Abstract
Objective
Transport of pyruvate into the mitochondrial matrix by the Mitochondrial Pyruvate Carrier (MPC) is an important and rate-limiting step in its metabolism. In pancreatic β-cells, mitochondrial pyruvate metabolism is thought to be important for glucose sensing and glucose-stimulated insulin secretion.
Methods
To evaluate the role that the MPC plays in maintaining systemic glucose homeostasis, we used genetically-engineered Drosophila and mice with loss of MPC activity in insulin-producing cells.
Results
In both species, MPC deficiency results in elevated blood sugar concentrations and glucose intolerance accompanied by impaired glucose-stimulated insulin secretion. In mouse islets, β-cell MPC-deficiency resulted in decreased respiration with glucose, ATP-sensitive potassium (KATP) channel hyperactivity, and impaired insulin release. Moreover, treatment of pancreas-specific MPC knockout mice with glibenclamide, a sulfonylurea KATP channel inhibitor, improved defects in islet insulin secretion and abnormalities in glucose homeostasis in vivo. Finally, using a recently-developed biosensor for MPC activity, we show that the MPC is rapidly stimulated by glucose treatment in INS-1 insulinoma cells suggesting that glucose sensing is coupled to mitochondrial pyruvate carrier activity.
Conclusions
Altogether, these studies suggest that the MPC plays an important and ancestral role in insulin-secreting cells in mediating glucose sensing, regulating insulin secretion, and controlling systemic glycemia.
Keywords: Stimulus-coupled secretion, Insulin, β-Cell, Diabetes, Pyruvate, Mitochondria, Drosophila
Abbreviations: IMM, inner mitochondrial membrane; MPC1 and MPC2, Mitochondrial Pyruvate Carrier 1 and 2; GSIS, glucose-stimulated insulin secretion; IPCs, Insulin-producing Cells; OCR, oxygen consumption rates; DILP2, Drosophila insulin-like peptide 2; Pdx1, pancreatic and duodenal homeobox 1; GTT, glucose tolerance test; ITT, insulin tolerance test; RESPYR, REporter Sensitive to PYRuvate
Highlights
-
•
Loss of MPC activity in Drosophila leads to hyperglycemia.
-
•
Glucose-stimulated insulin secretion is impaired in Drosophila lacking MPC.
-
•
β-cell-specific MPC deletion in mice also results in impaired insulin secretion.
-
•
The MPC may be a sensor for glucose concentration in β-cells and couple pyruvate production to insulin secretion.
1. Introduction
Mitochondrial pyruvate metabolism requires transport across the impermeable inner mitochondrial membrane (IMM). Recent work has shown that mitochondrial pyruvate import is mediated by two proteins, the Mitochondrial Pyruvate Carrier 1 and 2 (MPC1 and MPC2), which form a hetero-oligomeric complex in the IMM [1], [2]. Deletion of either MPC1 or MPC2 leads to destabilization and degradation of the complex, effectively resulting in a MPC double knockout and significantly reduced mitochondrial pyruvate uptake [1], [2], [3]. Mice with partial loss of MPC function [4], Drosophila with constitutive MPC1 deletion [2], and mammalian cells with MPC knockdown via RNAi [5] are viable and outwardly normal. However, global and constitutive loss of MPC1 [6] or MPC2 [4] in mice leads to lethality at early embryonic stages.
Mitochondrial pyruvate metabolism is thought to play an important role in the ability of pancreatic β-cells to respond appropriately to increased glucose concentrations by secreting insulin [7], [8], [9]. Oxidation of pyruvate by the pyruvate dehydrogenase complex results in increased ATP production, which inhibits ATP-sensitive potassium (KATP) channels, depolarizes the β-cell, and promotes calcium influx to drive insulin release. In addition, several studies have shown that the production of anaplerotic products by pyruvate carboxylation in the mitochondrial matrix promotes insulin granule exocytosis by Ca2+-independent mechanisms [7], [10], [11], [12]. Chemical inhibition or RNAi-mediated knockdown of the MPC in INS-1 cells and isolated rat islets reduced oxygen consumption rates, ATP content, and glucose-stimulated insulin secretion (GSIS) [13]. While relatively little is known about the role of the MPC in human islet insulin secretion, this same study showed that MPC inhibition in isolated human islets produced effects similar to those seen in rat islets [13]. In addition, mutant mice that carry a partial loss-of-function mutation in Mpc2 are also hypoinsulinemic and glucose intolerant [4]. These studies support the model that MPC function is required in the β-cell for GSIS and proper glucose homeostasis. Validation of this model in vivo, however, requires the characterization of the phenotypes associated with β-cell-specific MPC knockout. To complicate this issue further, recent work has shown that with marked depletion or even complete deletion of MPC proteins, adaptive mechanisms exist that can circumvent the block in pyruvate import [3], [14], [15], [16]. For instance, conversion of pyruvate into amino acids, especially alanine, was shown to support hepatic gluconeogenesis in liver MPC knockout mice by bypassing the need for MPC activity [3], [14]. Thus, we sought to evaluate the role of the MPC in GSIS in β-cells in a comprehensive manner by using both Drosophila and mouse models.
Herein, we demonstrate that the MPC plays a central role in GSIS and systemic glucose homeostasis. MPC deficiency in Drosophila or the β-cells of mice led to elevated blood glucose concentrations, glucose intolerance, and reduced GSIS. Pancreas-specific MPC deficiency resulted in impaired islet glucose metabolism and KATP channel hyperactivity. Moreover, treatment with the KATP channel inhibitor glibenclamide rescued the defects in GSIS both in vitro and in vivo. Finally, glucose increased MPC activity in cultured INS-1 cells in a concentration-dependent manner, suggesting that glucose sensing is coupled to mitochondrial pyruvate transport and utilization to support efficient GSIS. Taken together with previous work in human islets [13], these data demonstrate an important and ancestral role for the MPC in glucose sensing and the regulation of insulin secretion that is conserved from Drosophila to humans.
2. Materials and methods
2.1. Animal studies
Drosophila dMPC1 mutants (dMPC11/dMPC12 transheterozygotes) and genetically-matched precise-excision control strains have been described previously [2]. Unless otherwise noted, experiments were conducted with 6–12 week old mice of both sexes. All vertebrate animal experiments were approved by the Animal Studies Committee of Washington University School of Medicine.
2.2. Drosophila dietary treatments
Drosophila stocks were maintained on a standard cornmeal-molasses diet at 25 °C. To alter dietary sugar concentrations, media was prepared using either low (2% sucrose) or high (18%) sugar concentrations along with 10% yeast in water. For the lifespan studies, males were transferred to the indicated diet within one day of eclosion, then transferred to new vials every 2–5 days. To assay the effect of dietary sugar concentrations on metabolite levels, animals were raised on standard media and transferred to the indicated diet within 2–4 days of eclosion. Metabolites were measured within 8–12 days of transfer.
2.3. Fly metabolite measurements
Whole-animal glucose, trehalose, triacylglycerol, glycogen, and protein measurements were performed using standard colorimetric assays [17]. All other metabolites were measured by metabolomic profiling using gas chromatography/mass spectrometry as described [17]. To measure circulating glucose in Drosophila, ∼30 adult females were punctured in the thorax with a tungsten needle and centrifuged at 13,000 g through DNA columns (Zymo- Spin IIIC #C1006-50) twice in a centrifuge at 4 °C. Hemolymph was then diluted 1/200 in PBS and heat-treated at 70 °C for 10 min to inactivate enzymes, after which the glucose concentration was measured as described [2].
2.4. Western blot analysis
To assay AKT phosphorylation, flies were aged for 8–12 days on high sugar media and then homogenized. Protein was resolved by SDS-PAGE and immunoblotted using standard methods with antibodies to phospho-AKT (Cell Signaling #4691, 1:1000 dilution), pan-AKT (Cell Signaling #4054, 1:1000 dilution), and beta-Tubulin (Chemicon MAB380, 1:100,000 dilution), followed by chemiluminescent detection.
2.5. Immunostaining of Drosophila insulin-producing cells (IPCs)
Brains were dissected in cold PBS and fixed for 20 min at room temperature in 4% paraformaldehyde in PBS. Following several washes in PAT (PBS + 0.5% Triton X-100) brains were blocked with 5% normal donkey serum overnight. Primary antibodies directed against DILP2 [18] and dMPC1 [5] were used at 1:500 concentration for 24 h at 4 °C. Rat Alexa 488-conjugated secondary antibodies (Jackson 212-545-168) and rabbit Cy3-conjugated secondary antibodies (Jackson 711-165-152) were used at 1:800 dilution at 4 °C. Brains were mounted in SlowFade Gold™ (Invitrogen) and imaged using an Olympus FV1000 confocal microscope. Z stack images were taken through the depth of fluorescence of the IPCs using identical settings.
2.6. DILP2 secretion assay
For measuring circulating DILP2 in dMPC1 mutants, the HA-FLAG tagged DILP2 (DILP2-HF) transgene in a dilp21 mutant background was recombined into a dMPC11/dMPC12 transheterozygote mutant background [19]. To disrupt MPC function in the IPCs, UAS-Dcr2; dilp21 DILP2HF dilp2-GAL4/UAS-MPC1 RNAi; dilp21 DILP2HF dilp2-GAL4 flies were used as described along with dilp21 DILP2HF controls [19]. Briefly, adult male progeny were fasted for 16 h and then fed 2 M glucose for 30 min. The posterior end of the abdomen was dissected to collect circulating hemolymph in PBS and the remaining carcasses were homogenized in PBS containing 1% Triton X-100 to assay for total remaining DILP2-HF. HA-FLAG peptide standards from 0, 20, 40 80, 160, 320 and 640 pg/ml were generated for a linear standard curve. 96-well ELISA plate (Thermo Scientific MaxiSorp Immulon 4 HBX, Cat# 3855) coated with mouse anti-FLAG antibody (Sigma F1804, M2 monoclonal) and 1-Step Ultra TMB ELISA Substrate (Thermo Scientific 34029) were utilized for the ELISA assays. Circulating DILP2-HF (pg/fly) versus total remaining peptide was calculated to determine the percent secretion relative to controls (n ≥ 4 biological replicates per condition).
2.7. Generation of β-cell specific Mpc2 deficient mice
Mice harboring a conditional floxed Mpc2 allele have been previously described [3]. Mpc2 floxed mice were crossed with mice expressing Cre recombinase driven by the rat insulin promoter [20] (RipCreMpc2−/− mice), the pancreatic and duodenal homeobox 1 (Pdx1) promoter [21] (PdxCreMpc2−/− mice), or a Pdx1 promoter-driven tamoxifen-regulated Cre [22] (PdxCreERMpc2−/− mice). To conditionally-delete Mpc2, PdxCreERMpc2−/− mice were injected i.p. with tamoxifen (50 μg/g body weight) for 5 consecutive days commencing at 5–6 weeks of age. Littermate Mpc2 floxed mice not expressing Cre (fl/fl) were used for controls in all experiments.
2.8. Glucose and insulin tolerance tests
For the Drosophila glucose tolerance test (GTT), adult male flies were aged 5–9 days on standard media, fasted overnight on fasting media (1% agar in water), fed 10% glucose/1% agar for 2 h, and subsequently transferred to fasting media for two or 4 h. Glucose measurements were performed at each time point as described [17]. Mouse glucose and insulin tolerance tests were performed as previously reported [3], [4].
2.9. Plasma insulin measurements
Fifteen minutes post 1.5 g/kg body weight glucose bolus, mice were sacrificed by CO2 asphyxiation and blood was collected by cannulation of the inferior vena cava. Plasma was collected after blood centrifugation and insulin content was analyzed by Singulex assay by the Washington University Immunoassay Core of the Diabetes Research Center.
2.10. Pancreas immunohistochemistry
Insulin and glucagon protein abundance was assessed using paraffin-embedded pancreatic sections from WT, PdxCreMpc2−/−, and RipCreMpc2−/− mice. Slides were rehydrated, permeabilized with 1 mg/ml trypsin, blocked in 3% BSA, and probed with guinea pig anti-insulin (Abcam #ab10988, 1:100 dilution) and mouse anti-glucagon (Abcam #ab7842, 1:100 dilution) antibodies, in 3% BSA overnight. Slides were washed and probed with Alexa Fluor® 488 goat anti-guinea pig (Invitrogen #A11073, 1:1000 dilution) and Alexa Fluor® 594 goat anti-mouse secondary (Invitrogen #A21125, 1:1000 dilution) antibodies in 3% BSA for 1 h. Coverslips were fixed with ProLong® Gold antifade reagent with DAPI (Life Technologies). Stained pancreatic sections were then imaged on an EVOS FL digital inverted fluorescence microscope (Invitrogen). Islet number and cross-sectional areas were determined using ImageJ.
2.11. Islet isolation and insulin secretion and rubidium efflux assays
Islets were isolated from PdxCreMpc2−/− or PdxCreERMpc2−/− and littermate WT mice following cannulation of the pancreas with Hank's balanced salt solution containing collagenase as previously described [4], [23] and then maintained in RMPI 1640 media. After incubation with increasing glucose concentrations, insulin concentrations in the media were assayed by ELISA [4]. Subsets of islets were treated with 30 mM KCl + 1 mM glucose, or 10 mM glutamine + 23 mM glucose. Insulin secretion treatments were performed in duplicate from 2 to 3 mice per genotype. For rubidium efflux assays, islets were preincubated for 6 h with culture media containing 86RbCl (1 μCi/mL). After stimulation with glucose, medium was aspirated and replaced with fresh solution at 5 min intervals. At 65 min, cells were lysed with 2% SDS. The amount of 86Rb+ in each aspirated solution and in final cell lysate was determined as previously described [24]. Five experimental replicates were performed of the rubidium efflux assays using 2–3 mice of each genotype for each replicate.
2.12. Islet respiration assay
Islets were isolated from PdxCreMpc2−/− and WT mice as above and 75 equal-sized islets were spun to the bottom of wells of Seahorse XF-24 plates. Oxygen consumption rates (OCR) were determined using an XF-24 Extracellular Flux Analyzer (Seahorse Bioscience) and normalized to protein content. Basal measurements were performed with 2 mM glucose and then islets were stimulated with 20 mM glucose. To characterize mitochondrial function, 1 μM oligomycin, 1.5 μM FCCP, and 1 μM antimycin A plus 100 nM rotenone were added sequentially. Islets were isolated and OCR measured from 4 to 5 mice per genotype.
2.13. REporter sensitive to PYRuvate (RESPYR) assay
pWPT lentiviral vectors expressing cDNA encoding human MPC2 fused to RLuc8 (BRET donor) and human MPC1 fused to Venus (BRET acceptor) [25] were transduced into INS-1 cells. INS-1 cells were seeded in white 96-well plates 48 h before recording using RPMI 1640 media supplemented with 10% FBS, 5 mM sodium pyruvate, 10 mM l-glutamine and 0.25 mM β-mercaptoethanol. Medium was replaced with pyruvate- and glucose-free RPMI 4 h prior to assays, which were performed and analyzed as described [25]. Luminescence was measured using a plate reader (Biotek Synergy 2) at 460 and 528 nm at 37 °C.
2.14. Statistical analyses
P values for pairwise comparisons were calculated using a Student's t test. P values for Kaplan Meier survival curves were calculated using a log rank test. P values for RESPYR curves were calculated using 2-way ANOVA coupled to Tukey's multiple comparison tests. In all experiments, P ≤ 0.05 was used to determine significant difference. All quantitative data is represented as mean ± SEM.
3. Results
3.1. Drosophila MPC1 null mutants exhibit elevated carbohydrates and premature death on a high-sugar diet
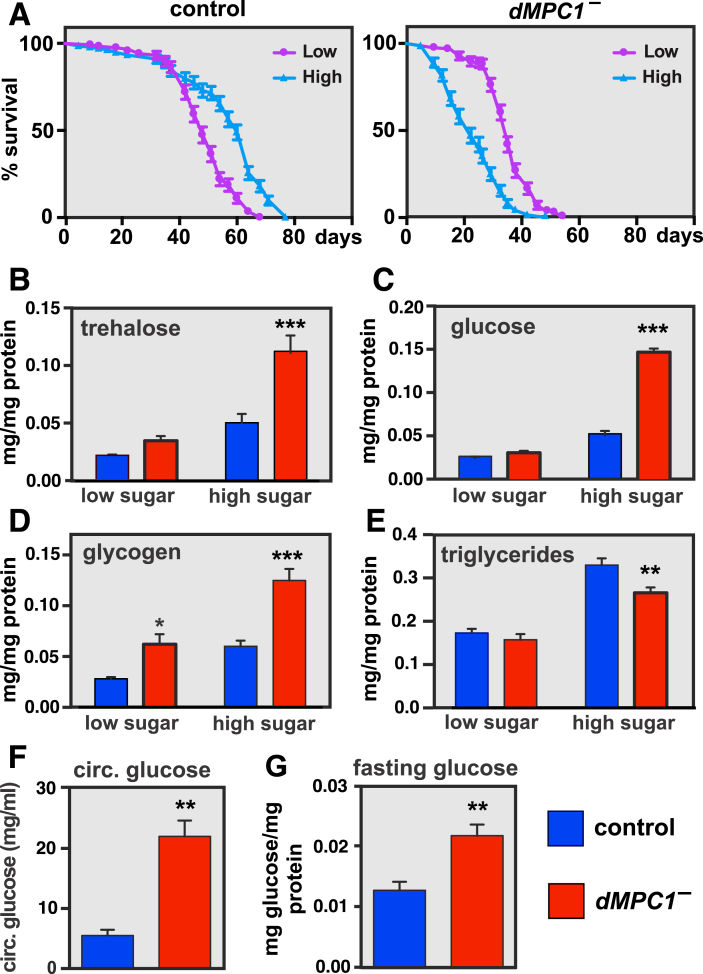
Our previous studies have shown that Drosophila MPC1 null mutants on normal laboratory medium or a diet consisting of only sugar have elevated levels of glucose and the glucose disaccharide trehalose, which is consistent with the manifestation of diabetes in Drosophila [2], [26], [27], [28]. In addition, dMPC1 mutants are sensitive to dietary sugar. Raising control flies on a low or high sugar diet (2% or 18% dietary sugar, respectively) has little effect on their lifespan (Figure 1A, left panel). In contrast, dMPC1 mutants on the low sugar diet have approximately half the lifespan of controls (Figure 1A, purple curves), and this becomes significantly worse on the high sugar diet (Figure 1A, light blue curves). Analysis of whole-body metabolites demonstrated that dMPC1 mutants display normal trehalose, glucose, and triglycerides on the low sugar diet, along with an increase in glycogen, but significantly elevated carbohydrates and decreased triglycerides on the high sugar diet (Figure 1B–E). This diet-dependent effect on carbohydrate levels is reminiscent of the beneficial impact of a ketogenic diet on diabetic patients.
Figure 1.
Drosophila MPC1 mutants are hyperglycemic and sensitive to dietary sugar. A: dMPC1 mutants die more rapidly as dietary sugar is increased. The percentage of surviving adult control (left panel) or dMPC1 mutants (right panel) fed a low (2% sucrose, 10% yeast; purple lines) or high (18% sucrose, 10% yeast; light blue lines) sugar diet was assayed every 2–5 days. N > 115 male flies per genotype under each feeding condition. Mean ± SEM is shown. B–E: Control (blue) or dMPC1 mutants (red) were aged 8–12 days on the low or high sugar diet and whole animal metabolite levels were measured to determine the abundance of B: trehalose C: glucose, D: glycogen, or E: triglycerides (TAG), all normalized to protein. N = 5–6 samples per genotype. Mean ± SEM is shown. *p < 0.05, **p < 0.01, and ***p < 0.001. F: Hemolymph glucose concentrations were determined in control and dMPC1 mutants aged 10 days on the high sugar media. N = 4 per genotype. **p < 0.01 G: Control and dMPC1 mutants were aged 5–9 days on standard laboratory media and fasted overnight for 16 h. After fasting, whole-animal glucose levels were determined and normalized to protein. N = 5 per genotype. **p < 0.01.
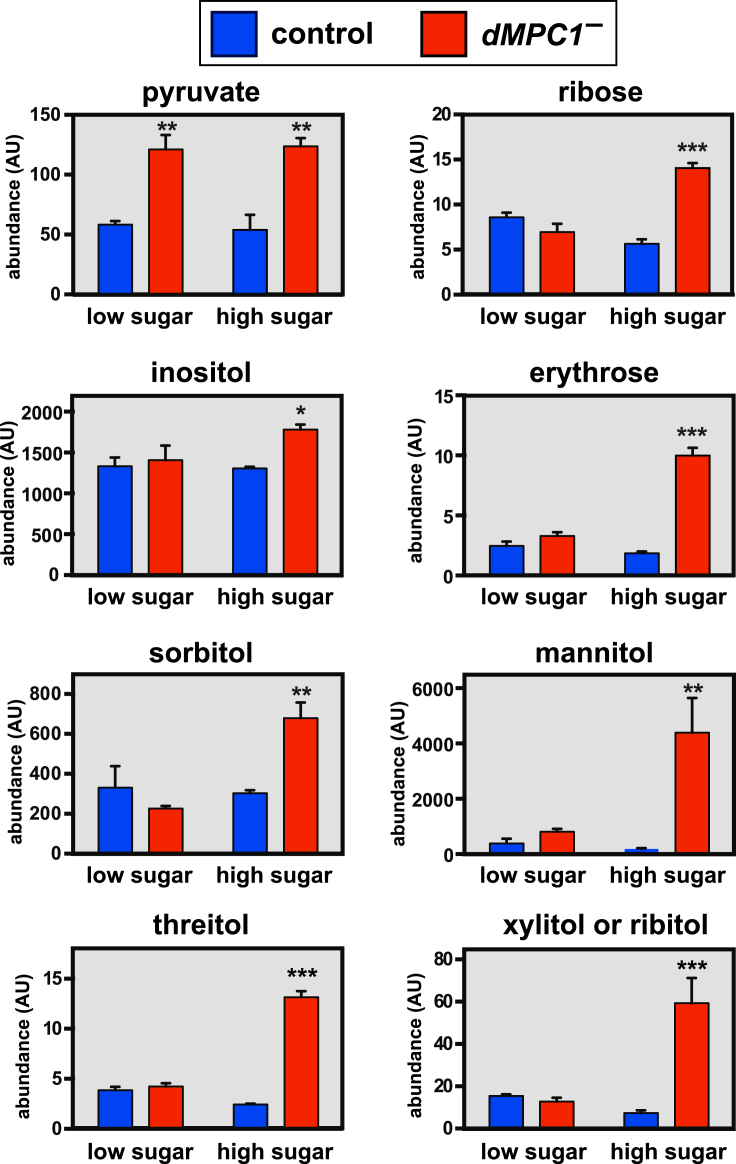
To further examine the effect of diets on the metabolic state of dMPC1 mutants, we conducted metabolomic profiling using small-molecule GC/MS (Figure 2). dMPC1 mutants exhibit a significant accumulation of the sugars inositol, erythrose, and ribose as well as the sugar alcohols sorbitol, mannitol, threitol, and xylitol (or ribitol) when maintained on the high sugar diet. Conversion of excess intracellular glucose to sorbitol through the polyol pathway is thought to provide an alternative pathway for glucose clearance and storage and is associated with neuropathy in diabetic patients [29]. In contrast, pyruvate accumulates to similar levels in dMPC1 mutants on both diets (Figure 2). These metabolic changes on the high sugar diet demonstrate that dMPC1 mutants are unable to maintain carbohydrate and lipid homeostasis in response to changes in dietary sugar.
Figure 2.
Sugars and sugar alcohols accumulate in dMPC1 mutants in response to dietary sugar. Control (blue) or dMPC1 mutants (dMPC1–)(red) were aged 8–12 days on the low or high sugar diets and whole animal metabolite levels were measured by GC/MS. The abundance of pyruvate, ribose, inositol, erythrose, sorbitol, mannitol, threitol, and xylitol or ribitol (which cannot be distinguished in our analysis) is shown. N = 3 biological replicates per condition. Mean ± SEM is shown. P values comparing mutants to controls under either condition were calculated by Student's t test. *p < 0.05, **p < 0.01, and ***p < 0.001.
3.2. Drosophila MPC1 null mutants are glucose-intolerant with decreased insulin-like peptide secretion
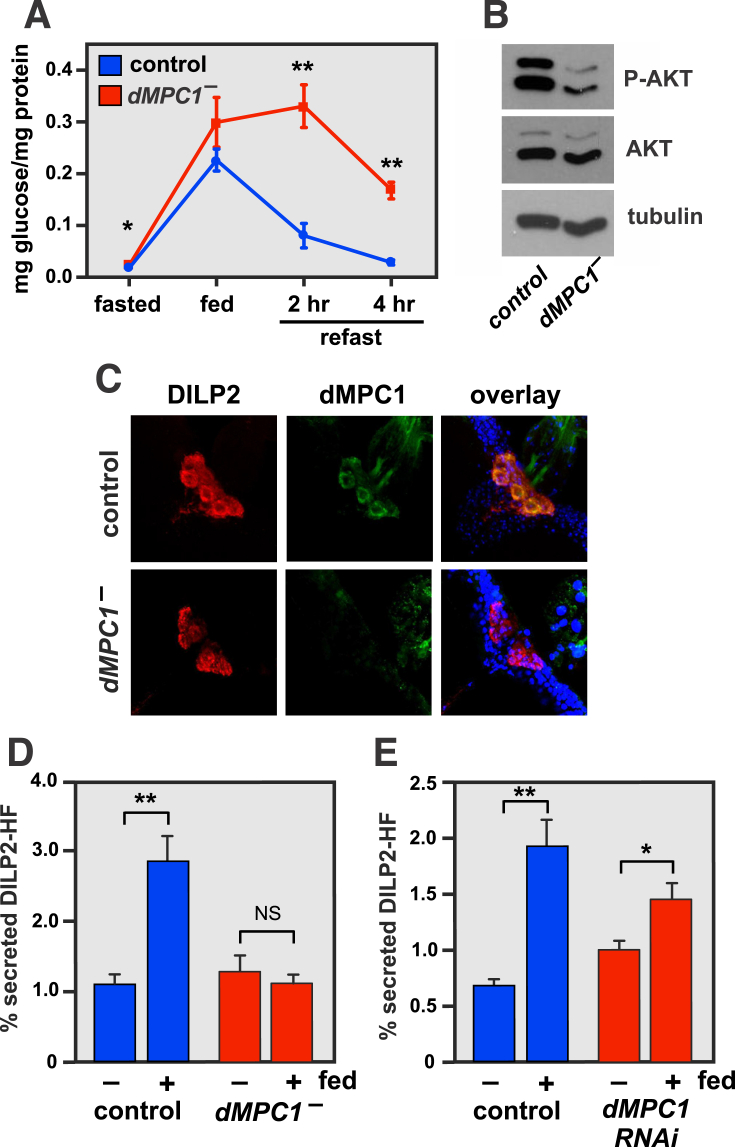
Consistent with the idea that dMPC1 mutants suffer from an inherited form of diabetes, dMPC1 mutants display elevated circulating glucose levels on the high sugar diet (Figure 1F), as well as fasting hyperglycemia (Figure 1G). Adult flies were subjected to an oral GTT by fasting control and dMPC1 mutants overnight, transferring them to a 10% glucose diet for 2 h, and then re-fasting for two or 4 h. Although dMPC1 mutants display a normal postprandial spike in free glucose levels after feeding, glucose clearance is significantly impaired in these animals, indicating glucose intolerance (Figure 3A). Taken together, these data indicate that dMPC1 is required to maintain proper systemic glucose homeostasis and suppress diabetes.
Figure 3.
Drosophila MPC1 mutants are glucose intolerant and have reduced glucose-stimulated insulin secretion. A: Oral-glucose tolerance test performed on adult control (blue) and dMPC1 mutants (red) that were aged 5–9 days on standard laboratory media, fasted overnight, fed 10% glucose for 2 h, and then re-fasted for either 2 or 4 h. Data represents free glucose levels from whole animal homogenates normalized to protein concentration. N = 5 biological replicates per genotype at each timepoint. Mean ± SEM is shown. *p < 0.05 and ***p < 0.001. B: Control and dMPC1 mutants were aged 8–12 days on the high sugar diet and phosphorylated AKT (P-AKT), total AKT, and tubulin protein levels were determined by western blot analysis. C: The insulin-producing cells (IPCs) embedded in the adult brain were stained with antibodies directed against either DILP2 (left, red) or MPC1 (green, center) in either control or dMPC1 mutants. dMPC1 staining is evident in the IPCs of control, but not mutant, flies. D: dMPC1 mutants display defects in GSIS. Control and dMPC1 mutants carrying DILP2-HF in a dilp21 mutant background were aged 5–9 days on standard laboratory media, fasted overnight, and fed glucose for 30 min. Hemolymph was collected to measure circulating DILP2-HF under fasted and fed conditions. Y axis depicts the ratio of secreted DILP2-HF to total DILP2-HF. (fasted n = 4, fed n = 5). **p ≤ 0.01. NS = not significant. E: IPC-specific dMPC1 RNAi results in reduced GSIS. Levels of circulating DILP2-HF were assayed in controls (blue) or animals with IPC-specific RNAi against dMPC1 (red) using a dilp2-GAL4 driver. *p < 0.05, **p ≤ 0.01.
Western blot analysis of dMPC1 mutants revealed reduced whole-animal levels of phospho-AKT, indicating a decrease in systemic insulin signaling (Figure 3B). This observation raises the possibility that the defects in glucose homeostasis in dMPC1 mutants could arise, at least in part, through defects in GSIS. dMPC1 is expressed in the insulin producing cells (IPCs) of adult Drosophila as revealed by immunohistochemistry (Figure 3C). In addition, a direct assay to measure circulating levels of a tagged form of the major Drosophila Insulin-Like Peptide DILP2 (DILP2-HF) revealed that dMPC1 mutants have no response to glucose feeding whereas control animals display a normal increase in circulating DILP2 (Figure 3D) [19]. Finally, IPC-specific disruption of dMPC1 expression by RNAi results in a significant reduction in GSIS relative to controls (Figure 3E). Whereas control flies show a ∼2.5-fold increase in circulating DILP2-HF from the fasted to fed state, flies lacking dMPC1 in their IPCs show only a ∼1.5-fold response. These results indicate that the diabetic defects in dMPC1 mutants arise, at least in part, from a defect in GSIS. Previous studies have shown that the Drosophila homologs of the KATP channel subunits Sur1 and Kir6 are active in adult IPCs and that these cells respond to glucose or glibenclamide treatment by undergoing calcium influx and membrane depolarization similar to mammalian pancreatic β-cells [30]. Taken together with our studies of the MPC, this work suggests that efficient pyruvate oxidation in IPCs acts through the KATP channel to promote insulin release. Our studies of MPC function in mice, described below, support this model.
3.3. Loss of MPC2 in pancreas leads to glucose intolerance and impaired GSIS in mice
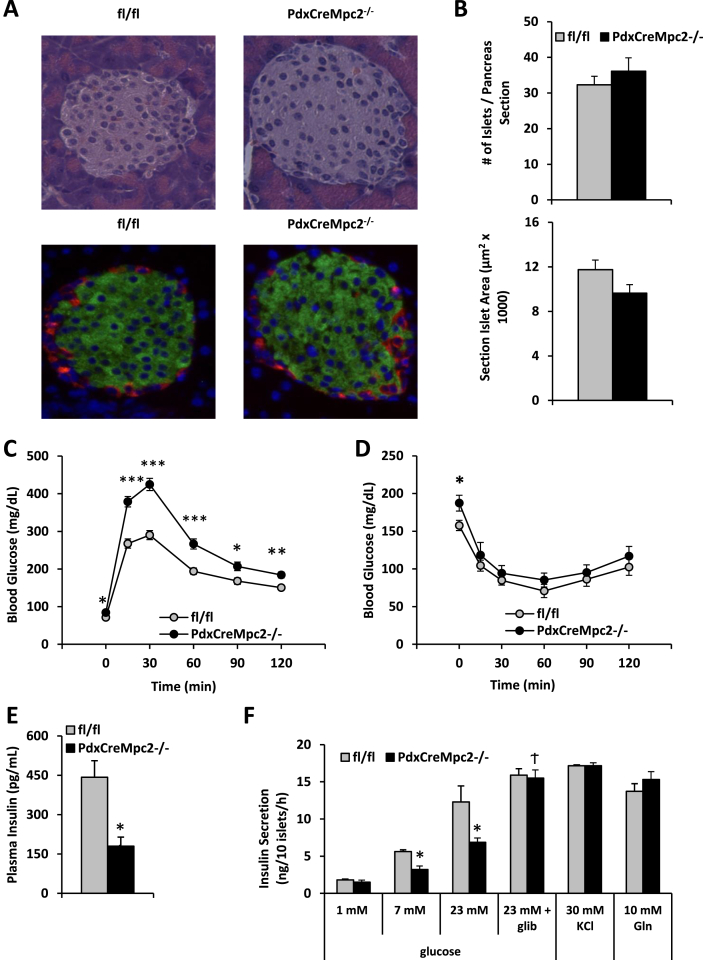
We generated mice with deletion of Mpc2 in pancreas by using the Pdx promoter to drive Cre expression in pancreatic pre-endoderm in mice with Mpc2 floxed alleles. PdxCreMpc2−/− mice were outwardly normal and viable. Deletion of Mpc2 did not affect islet number or cross-sectional area (Figure 4A and B), nor did it affect the intensity of staining for insulin or glucagon (Figure 4A). Like dMPC1 mutants, PdxCreMpc2−/− mice were glucose intolerant in a GTT (Figure 4C). This was likely due to diminished insulin secretion, since insulin tolerance was unchanged by loss of MPC (Figure 4D) and plasma insulin concentration 15 min after a bolus glucose injection was significantly reduced compared to control mice (Figure 4E). Consistent with a defect in GSIS, islets isolated from PdxCreMpc2−/− mice exhibited reduced insulin secretion in response to 7 or 23 mM, but not 1 mM, glucose (Figure 4F). Stimulation of insulin secretion by 30 mM KCl or with addition of 10 mM glutamine, an alternate mitochondrial energy substrate, was not affected by loss of MPC2.
Figure 4.
Mice with pancreas-specific MPC2 deletion have reduced GSIS despite normal insulin content. A: Immunohistochemical staining of islets showed no difference in immunodetectable insulin, glucagon, or β and α cell number. B: Histologic assessment of islet number and individual cross-sectional area detected no difference between WT and PdxCreMpc2−/− mice. C: Mice were fasted for 16 h and injected i.p. with 1.5 g/kg glucose. PdxCreMpc2−/− mice displayed significantly elevated blood glucose concentrations after the overnight fast and after bolus glucose injection. D: Mice were fasted for 4 h and injected i.p. with 0.5 U/kg insulin. PDXCreMpc2−/− mice displayed elevated basal blood glucose levels but similar insulin response curves. E: Plasma insulin concentrations were reduced in PdxCreMpc2−/− mice 15 min after bolus glucose injection. F: Loss of Mpc2 in beta cells impairs GSIS by isolated islets. Isolated islets from WT and PdxCreMpc2−/− mice were incubated with the indicated concentrations of glucose and insulin concentration of the medium determined. Insulin secretion was corrected in PdxCreMpc2−/− islets with either 1 μM glibenclamide treatment or stimulation with 30 mM KCl + 1 mM glucose. 10 mM glutamine + 23 mM glucose-stimulated insulin secretion was also normal. N = 10–12 mice per genotype with 10 pancreas sections analyzed per mouse in A and B. N = 10–13 mice per genotype in C and D. N = 5–6 mice per genotype in E. N = 2–3 mice per genotype and two technical replicates per mouse in F. Mean ± SEM is shown. P values comparing KOs to controls were calculated using a Student's t test. *p < 0.05, **p < 0.01, and ***p < 0.001, Ϯp < 0.05 compared to non-glib treatment.
3.4. Conditional deletion of MPC in β cells also leads to glucose intolerance and β cell dysfunction
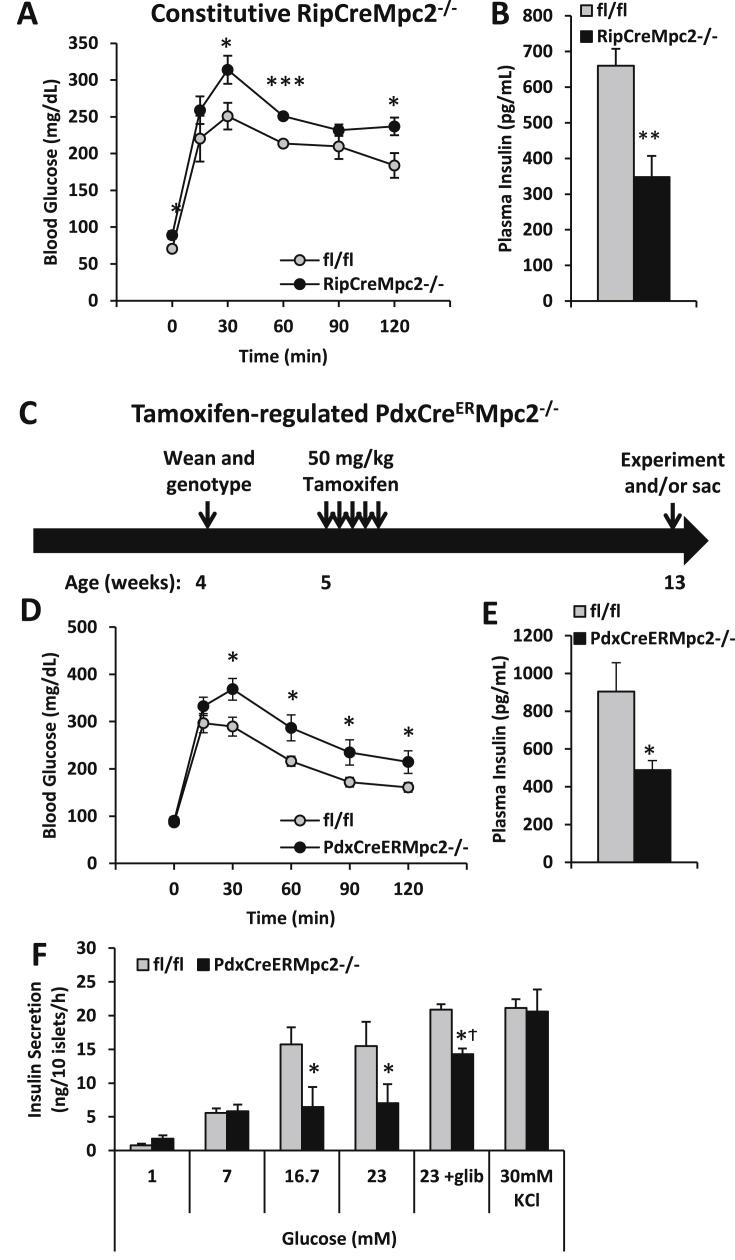
Previous work has demonstrated that some of the pancreas Cre drivers can have off-target or non-specific effects. These include gene recombination in the central nervous system [31], [32] and expression of human growth hormone due to the inclusion of a minigene encoding this secreted protein downstream of the Cre cDNA [33]. Because of these issues, we verified the above findings using distinct β-cell Cre drivers. Deletion of Mpc2 specifically in β-cells by using the rat insulin promoter-driven Cre also produced mice that were glucose intolerant (Figure 5A) with lower plasma insulin concentrations after bolus glucose injections (Figure 5B). Mpc2 was also inducibly-deleted in β-cells by using a tamoxifen-activated Cre recombinase (CreER) driven by the Pdx promoter in mice that do not harbor the growth hormone minigene by injecting mice with 50 mg/kg tamoxifen on 5 consecutive days (Figure 5C). Eight weeks after tamoxifen injection, PdxCreERMpc2−/− mice were glucose intolerant (Figure 5D) and had lower blood insulin concentration 15 min after bolus glucose administration (Figure 5E) compared to littermate controls. Islets isolated from PdxCreERMpc2−/− mice exhibited reduced insulin secretion in response to 16 and 23 mM glucose, but not 1 and 7 mM glucose (Figure 5F).
Figure 5.
Constitutive or Tamoxifen-inducible deletion of Mpc2 in pancreatic β-cells results in defective glucose-stimulated insulin secretion. A: Mice were fasted for 16 h and injected i.p. with 1.5 g/kg glucose. RipCreMpc2−/− mice display elevated blood glucose concentrations following bolus glucose injection. B: Plasma insulin concentration of RipCreMPC2−/− mice is reduced 15 min after bolus glucose injection C: Schematic of Tamoxifen-inducible Mpc2 deletion in PdxCreERMpc2−/− mice. Tamoxifen dose was 50 mg/kg i.p. for 5 consecutive days. D: Mice were fasted for 16 h and injected i.p. with 1.5 g/kg glucose. PdxCreERMpc2−/− mice displayed elevated blood glucose concentration during the GTT. Glucose AUC was also significantly elevated. E: Plasma insulin concentrations of PdxCreERMpc2−/− mice were reduced 15 min after i.p. injection of 1.5 g/kg glucose following a 6 h fast. F: Isolated islets from PdxCreERMpc2−/− mice contain normal insulin content, but defective glucose-stimulated insulin secretion. Insulin secretion was corrected in PdxCreERMpc2−/− islets with either 1 μM Glibenclamide treatment or stimulation with 30 mM KCl + 1 mM glucose, suggesting the defect prior to the membrane depolarization. N = 5–7 mice per group in A, B, and E. N = 11–12 mice per genotype in D, and N = 2–3 mice per genotype and two technical replicates per mouse in F. Mean ± SEM is shown. P values comparing KOs to controls were calculated using a Student's t test. *p < 0.05, **p < 0.01, and ***p < 0.001, Ϯp < 0.05 compared to non-glib treatment.
3.5. MPC deficiency impairs GSIS via KATP channel hyperactivity
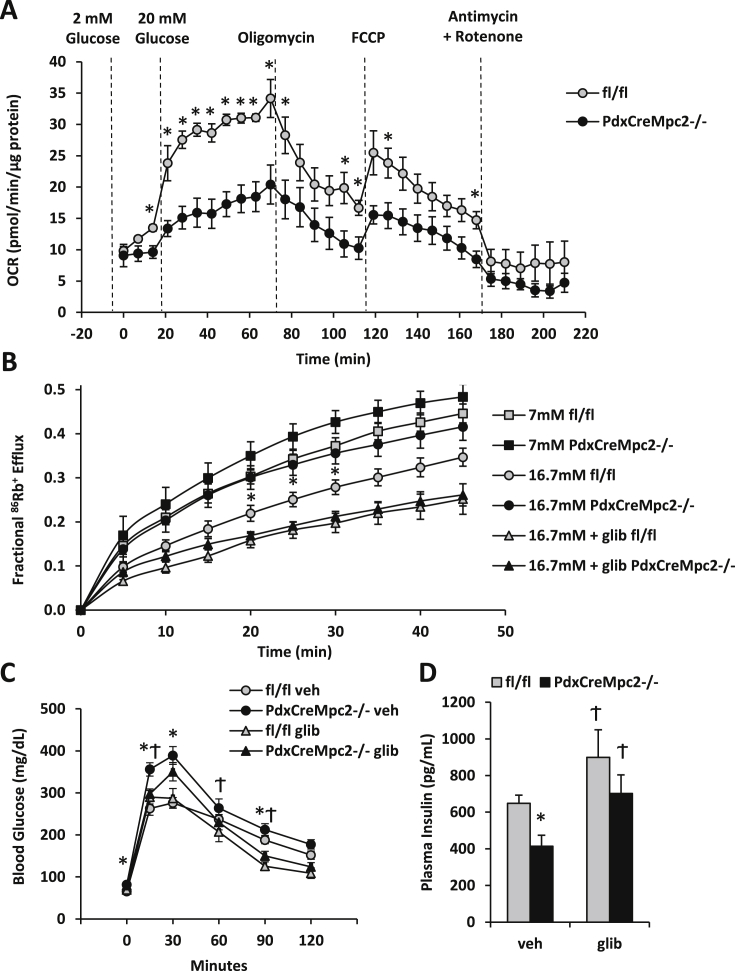
To confirm that mitochondrial metabolism in high glucose conditions was diminished by MPC-deficiency, oxygen consumption rates in islets from WT and PdxCreMpc2−/− mice were assessed using a Seahorse XF analyzer. While basal (low glucose) respiration was unaffected, high glucose stimulated oxygen consumption rates were significantly reduced in PdxCreMpc2−/− islets compared to WT controls (Figure 6A). Mpc2-deficient islets responded normally to traditional respiration inhibitors and uncoupling (Figure 6A).
Figure 6.
Defects in MPC2 KO islet KATPchannel activity and GSIS are correctable with sulfonylurea treatment. A: Isolated islets were stimulated with glucose and oxygen consumption rates (OCR) were determined. PdxCreMpc2−/− islets display reduced OCR in response to 20 mM glucose stimulation, but maintain similar responsiveness to oligomycin, FCCP, and Antimycin A + Rotenone treatment. B: PdxCreMpc2−/− islets display increased KATP channel activity in response to glucose stimulation. 86Rb+ efflux was measured in cultured islets following stimulation with glucose (7 mM or 16.7 mM) or glucose plus glibenclamide (16.7 mM + glib). C: Blood glucose concentrations during the GTT performed using fl/fl and PdxCreMpc2−/− mice. Mice were fasted for 16 h and injected i.p. with 1.5 g/kg glucose and co-injected with vehicle or the KATP channel inhibitor glibenclamide (0.1 mg/kg). D: Glibenclamide also corrected plasma insulin concentrations 15 min after glucose injection in PdxCreMpc2−/− mice. N = 4–5 mice per genotype in A, N = 5 experimental replicates using islets from 2 to 3 mice per genotype in B, N = 13–14 mice per GTT group in C, and N = 6 per insulin secretion group in D. *p < 0.05, Ϯp < 0.05 compared to vehicle treatment.
One mechanism by which increased glucose concentration is believed to regulate insulin secretion is via inhibition of KATP channel activity leading to membrane depolarization, Ca2+ influx, and insulin granule docking with the cell membrane and release. As a measure of KATP channel activity, we performed rubidium efflux (86RbCl) experiments (Rb+ as a surrogate for K+) [23], [34] using islets from PdxCreMpc2−/− and WT mice. 86Rb efflux was suppressed in a glucose concentration-dependent manner in WT islets (Figure 6B). However, the suppression of 86Rb efflux by higher glucose concentrations was impaired in islets from PdxCreMpc2−/− mice, suggesting KATP channel hyperactivity (Figure 6B).
We next assessed whether the sulfonylurea compound glibenclamide, which inhibits the KATP channel [35], could uncouple pyruvate metabolism and membrane depolarization and therefore correct GSIS in Mpc2-deficient islets and mice. The defects in GSIS (Figure 4, Figure 5F) and Rb+ efflux (Figure 6B) in Mpc2-deficient islets were reversed by incubation with glibenclamide. Moreover, the impairments in glucose intolerance and reduced plasma insulin concentration observed in PdxCreMpc2−/− mice were improved when glibenclamide was administered at the time of glucose bolus (Figure 6C and D). Altogether, these data suggest that the MPC complex and mitochondrial pyruvate metabolism regulates β-cell GSIS, at least in part, through regulation of KATP channel activity.
3.6. MPC activity is enhanced by high glucose in INS-1 cells
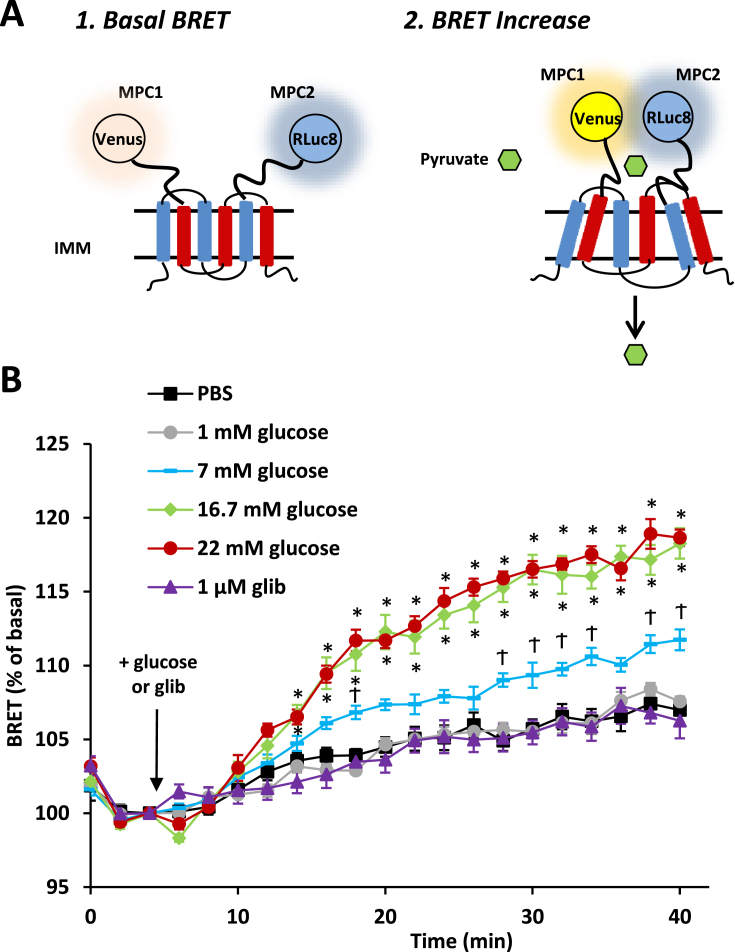
Recent work using a BRET-based reporter assay for MPC activity (REporter Sensitive to PYRuvate; RESPYR) has suggested that MPC complex activity is acutely regulated [25]. In this system, MPC2 protein is fused to modified Renilla luciferase (RLuc8; photon donor), while MPC1 is fused to a variant of yellow fluorescent protein (Venus; photon acceptor). Pyruvate transport induces a conformational change in the complex, reducing the distance between the donor and acceptor, and resulting in an increase in BRET (Figure 7A). Within minutes of administration, 7, 16, or 22 mM glucose increased the BRET signal compared to 0 or 1 mM glucose (Figure 7B) in INS-1 cells transduced with the RESPYR system. Glibenclamide had no effect on RESPYR activity (Figure 7B). The glucose concentration-dependent increase in RESPYR activity suggests that stimulation of MPC activity may be an important mediator of glucose sensing in insulin-producing cells.
Figure 7.
Glucose stimulation increases MPC activity in INS-1 cells. A: Schematic of the BRET-based RESPYR biosensor used to measure real time activity of the mitochondrial pyruvate carrier. RLuc8 is c-terminally fused to MPC2 and Venus is c-terminally fused to MPC1. BRET signal increases above basal levels as carrier transport activity increases. B: BRET kinetics of INS-1 cells expressing RESPYR. Cells were stimulated after 5 min with PBS (black squares), 1 mM glucose (gray circles), 7 mM glucose (blue rectangles), 16.7 mM glucose (green diamonds) or 22 mM glucose (red circles) or 1 μM glibenclamide (purple triangles) in PBS. Data were analyzed by repeated measures ANOVA in Prism. Post Hoc analysis was performed using Tukey's multiple comparison tests. Data represents mean ± SEM of six independent experiments with 4–5 technical replicates per experiment.*p < 0.05 compared to PBS, 1 mM glucose and 1 μM glib treatments, Ϯp < 0.05 compared to all other treatments.
4. Discussion
Our studies of a complete loss of MPC function in Drosophila and mouse pancreatic β-cells have demonstrated an evolutionarily-conserved role for the MPC in insulin-producing cells to support GSIS and suppress hyperglycemia. Moreover, our mechanistic analyses support the model that mitochondrial pyruvate metabolism is critical for inhibition of KATP channel activity and insulin release and provide new evidence that MPC activity is acutely regulated by glucose concentration in insulin-producing cells. Our studies, together with previous work, place the MPC at a critical nexus in GSIS that is conserved through evolution, from flies to humans.
At high concentrations of glucose, islets isolated from PdxCreMpc2−/− mice exhibited deficits in oxygen consumption rates. Previous work has shown that ATP produced from pyruvate oxidation suppresses KATP channel activity [36], [37], [38] and that genetic deletion of critical components of the pyruvate dehydrogenase complex, which is required for pyruvate oxidation, leads to overt hyperglycemia in mice [39]. The alternative metabolic fate of pyruvate in the mitochondrion, carboxylation, has also been shown to play an important role in regulating insulin secretion via production of intermediates that stimulate insulin granule release by Ca2+-independent mechanisms [7], [10], [11], [12], [40]. Because our genetic approach impacts both pyruvate oxidation and anaplerosis, it is not possible to distinguish which pathway is mediating the effects of MPC deficiency in β-cells. However, our studies assessing Rb+ efflux and using glibenclamide are consistent with the idea that KATP channel hyperactivity is at least partially responsible for the phenotype of these mice.
The present studies, together with other recent work, illustrate interesting species-specific and tissue-specific effects of MPC deficiency. Drosophila MPC1 mutants developed more severe diabetic phenotypes than pancreas-specific Mpc2 knockout mice, including more severe glucose intolerance. This is likely due to the systemic loss of MPC function in the Drosophila whole-animal mutants. Impaired mitochondrial pyruvate metabolism in glucose-utilizing myocytes or neurons might be predicted to impair glucose disposal. On the other hand, knockout of Mpc1 or Mpc2 in the liver of mice leads to hypoglycemia and protects mice from the development of diabetes [3], [14]. Pyruvate is a substrate for gluconeogenesis in hepatocytes and mitochondrial metabolism of pyruvate is required to convert pyruvate to new glucose, which contributes to hyperglycemia of diabetes when insulin is insufficient to suppress gluconeogenesis [3]. Thus, while the MPC could be considered an intriguing target for antidiabetic drugs, it is important to emphasize that it will be necessary to identify agents that act in a tissue-specific manner to ensure the intended effect. Targeting MPC function to improve β-cell GSIS would have to be performed with caution as increasing MPC activity in the liver would likely increase gluconeogenesis and worsen hyperglycemia [3], [14].
The MPC is amenable to pharmacological targeting. A number of specific MPC inhibitors (e.g. UK-5099) were identified years ago [41]. Interestingly, experimental thiazolidine compounds studied as KATP channel inhibitors can also act in an inhibitory fashion on the MPC [42]. More recently, thiazolidinedione (TZD) insulin-sensitizers (rosiglitazone, pioglitazone, MSDC-0602, and MSDC-0160) were shown to bind to [3], [5] and inhibit the MPC [3], [43]. However, it should be noted that treatment of primary human islets with MSDC-0160 did not affect GSIS and enhanced islet survival and insulin expression [44] in contrast with the results obtained with other genetic or pharmacologic inhibition studies [4], [13].
Lastly and importantly, using a recently-developed BRET-based sensor, our studies in INS-1 cells demonstrated that increasing concentrations of glucose dose-dependently increased MPC activity. This could suggest that the activity of this complex is a mechanistic detector of glucose concentration that contributes to the ability of β-cells to “sense” glucose. Future studies will be needed to determine whether the activity of this complex is deranged in insulin resistance and in type 2 diabetes and, if so, at what regulatory levels the complex is affected. It also remains to be determined whether genetic mutation or variations in the MPC could influence the onset of relative hypoglycemia that leads to diabetes. We believe that the MPC may be an important target to pharmacologically-enhance GSIS in insulin-resistant states and develop new classes of anti-diabetic drugs.
Acknowledgments
We thank P. Leopold for providing DILP2 antibodies, Dr. J. Cox at the University of Utah Metabolomics Core Facility for performing the GC/MS metabolomic analysis, the Bloomington Stock Center for providing stocks, and FlyBase for critical information that made these studies possible. We also thank J.C. Martinou for the gift of RESPYR plasmids, W. McDonald for the Drosophila MPC1 antibodies, and J. Rutter and his lab (University of Utah) for helpful discussions. This research was supported by NIH grants R01 GM094232 (C.S.T.), R01 DK098584 (M.S.R.), R01 DK104735 (B.N.F.), and pilot and feasibility funding from the Diabetes Research Center to B.N.F (P30 DK020579). The Core services of the Diabetes Research Center (P30 DK020579) and the Nutrition Obesity Research Center (P30 DK56341) at the Washington University School of Medicine also supported this work. K.S.M. is a Diabetes Research Postdoctoral Training Program fellow (T32 DK007296 and DK007120). None of the funding sources played a role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Contributor Information
Carl S. Thummel, Email: carl.thummel@genetics.utah.edu.
Brian N. Finck, Email: bfinck@dom.wustl.edu.
Conflict of interest
None declared.
References
- 1.Herzig S., Raemy E., Montessuit S., Veuthey J.L., Zamboni N., Westermann B. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 2.Bricker D.K., Taylor E.B., Schell J.C., Orsak T., Boutron A., Chen Y.C. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCommis K.S., Chen Z., Fu X., McDonald W.G., Colca J.R., Kletzien R.F. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metabolism. 2015;22:682–694. doi: 10.1016/j.cmet.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vigueira P.A., McCommis K.S., Schweitzer G.G., Remedi M.S., Chambers K.T., Fu X. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Reports. 2014;7:2042–2053. doi: 10.1016/j.celrep.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colca J.R., McDonald W.G., Cavey G.S., Cole S.L., Holewa D.D., Brightwell-Conrad A.S. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)–relationship to newly identified mitochondrial pyruvate carrier proteins. PloS One. 2013;8:e61551. doi: 10.1371/journal.pone.0061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanderperre B., Bender T., Kunji E.R., Martinou J.C. Mitochondrial pyruvate import and its effects on homeostasis. Current Opinion in Cell Biology. 2014;33C:35–41. doi: 10.1016/j.ceb.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Jensen M.V., Joseph J.W., Ronnebaum S.M., Burgess S.C., Sherry A.D., Newgard C.B. Metabolic cycling in control of glucose-stimulated insulin secretion. American Journal of Physiology Endocrinology and Metabolism. 2008;295:E1287–E1297. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prentki M., Matschinsky F.M., Madiraju S.R. Metabolic signaling in fuel-induced insulin secretion. Cell Metabolism. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Sugden M.C., Holness M.J. The pyruvate carboxylase-pyruvate dehydrogenase axis in islet pyruvate metabolism: going round in circles? Islets. 2011;3:302–319. doi: 10.4161/isl.3.6.17806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J., Han J., Long Y.S., Epstein P.N., Liu Y.Q. The role of pyruvate carboxylase in insulin secretion and proliferation in rat pancreatic beta cells. Diabetologia. 2008;51:2022–2030. doi: 10.1007/s00125-008-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan N.M., Longacre M.J., Stoker S.W., Boonsaen T., Jitrapakdee S., Kendrick M.A. Impaired anaplerosis and insulin secretion in insulinoma cells caused by small interfering RNA-mediated suppression of pyruvate carboxylase. The Journal of Biological Chemistry. 2008;283:28048–28059. doi: 10.1074/jbc.M804170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farfari S., Schulz V., Corkey B., Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 13.Patterson J.N., Cousteils K., Lou J.W., Manning Fox J.E., Macdonald P.E., Joseph J.W. Mitochondrial metabolism of pyruvate is essential for regulating glucose-stimulated insulin secretion. The Journal of Biological Chemistry. 2014;289:13335–13346. doi: 10.1074/jbc.M113.521666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray L.R., Sultana M.R., Rauckhorst A.J., Oonthonpan L., Tompkins S.C., Sharma A. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metabolism. 2015;22:669–681. doi: 10.1016/j.cmet.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vacanti N.M., Divakaruni A.S., Green C.R., Parker S.J., Henry R.R., Ciaraldi T.P. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Molecular Cell. 2014;56:425–435. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang C., Ko B., Hensley C.T., Jiang L., Wasti A.T., Kim J. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Molecular Cell. 2014;56:414–424. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tennessen J.M., Barry W.E., Cox J., Thummel C.S. Methods for studying metabolism in Drosophila. Methods. 2014;68:105–115. doi: 10.1016/j.ymeth.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geminard C., Rulifson E.J., Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metabolism. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Park S., Alfa R.W., Topper S.M., Kim G.E., Kockel L., Kim S.K. A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genetics. 2014;10:e1004555. doi: 10.1371/journal.pgen.1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 21.Hingorani S.R., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H., Fujitani Y., Wright C.V., Gannon M. Efficient recombination in pancreatic islets by a tamoxifen-inducible Cre-recombinase. Genesis. 2005;42:210–217. doi: 10.1002/gene.20137. [DOI] [PubMed] [Google Scholar]
- 23.Remedi M.S., Rocheleau J.V., Tong A., Patton B.L., McDaniel M.L., Piston D.W. Hyperinsulinism in mice with heterozygous loss of K(ATP) channels. Diabetologia. 2006;49:2368–2378. doi: 10.1007/s00125-006-0367-4. [DOI] [PubMed] [Google Scholar]
- 24.Finol-Urdaneta R.K., Remedi M.S., Raasch W., Becker S., Clark R.B., Struver N. Block of Kv1.7 potassium currents increases glucose-stimulated insulin secretion. EMBO Molecular Medicine. 2012;4:424–434. doi: 10.1002/emmm.201200218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compan V., Pierredon S., Vanderperre B., Krznar P., Marchiq I., Zamboni N. Monitoring mitochondrial pyruvate carrier activity in real time using a Bret-based biosensor: investigation of the Warburg effect. Molecular Cell. 2015;59:491–501. doi: 10.1016/j.molcel.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh A.C., O'Connor M.B. Systemic activin signaling independently regulates sugar homeostasis, cellular metabolism, and ph balance in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5729–5734. doi: 10.1073/pnas.1319116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Havula E., Teesalu M., Hyotylainen T., Seppala H., Hasygar K., Auvinen P. Mondo/Chrebp-Mlx-regulated transcriptional network is essential for dietary sugar tolerance in Drosophila. PLoS Genetics. 2013;9:e1003438. doi: 10.1371/journal.pgen.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ugrankar R., Berglund E., Akdemir F., Tran C., Kim M.S., Noh J. Drosophila glucome screening identifies ck1alpha as a regulator of mammalian glucose metabolism. Nature Communications. 2015;6:7102. doi: 10.1038/ncomms8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 30.Kreneisz O., Chen X., Fridell Y.W., Mulkey D.K. Glucose increases activity and Ca2+ in insulin-producing cells of adult Drosophila. Neuroreport. 2010;21:1116–1120. doi: 10.1097/WNR.0b013e3283409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wicksteed B., Brissova M., Yan W., Opland D.M., Plank J.L., Reinert R.B. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59:3090–3098. doi: 10.2337/db10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song J., Xu Y., Hu X., Choi B., Tong Q. Brain expression of cre recombinase driven by pancreas-specific promoters. Genesis. 2010;48:628–634. doi: 10.1002/dvg.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouwers B., de Faudeur G., Osipovich A.B., Goyvaerts L., Lemaire K., Boesmans L. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metabolism. 2014;20:979–990. doi: 10.1016/j.cmet.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koster J.C., Remedi M.S., Dao C., Nichols C.G. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes. 2005;54:2645–2654. doi: 10.2337/diabetes.54.9.2645. [DOI] [PubMed] [Google Scholar]
- 35.Boyd A.E., 3rd Sulfonylurea receptors, ion channels, and fruit flies. Diabetes. 1988;37:847–850. doi: 10.2337/diab.37.7.847. [DOI] [PubMed] [Google Scholar]
- 36.Ashcroft F.M., Proks P., Smith P.A., Ammala C., Bokvist K., Rorsman P. Stimulus-secretion coupling in pancreatic beta cells. Journal of Cellular Biochemistry. 1994;55(Suppl):54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- 37.Detimary P., Van den Berghe G., Henquin J.C. Concentration dependence and time course of the effects of glucose on adenine and guanine nucleotides in mouse pancreatic islets. The Journal of Biological Chemistry. 1996;271:20559–20565. doi: 10.1074/jbc.271.34.20559. [DOI] [PubMed] [Google Scholar]
- 38.Maechler P., Wang H., Wollheim C.B. Continuous monitoring of ATP levels in living insulin secreting cells expressing cytosolic firefly luciferase. FEBS Letters. 1998;422:328–332. doi: 10.1016/s0014-5793(97)01618-9. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan M., Choi C.S., Ghoshal P., Pliss L., Pandya J.D., Hill D. β-cell-specific pyruvate dehydrogenase deficiency impairs glucose-stimulated insulin secretion. American Journal of Physiology Endocrinology and metabolism. 2010;299:E910–E917. doi: 10.1152/ajpendo.00339.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu D., Mulder H., Zhao P., Burgess S.C., Jensen M.V., Kamzolova S. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2708–2713. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halestrap A.P. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. The Biochemical Journal. 1975;148:85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hildyard J.C., Ammala C., Dukes I.D., Thomson S.A., Halestrap A.P. Identification and characterisation of a new class of highly specific and potent inhibitors of the mitochondrial pyruvate carrier. Biochimica et Biophysica Acta. 2005;1707:221–230. doi: 10.1016/j.bbabio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Divakaruni A.S., Wiley S.E., Rogers G.W., Andreyev A.Y., Petrosyan S., Loviscach M. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohatgi N., Aly H., Marshall C.A., McDonald W.G., Kletzien R.F., Colca J.R. Novel insulin sensitizer modulates nutrient sensing pathways and maintains beta-cell phenotype in human islets. PloS One. 2013;8:e62012. doi: 10.1371/journal.pone.0062012. [DOI] [PMC free article] [PubMed] [Google Scholar]