TNBS-control group reached 55% by day 8.
1.4. Induction of TNBS-colitis
Before the induction of experimental colitis, a short fasting precedes. The duration ranges from 12 to 24 h. Subsequently, mild anesthesia is induced by means such as ether, sevoflurane and halothane. The next step includes administering TNBS dissolved in ethanol per rectum to induce the experimental colitis. TNBS is instilled by a suitable medical-grade polyurethane catheter for enteral feeding approximately 4–8 cm proximal to anal verge. After that, the rodents are maintained for some minutes at a head down position in order to avoid expulsion of the fluid and ensure even distribution of the chemical. Dosing of TNBS and ethanol is subject to many variations as evident in the literature. Specifically, a range of 50–150 mg/kg has been utilized by various researchers. Morris et al. who established the model used a dose of 100 mg/kg.
Colons of mice receiving exclusively ethanol showed a normal macroscopic appearance, confirmed by a histological score of 0. A TNBS dose of 50 mg/kg body weight resulted in a very low level of colitis (“histological colitis”), associated with a mean Wallace score of about 2, while a dose of 150 mg/kg body weight induced a 20–30% mortality rate, associated with high to very high levels of colitis (minimum Wallace score of 5). The mean macroscopic scores did not show marked differences in the range of 100–150 mg TNBS/kg. Death resulted mainly from an excessive inflammatory reaction, as assessed by postmortem autopsy. For a TNBS dose varying from 100 to 150 mg/kg, with 10 mice per group, the reproducibility of the mean Wallace score as well as the spread per experimental group appeared to be quite stable, with an average score of 3.6 ± 0.6, ranging from 2.8 to 4.9 (for 26 experiments). In experiments with the standard dose of 100 mg/kg, more than 90% of the mice showed a slight thickening of the colon, with focal hyperemia and intestinal ulcerations (Wallace score ≥3) in approximately 65% of the animals. Consequently, in each experiment, a small proportion of mice can be expected to display low levels of inflammation, while another small number of animals will show “close-to-death-scores” or will die during the 48 h following TNBS administration. This observation leads us to recommend the use of a minimum of 10 mice per group. This was confirmed by a power calculation value of 80%, meaning that a “significant” decrease of 30% of a mean Wallace score of 4, with a standard deviation (SD) of around 1.2, will require at least 10 mice per group in order to guarantee that this effect is detected in 80% of cases with a P value of 0.05.
The difference in responsiveness has been observed before in this model as well as in many other animal models, i.e., TNBS in rats, DSS in mice, and transgenic mice models, but was never thought to have a major influence on the final average score, provided that a sufficiently high number of mice was included in each group. The observed variation, even within a given experiment, could probably be attributed to differences in individual responsiveness of the mice rather than to a lack of standardization of the experimental parameters in the model. The observed distribution of the scores of individual mice within a given experimental setup was independent of the TNBS dose administered and did not change significantly with prolonged exposure time (comparison of damage at 24, 48, and 72 h for some representative samples; data not shown). While no weight loses were generally seen with a TNBS dose of 50 mg/kg, doses varying from 100 to 150 mg/kg increased the individual body weight loss concomitantly from 10 to 20%. In order to better define the severity of colitis, linked to the TNBS dose used, we summarized the relationship among the different experimental parameters such as TNBS dose, mortality rate, weight loss, and mean Wallace score (Table 1). Despite relative overlaps and realistic ranges of values, four levels of colitis could be established, corresponding to weak, moderate, strong, or severe colitis. Table 1 can be used to facilitate interpretation of results obtained in such a model, where variation of TNBS-positive controls is inherent and should, therefore, ensure that only results of equivalent ranges of colitis are compared.
Table 1.
Animal models of TNBS induced colitis.
| Study | Mouse/Rat strain | Age of the mice-rat | TNBS dose, mg | Alcohol, % | Times administered | Day of death | Study conclusions |
|---|---|---|---|---|---|---|---|
| Zhao et al. [27] | Sprague–Dawley (SD) rats | 6–8 weeks old | 400 ll of 2.5 mg/ml TNBS | 50% | 1 | 7 d | marked decrease in both the mRNA and protein expression |
| Cury et al. [28] | Wistar rats | (240–280 g) | 25 mg TNBS (0.6 ml) | 50% | 1 | 12 d | calprotectin levels correlate with biochemical and histological markers of inflammation |
| Vermeulen et al. [29] | Wistar rats | (180–220 g) | 7.5 mg | 40% | 1 | – | TRPV1 and TRPA1 may be promising targets in pain modulation. |
| Abad et al. [30] | BALB/c mice | 6–8 weeks | 3-mg | 50% | 2 | 3–10 d | VIP has beneficial prophylactic and therapeutic effects in TNBS-induced colitis |
| Petrella et al. [31] | Wistar rats | (200–250 g) | 60 mg/kg | 1 | 4 d | Low doses of N/OFQ significantly decrease the colonic inflammatory profile | |
| Chen et al. [32] | Rats; | 0.6 mL | 50% | 1 | 1–21 d | NF-κB also regulates ICAM-1 expression during colonic inflammation. Pretreatment of PDTC may attenuate the inflammation development. | |
| Fitzpatrick et al. [33] | Wistar rats | Neonatal rats-3w. | 8 to 32 mg | 40% | 1, 3, or 4 | 23, 35, 48, and 59 d | This chronic TNBS model may be useful for studying the development of inflammation and fibrosis in preadult animals |
| Rivera et al. [34] | Sprague-Dawley rats | (220–265 g) | 30 mg | 50% | 1 | 3 d | 9 of 10 critical genes identified by DNA microarray. |
| Pohlmann et al. [35] | Wistar rats | (297 5 g; mean) | 17.5 mg | 30% | 1 | 10 d | this technique could provide useful information for the evaluation of potential new treatments for IBD |
| Charpentier et al. [36] | Sprague–Dawley rats | (200–250 g) | 25 mg | 50% | 1 | 2 d | Minimal bowel wall thickness and wall signal intensity on T2w images were associated with histological score |
| Foligne et al. | BALB/C mice | 7–8 weeks | 50 to 150 mg/kg | 50% | 1 | 2 d | The use of the model as described above allows the construction of long-term databases which can serve as a reference for future strain comparisons |
| Natah et al. [37] | Sprague–Dawley rats | (250–300 g) | 30 mg | 50% | 1 | 1, 2, 3, 7 and 21 d | colitis transiently increased permeability to sodium fluorescein and led to a mild disruption of BBB |
| Motavallian et al. | Wistar rats | (200 ± 20 g) | 10 mg | 50% | 1 | 6 d | administration of cisapride, a 5HT4 receptor agonist, does not aggravate the severity of colonic injuries in TNBS-induced colitis in rat |
| El-Salhy et al. [38] | Wistar rats | (166–246 g) | 25 mg | 50% | 1 | 3 d | colonoscopy provides a reliable method for following up |
| Lohman et al. [39] | Wistar rats | 8–9 weeks | 80 mg/kg | 50% | 1 | 8 d | The current results connect PAR2 activation to -tryptase release and mast cells in the pathogenesis of TNBS colitis |
| Yue et al. [40] | Sprague-Dawley rats | (300–350 g) | 25 mg/ml | 50% | 1 | 1 d | local elevated level of peroxynitrite is a major contributor to colon epithelial apoptosis |
| Pilichos et al. [41] | Wistar rats | 300-450 g | 60 mg/ml | 30% | 1 | 10 d | NOS inhibitors might prove to be promising therapeutic agents for IBD |
| Brenna et al. [42] | Sprague-Dawley rats | 8 weeks | 30 mg/ml | 50% | 1 | 12 d | Endoscopy with biopsies in TNBS-colitis is useful to follow temporal changes of inflammation |
1.5. Pathological characteristics of TNBS colitis
TNBS colitis invokes certain lesions in a histopathological level [21]. Those lesions can be assessed either macroscopically or microscopically. Multiple scores and assessment tools have been developed in an effort to quantify the impact of inflammation on colonic tissue and furthermore evaluate the effect of various compounds in preventing or treating those lesions. Macroscopically, inflammation is spreading in a transverse fashion and ends up to the development of transmural colitis [22]. That is a shared feature with the hallmark of human Crohn's disease. Moreover, mucosal edema, distortion of crypts and the formation of abscesses occur during the course of the disease [23], [24]. Macroscopic evaluation of the colon and rectum up to 24 h after TNBS treatment revealed the presence of mucosal edema and hemorrhagic ulcerations. Conglutination was obvious later.
Histologic examination has showed predominant infiltration of leukocytes and erythrocytes in mucosa and submucosa in the first week. Neutrophils, macrophages, lymphocytes infiltrated in mucosa, submucosa and muscularis propria 14 d later. Fibroblasts and granuloma-like structures were also obviously seen. No damage was seen in NS group. The macroscopic and microscopic damage score showed the peak at d 3, then decreased (scores in T/E group at various time points vs scores in NS group, P < 0.01.
Administration of repetitive TNBS enemas to young rats results in the appearance of cobblestone-like ulceration in the distal colon. This pattern of intestinal ulceration is commonly observed and considered characteristic of macroscopic colonic changes in patients with CD (19,20). In general, the colonic submucosa shows only limited collagen staining in the absence of TNBS administration. In contrast, following repeated administration of TNBS there is a clear and profound increase in the extent of green trichrome-stained collagen. Mice treated with TNBS in 50% ethanol developed severe bloody diarrhea and rectal prolapse accompanied by an extensive wasting disease. The histopathologic analysis during the first days after induction of colitis showed infiltration of neutrophils and macrophages into the colonic mucosa and submucosa layers. By day 3, transmural inflammation, characterized by neutrophilic infiltration, was associated with a thickening of the colon wall, ulcerations, loss of goblet cells, and fibrosis found through the colon. By day 7, massive infiltration of lymphocytes characterized the colon sections studied, which is a major sign of the chronic inflammation in the late stages of this colitis model.
To assess the severity of colonic inflammation histopathologically, HPS was calculated by two different pathologists. Ulceration, massive inflammatory cell infiltration, colon structure disorganization, goblet cell depletion, submucosa edema and extensive fibrosis were found throughout the colons in the model group, while no or very little mucosal lesions were observed in the colons from the other two groups. The CMDI and HPS in the model group were significantly higher than those in the NC and EC group, but there was no difference between the NC and EC group (P < 0.05).
CMDI was used to evaluate the gross appearance of the colon and assess the severity of intestinal injury. The colons from rats in the model group were adherent to other tissues and organs with marked hyperemia, hemorrhage, inflammation, necrosis, ulcers and bowel wall thickening, whereas the colons from the rats in the NC and EC groups showed no or only slight inflammation.
Susceptibility to TNBS varies according to different species and moreover different strains of the same species. Of note, Balb/c mice appear to develop Th2 inflammation instead of Th1 and rather represent an animal model of ulcerative colitis and not Crohn's disease.
1.6. Suitability of TNBS-induced colitis for therapeutic studies
The use of TNBS colitis has been valuable in elucidating the mechanisms that mediate disease pathogenesis and consequently has provided the scientific community with information that have profoundly enhanced our knowledge. Besides its use as a means to study the aetiopathogenesis of Crohns's disease TNBS colitis is a valid and largely inexpensive way to assess compounds with potential therapeutic effects such as anti-TNF-a, corticosteroids, natural compounds and traditional medicine.
2. Conclusion
TNBS induced colitis is a commonly utilized animal model that shares significant properties with human Crohn's disease. The advantages of its use include the reproducibility, the technical simplicity and the low cost [25]. The model is applicable in both rodents and guinea pigs with significant strain-dependent differences. Collectively, despite the introduction of genetic and spontaneous models that mimic Crohn's disease, TNBS-colitis remains a potent tool in our armamentorium to study immunopathogenesis and potential treatments of the disease. Of note, the model has several inherent limitations and does not recapitulate the disease in terms of aetiopathogenesis. Being aware of the model's limitations will enable researchers to properly use the model. More research is warranted to shed light on the exact mechanisms that underlie its pathogenesis and specifically the role of gut microbiota.
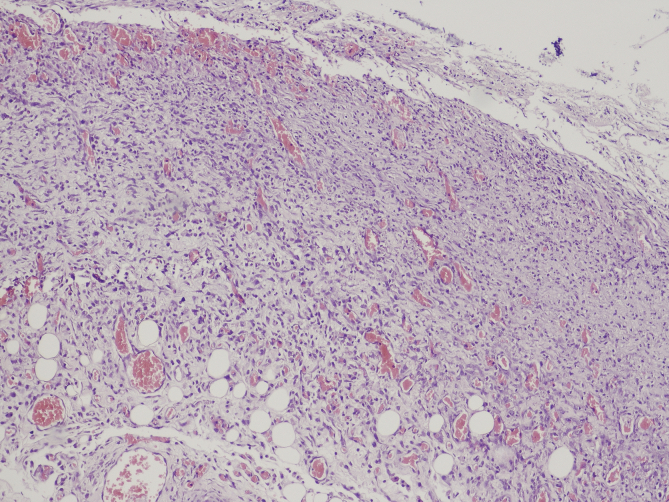
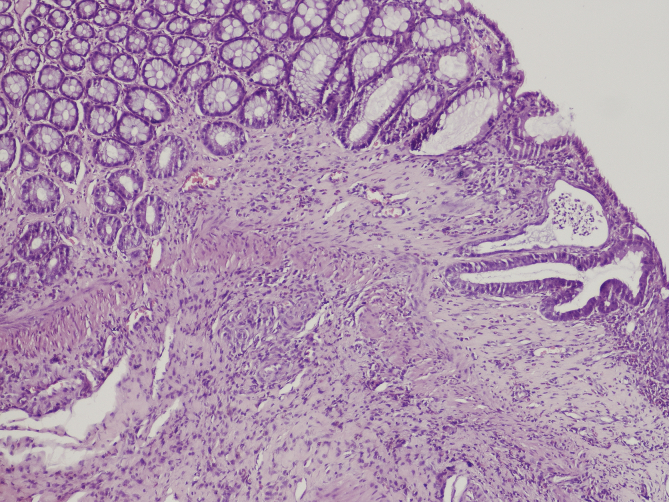
Fig. 1, Fig. 2 [26] Changes into the large bowel wall, eosin – hematoxylin stain, 100× original magnification. Severe colitis with ulceration (I) and colitis with intraluminal abscesses (II – arrow).
Fig. 1.
Severe colitis with ulceration.
Fig. 2.
Colitis with intraluminal abscesses.
Ethical approval
None.
Sources of funding
None.
Author contribution
Efstathios Antoniou: study design, data analysis, writing.
Georgios Antonios Margonis: study design, data collections, data analysis, writing.
Anastasios Angelou: study design, data collections, data analysis, writing.
Pikouli Anastasia: data collections.
Paraskevi Argiri: data collections, data analysis.
Ioannis Karavokyros: data analysis, writing.
Apostolos Papalois: data analysis.
Emmanouil Pikoulis: study design, data analysis, writing.
Conflicts of interest
None.
Trial registry number
None.
Guarantor
Emmanouil Pikoulis.
References
- 1.Baumgart D.C., Sandborn W.J. Crohn's disease. Lancet. 2012;380(9853):1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [PMID: 22914295] [DOI] [PubMed] [Google Scholar]
- 2.Fuss I.J., Heller F., Boirivant M., Leon F., Yoshida M., Fichtner-Feigl S., Yang Z., Exley M., Kitani A., Blumberg R.S., Mannon P., Strober W. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Investig. 2004;113(10):1490–1497. doi: 10.1172/JCI19836. [PMID: 15146247 PMCID: 406524] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncada D.M., Kammanadiminti S.J., Chadee K. Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol. 2003;19(7):305–311. doi: 10.1016/s1471-4922(03)00122-3. [PMID: 12855381] [DOI] [PubMed] [Google Scholar]
- 4.Zhang H.Q., Ding T.T., Zhao J.S., Yang X., Zhang H.X., Zhang J.J., Cui Y.L. Therapeutic effects of Clostridium butyricum on experimental colitis induced by oxazolone in rats. World J. Gastroenterol. WJG. 2009;15(15):1821–1828. doi: 10.3748/wjg.15.1821. [PMID: 19370778 PMCID: 2670408] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iseri S.O., Ersoy Y., Ercan F., Yuksel M., Atukeren P., Gumustas K., Alican I. The effect of sildenafil, a phosphodiesterase-5 inhibitor, on acetic acid-induced colonic inflammation in the rat. J. Gastroenterol. Hepatol. 2009;24(6):1142–1148. doi: 10.1111/j.1440-1746.2009.05797.x. [PMID: 19638092] [DOI] [PubMed] [Google Scholar]
- 6.Watanabe T., Asano N., Murray P.J., Ozato K., Tailor P., Fuss I.J., Kitani A., Strober W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J. Clin. Investig. 2008;118(2):545–559. doi: 10.1172/JCI33145. [PMID: 18188453 PMCID: 2176188] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z., Fuss I.J., Watanabe T., Asano N., Davey M.P., Rosenbaum J.T., Strober W., Kitani A. NOD2 transgenic mice exhibit enhanced MDP-mediated down-regulation of TLR2 responses and resistance to colitis induction. Gastroenterology. 2007;133(5):1510–1521. doi: 10.1053/j.gastro.2007.07.025. [PMID: 17915219 PMCID: 2134971] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris G.P., Beck P.L., Herridge M.S., Depew W.T., Szewczuk M.R., Wallace J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. [PMID: 2914642] [PubMed] [Google Scholar]
- 9.Neurath M.F., Fuss I., Kelsall B.L., Stuber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [PMID: 7595199 PMCID: 2192205] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda M., Takeshima F., Isomoto H., Shikuwa S., Mizuta Y., Ozono Y., Kohno S. Simvastatin attenuates trinitrobenzene sulfonic acid-induced colitis, but not oxazalone-induced colitis. Dig. Dis. Sci. 2008;53(7):1869–1875. doi: 10.1007/s10620-007-0102-0. [PMID: 18049901] [DOI] [PubMed] [Google Scholar]
- 11.Little J.R., Eisen H.N. Preparation and characterization of antibodies specific for the 2,4,6-trinitrophenyl group. Biochemistry. 1966;5(11):3385–3395. doi: 10.1021/bi00875a001. [PMID: 4165954] [DOI] [PubMed] [Google Scholar]
- 12.Elson C.O., Sartor R.B., Tennyson G.S., Riddell R.H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [PMID: 7557106] [DOI] [PubMed] [Google Scholar]
- 13.da Silva M.S., Sanchez-Fidalgo S., Talero E., Cardeno A., da Silva M.A., Villegas W., Souza Brito A.R., de La Lastra C.A. Anti-inflammatory intestinal activity of Abarema cochliacarpos (Gomes) Barneby & Grimes in TNBS colitis model. J. Ethnopharmacol. 2010;128(2):467–475. doi: 10.1016/j.jep.2010.01.024. [PMID: 20083187] [DOI] [PubMed] [Google Scholar]
- 14.Takagi T., Naito Y., Mizushima K., Akagiri S., Suzuki T., Hirata I., Omatsu T., Handa O., Kokura S., Ichikawa H., Yoshikawa T. Inhalation of carbon monoxide ameliorates TNBS-induced colitis in mice through the inhibition of TNF-alpha expression. Dig. Dis. Sci. 2010;55(10):2797–2804. doi: 10.1007/s10620-009-1112-x. [PMID: 20094779] [DOI] [PubMed] [Google Scholar]
- 15.Mannon P.J., Fuss I.J., Mayer L., Elson C.O., Sandborn W.J., Present D., Dolin B., Goodman N., Groden C., Hornung R.L., Quezado M., Yang Z., Neurath M.F., Salfeld J., Veldman G.M., Schwertschlag U., Strober W. Anti ILCsDSG. Anti-interleukin-12 antibody for active Crohn's disease. N. Engl. J. Med. 2004;351(20):2069–2079. doi: 10.1056/NEJMoa033402. [PMID: 15537905] [DOI] [PubMed] [Google Scholar]
- 16.Camoglio L., te Velde A.A., de Boer A., ten Kate F.J., Kopf M., van Deventer S.J. Hapten-induced colitis associated with maintained Th1 and inflammatory responses in IFN-gamma receptor-deficient mice. Eur. J. Immunol. 2000;30(5):1486–1495. doi: 10.1002/(SICI)1521-4141(200005)30:5<1486::AID-IMMU1486>3.0.CO;2-8. [PMID: 10820397] [DOI] [PubMed] [Google Scholar]
- 17.Lees C.W., Barrett J.C., Parkes M., Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–1753. doi: 10.1136/gut.2009.199679. [PMID: 21300624] [DOI] [PubMed] [Google Scholar]
- 18.Khor B., Gardet A., Xavier R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–317. doi: 10.1038/nature10209. [PMID: 21677747 PMCID: 3204665] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker C., Dornhoff H., Neufert C., Fantini M.C., Wirtz S., Huebner S., Nikolaev A., Lehr H.A., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Galle P.R., Karow M., Neurath M.F. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J. Immunol. 2006;177(5):2760–2764. doi: 10.4049/jimmunol.177.5.2760. [PMID: 16920909] [DOI] [PubMed] [Google Scholar]
- 20.Buonocore S., Ahern P.P., Uhlig H.H., Ivanov I.I., Littman D.R., Maloy K.J., Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [PMID: 20393462 PMCID: 3796764] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Almeida A.B., Sanchez-Hidalgo M., Martin A.R., Luiz-Ferreira A., Trigo J.R., Vilegas W., dos Santos L.C., Souza-Brito A.R., de la Lastra C.A. Anti-inflammatory intestinal activity of Arctium lappa L. (Asteraceae) in TNBS colitis model. J. Ethnopharmacol. 2013;146(1):300–310. doi: 10.1016/j.jep.2012.12.048. [PMID: 23313393] [DOI] [PubMed] [Google Scholar]
- 22.Isik F., Tunali Akbay T., Yarat A., Genc Z., Pisiriciler R., Caliskan-Ak E., Cetinel S., Altintas A., Sener G. Protective effects of black cumin (Nigella sativa) oil on TNBS-induced experimental colitis in rats. Dig. Dis. Sci. 2011;56(3):721–730. doi: 10.1007/s10620-010-1333-z. [PMID: 20658190] [DOI] [PubMed] [Google Scholar]
- 23.Cheon G.J., Cui Y., Yeon D.S., Kwon S.C., Park B.G. Mechanisms of motility change on trinitrobenzenesulfonic Acid-induced colonic inflammation in mice. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2012;16(6):437–446. doi: 10.4196/kjpp.2012.16.6.437. [PMID: 23269907 PMCID: 3526749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin H.Y., Wu J.C., Tong X.D., Sung J.J., Xu H.X., Bian Z.X. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J. Gastroenterol. 2011;46(2):164–174. doi: 10.1007/s00535-010-0321-6. [PMID: 20848144] [DOI] [PubMed] [Google Scholar]
- 25.Randhawa P.K., Singh K., Singh N., Jaggi A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014;18(4):279–288. doi: 10.4196/kjpp.2014.18.4.279. [PMID: 25177159 PMCID: 4146629] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margonis G.A., Christoloukas N., Antoniou E., Arkadopoulos N., Theodoropoulos G., Agrogiannis G., Pikoulis E., Patsouris E.S., Zografos G.C., Papalois A.E. Effectiveness of sildenafil and U-74389G in a rat model of colitis. J. Surg. Res. 2015;193(2):667–674. doi: 10.1016/j.jss.2014.08.064. [PMID: 25277360] [DOI] [PubMed] [Google Scholar]
- 27.Zhao G., Li J., Wang J., Shen X., Sun J. Aquaporin 3 and 8 are down-regulated in TNBS-induced rat colitis. Biochem. Biophys. Res. Commun. 2014;443(1):161–166. doi: 10.1016/j.bbrc.2013.11.067. [PMID: 24286754] [DOI] [PubMed] [Google Scholar]
- 28.Cury D.B., Mizsputen S.J., Versolato C., Miiji L.O., Pereira E., Delboni M.A., Schor N., Moss A.C. Serum calprotectin levels correlate with biochemical and histological markers of disease activity in TNBS colitis. Cell. Immunol. 2013;282(1):66–70. doi: 10.1016/j.cellimm.2013.04.004. [PMID: 23685388 PMCID: 4080304] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermeulen W., De Man J.G., De Schepper H.U., Bult H., Moreels T.G., Pelckmans P.A., De Winter B.Y. Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur. J. Pharmacol. 2013;698(1–3):404–412. doi: 10.1016/j.ejphar.2012.10.014. [PMID: 23099257] [DOI] [PubMed] [Google Scholar]
- 30.Abad C., Martinez C., Juarranz M.G., Arranz A., Leceta J., Delgado M., Gomariz R.P. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124(4):961–971. doi: 10.1053/gast.2003.50141. [PMID: 12671893] [DOI] [PubMed] [Google Scholar]
- 31.Petrella C., Giuli C., Broccardo M., Eutamene H., Cartier C., Leveque M., Bedini A., Spampinato S., Bueno L., Theodorou V., Improta G., Agostini S. Protective and worsening peripheral nociceptin/orphanin FQ receptor-mediated effect in a rat model of experimental colitis. Pharmacol. Res. Off. J. Italian Pharmacol. Soc. 2013;70(1):72–79. doi: 10.1016/j.phrs.2013.01.004. [PMID: 23353033] [DOI] [PubMed] [Google Scholar]
- 32.Chen K., Long Y.M., Wang H., Lan L., Lin Z.H. Activation of nuclear factor-kappa B and effects of pyrrolidine dithiocarbamate on TNBS-induced rat colitis. World J. Gastroenterol. WJG. 2005;11(10):1508–1514. doi: 10.3748/wjg.v11.i10.1508. [PMID: 15770728 PMCID: 4305694] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzpatrick L.R., Meirelles K., Small J.S., Puleo F.J., Koltun W.A., Cooney R.N. A new model of chronic hapten-induced colitis in young rats. J. Pediatr. Gastroenterol. Nutr. 2010;50(3):240–250. doi: 10.1097/MPG.0b013e3181cb8f4a. [PMID: 20118800] [DOI] [PubMed] [Google Scholar]
- 34.Rivera E., Flores I., Rivera E., Appleyard C.B. Molecular profiling of a rat model of colitis: validation of known inflammatory genes and identification of novel disease-associated targets. Inflamm. Bowel Dis. 2006;12(10):950–966. doi: 10.1097/01.mib.0000231575.11678.8c. [PMID: 17012966] [DOI] [PubMed] [Google Scholar]
- 35.Pohlmann A., Tilling L.C., Robinson A., Woolmer O., McCleary S., Kruidenier L., Warnock L.C., Lewis H.D., Hobson A.R., James M.F. Progression and variability of TNBS colitis-associated inflammation in rats assessed by contrast-enhanced and T2-weighted MRI. Inflamm. Bowel Dis. 2009;15(4):534–545. doi: 10.1002/ibd.20800. [PMID: 19058230] [DOI] [PubMed] [Google Scholar]
- 36.Charpentier C., Marion-Letellier R., Savoye G., Nicol L., Mulder P., Aziz M., Vera P., Dechelotte P., Savoye-Collet C. Magnetic resonance colonography in rats with TNBS-induced colitis: a feasibility and validation study. Inflamm. Bowel Dis. 2012;18(10):1940–1949. doi: 10.1002/ibd.22897. [PMID: 22262626] [DOI] [PubMed] [Google Scholar]
- 37.Natah S.S., Mouihate A., Pittman Q.J., Sharkey K.A. Disruption of the blood-brain barrier during TNBS colitis. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2005;17(3):433–446. doi: 10.1111/j.1365-2982.2005.00654.x. [PMID: 15916631] [DOI] [PubMed] [Google Scholar]
- 38.El-Salhy M., Wendelbo I.H., Gundersen D., Hatlebakk J.G., Hausken T. Evaluation of the usefulness of colonoscopy with mucosal biopsies in the follow-up of TNBS-induced colitis in rats. Mol. Med. Rep. 2013;8(2):446–450. doi: 10.3892/mmr.2013.1528. [PMID: 23778962] [DOI] [PubMed] [Google Scholar]
- 39.Lohman R.J., Cotterell A.J., Suen J., Liu L., Do A.T., Vesey D.A., Fairlie D.P. Antagonism of protease-activated receptor 2 protects against experimental colitis. J. Pharmacol. Exp. Ther. 2012;340(2):256–265. doi: 10.1124/jpet.111.187062. [PMID: 22028393] [DOI] [PubMed] [Google Scholar]
- 40.Yue G., Lai P.S., Yin K., Sun F.F., Nagele R.G., Liu X., Linask K.K., Wang C., Lin K.T., Wong P.Y. Colon epithelial cell death in 2,4,6-trinitrobenzenesulfonic acid-induced colitis is associated with increased inducible nitric-oxide synthase expression and peroxynitrite production. J. Pharmacol. Exp. Ther. 2001;297(3):915–925. [PMID: 11356911] [PubMed] [Google Scholar]
- 41.Pilichos C.J., Kouerinis I.A., Zografos G.C., Korkolis D.P., Preza A.A., Gazouli M., Menenakos E.I., Loutsidis A.E., Zagouri F., Gorgoulis V.G., Fotiadis C.I. The effect of nitric oxide synthases inhibitors on inflammatory bowel disease in a rat model. in vivo. 2004;18(4):513–516. [PMID: 15369194] [PubMed] [Google Scholar]
- 42.Brenna O., Furnes M.W., Drozdov I., van Beelen Granlund A., Flatberg A., Sandvik A.K., Zwiggelaar R.T., Marvik R., Nordrum I.S., Kidd M., Gustafsson B.I. Relevance of TNBS-colitis in rats: a methodological study with endoscopic, histologic and Transcriptomic [corrected] characterization and correlation to IBD. PloS one. 2013;8(1):e54543. doi: 10.1371/journal.pone.0054543. [PMID: 23382912 PMCID: 3561356] [DOI] [PMC free article] [PubMed] [Google Scholar]