Abstract
Background
Hypomagnesemia is a common adverse event during cetuximab (Cmab) treatment. However, few reports have investigated the incidence and risk factors of hypomagnesemia in head and neck cancer patients treated with Cmab.
Methods
We retrospectively reviewed 131 head and neck cancer patients who received Cmab-containing therapy. Main eligibility criteria were ≥3 Cmab administrations, no prior EGFR-directed therapy, and no prophylactic Mg supplementation.
Results
Median baseline serum Mg level and number of Cmab administrations were 2.2 mg/dl and 8, respectively. Overall incidence of hypomagnesemia was 50.4% (grade 1, 46.6%; grade 2, 3.1%; grade 3, 0%; and grade 4, 0.8%) and differed between patients treated with palliative chemotherapy and bioradiation (Cmab and radiation) (63 versus 24%; P < 0.01). Independent risk factors were low baseline serum Mg [odds ratio (OR) 161.988, 95% confidence interval (CI) 9.436–2780.895], ≥7 Cmab administrations (OR 3.56, 95% CI 1.16–13.98), and concurrent administration of platinum (cisplatin; OR 23.695, 95% CI 5.219–107.574, carboplatin; OR 5.487, 95% CI 1.831–16.439). Respective incidence of hypomagnesemia in patients in high- (concurrent platinum and ≥7 Cmab administrations) and low-risk (no concurrent platinum and <7 Cmab administrations) groups was 66.0 and 6.6% (P < 0.001, OR 28.0).
Conclusion
Cmab is associated with a significant risk of hypomagnesemia in patients with head and neck cancer with longer term administration and concurrent platinum therapy. High-risk patients should be treated with particular care.
Keywords: hypomagnesemia, cetuximab, head and neck cancer, squamous cell carcinoma, chemotherapy, radiotherapy
Introduction
Cetuximab (Cmab) is a human–murine monoclonal antibody directed against the EGFR protein and is the only approved molecular targeted drug for the treatment of squamous cell carcinoma of the head and neck. Cmab has been demonstrated to enhance sensitivity to radiotherapy and chemotherapy and improve overall survival (1, 2). As a single agent, Cmab has a response rate of 13% in patients with platinum-refractory head and neck squamous cell carcinoma (3). This background will likely increase the use of this agent and in turn increase the incidence of toxicities associated with the prolonged use of Cmab.
The use of anti-EGFR mAbs is associated with a number of unique adverse events. Previous meta-analyses have shown an increased risk of rash, nail changes, and venous thromboembolism (4–6). Hypomagnesemia associated with Cmab has also been reported. Hypomagnesemia can result in cardiac arrhythmia, coronary artery vasospasm, and sudden cardiac death, and is a serious adverse event in patients treated with Cmab. However, the symptoms of hypomagnesemia may be fairly non-specific, including irritability, paresthesia, and severe fatigue, which are easily attributable to the underlying tumor or to previous chemotherapy regimens (7). Thus, the diagnosis of Cmab-induced hypomagnesemia may be overlooked, and the impact of the condition may be underestimated. Moreover, few studies that focused on the incidence and risk factors of hypomagnesemia in head and neck cancer patients treated with Cmab.
Here, we retrospectively reviewed the incidence and effects of hypomagnesemia in a series of head and neck cancer patients who received Cmab-containing therapy.
Patients and Methods
We reviewed the medical records of patients treated with Cmab-containing treatment at the National Cancer Center Hospital East Japan between February 2012 and March 2014. Main eligibility criteria included age ≥20 years, no prior history of EGFR-directed therapy, ≥3 Cmab administrations, and no prophylactic Mg supplementation and stage III or IV advanced head and neck cancer. All patients received Cmab at the dose of 400 mg/m2 IV on day 1 and 250 mg/m2 weekly thereafter. In the Cmab plus radiation (bioradiation) group, Cmab was given for the duration of radiotherapy. Patients treated with palliative chemotherapy were eligible to receive a platinum agent (cisplatin, CDDP; or carboplatin, CBDCA). In these settings, patients who had at least stable disease received Cmab monotherapy until disease progression or until unacceptable toxic effects after a maximum of six cycles of platinum administration. Serum Mg levels were recorded before administration at least once every week during the treatment of Cmab. The study was approved by the Clinical Research and Ethical Review Board of our institutional hospital (task number: 2013-283).
Statistical Analysis
The incidence of hypomagnesemia was calculated using the number of patients with hypomagnesemia and the total number of patients receiving Cmab treatment. The primary endpoint was the incidence of hypomagnesemia (i.e., Mg concentration below the lower limit of normal), and grade was recorded according to CTCAE version 4.0. The predictive value for hypomagnesemia was assessed by Cox regression models in multivariate analysis, with adjustment for the following potential prognostic variables: baseline serum Mg and calcium level, baseline creatinine clearance calculated from the Cockcroft–Gault formula (≥60 versus <60 ml/min), gender (male versus female), age (<60 versus ≥60 years), nutrition risk index calculated from serum albumin and body weight (8) (≥100 versus <100), number of Cmab administrations (<7 versus ≥7), concurrent platinum administration (absent versus present), history of platinum administration (absent versus present), and grade of rash (0–1 versus ≥2). SPSS version 21 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Patient and Treatment Characteristics
A total of 131 patient cases were available for analysis. Most patients were men (79%) with a median age of 63 years (range 24–79 years). Main primary disease sites included the hypopharynx (24%) and oropharynx (20%). Eight patients (6%) had stage III disease and the remainder (94%) had stage IV. Over half of the patients had a history of platinum administration; among these, median time from the last administration of platinum to the initiation of Cmab was 154 days (range 7–2650). Median baseline serum Mg level, serum Ca level, and creatinine clearance were 2.2 mg/dl (range 1.8–2.6), 9.4 mg/dl (range 7.4–11.4), and 74.5 ml/min (range 29.7–195.0), respectively (Table 1). The most frequent treatment form was palliative chemotherapy (48%), and the median number of cycles of Cmab administration was 8 (range 3–65) (Table 2).
Table 1.
Patient and disease characteristics (N = 131).
| Characteristic | |
|---|---|
| Median age (years) (range) | 63 (24–79) |
| Sex, n (%) | |
| Male/female | 103 (79)/28 (21) |
| Stage, n (%) | |
| III/IV | 8 (6)/123 (94) |
| Primary site, n (%) | |
| Oral cavity | 17 (13) |
| Oropharynx | 26 (20) |
| Hypopharynx | 32 (24) |
| Nasopharynx | 21 (16) |
| Larynx | 11 (8) |
| Nasal cavity/paranasal sinus | 16 (12) |
| Salivary gland | 6 (5) |
| Unknown primary site | 1 (1) |
| Others | 1 (1) |
| Initial median Mg level (mg/dl) (range) | 2.2 (1.8–2.6) |
| Initial median adjusted Ca level (mg/dl) (range) | 9.4 (7.4–11.4) |
| Initial median CCra (ml/min) (range) | 74.5 (29.7–195.0) |
| Nutrition risk indexb (range) | 104 (66–132) |
| Platinum history, n (%) | |
| Yes | 48 (37) |
| No | 83 (63) |
CCr, creatinine clearance.
aCockcroft–Gault equation.
b1.519 × serum albumin + 41.7 × actual weight/ideal weight.
Table 2.
Treatment characteristics (N = 131).
| Characteristic | |
|---|---|
| Treatment form, n (%) | |
| Bioradiation | 25 (19) |
| Induction chemotherapy | |
| PTX + CBDCA + Cmab | 30 (23) |
| DTX + CDDP + Cmab | 10 (8) |
| PTX + Cmab | 1 (1) |
| CDDP + 5-FU + Cmab | 1 (1) |
| Palliative chemotherapy | |
| PTX + CBDCA + Cmab | 25 (19) |
| Cmab alone | 16 (12) |
| CDDP + 5-FU + Cmab | 16 (12) |
| PTX + Cmab | 5 (4) |
| DTX + Cmab | 1 (1) |
| CBDCA + 5-FU + Cmab | 1 (1) |
| Median no. of Cmab administrations, n (range) | 8 (3–60) |
| Concurrent platinum (%) | |
| Yes | 83 (63) |
| No | 48 (37) |
PTX, paclitaxel; CBDCA, carboplatin; Cmab, Cetuximab; DTX, docetaxel; CDDP, cisplatin; 5-FU, 5-fluorouracil.
Incidence and Risk Factors of Hypomagnesemia
Almost all patients (96%) had a decrease in serum Mg level during treatment compared with baseline measurements. The median Mg reduction was 0.4 mg/dl (range 0 to −1.2), which was corresponding to 20% (0–67). A higher (≥2.2 mg/dl) baseline serum Mg level was associated with a steeper slope (mean reduction: −0.51 versus −0.39 mg/dl; P = 0.012). The overall incidence of hypomagnesemia was 50.4% (grade 1, 46.6%; grade 2, 3.1%; grade 3, 0%; and grade 4, 0.8%), with a median follow up of 76 days (range 20–624). Median time to the onset of hypomagnesemia was 32 days (range 8–427), and the most severe decrease was seen at 55 days (range 10–455) (Figure 1). The incidence of hypomagnesemia varied according to treatment form, being higher in patients treated with palliative chemotherapy than in those treated with bioradiation (63 versus 24%; P < 0.001, Table 3). On multivariate analysis, low baseline serum Mg [odds ratio (OR) 161.988, 95% confidence interval (CI) 9.436–2780.895], ≥7 Cmab administrations (OR 3.556, 95% CI 1.16–13.98), and concurrent administration of CDDP (OR 23.695, 95% CI 5.219–107.574) or CBDCA (OR 5.487, 95% CI 1.831–16.439) were associated with hypomagnesemia (Table 4). Respective incidence of hypomagnesemia in patients in the high- (concurrent platinum and ≥7 Cmab administrations) and low-risk (no concurrent platinum and <7 Cmab administrations) groups was 66.0 and 6.6% (P < 0.001, OR 28.0; Table 5), respectively. Serum Ca level, which influences serum Mg level, was assessed in all patients at the time of the worst hypomagnesemia. The overall incidence of hypocalcemia was low (grade 1, 2%; grade 2, 2%), and a statistically significant correlation between these decreases during treatment was not seen (Spearman’s rho = 0.154, P = 0.104). The most commonly observed symptom considered to be related to hypomagnesemia was cramps (23%). No mental alteration or seizures were recorded.
Figure 1.
Time to appearance (left) and the worst degree (right) of hypomagnesemia.
Table 3.
Incidence of hypomagnesemia according to treatment form.
| Treatment form | n (%) |
|---|---|
| Bioradiation (n = 25) | 6 (24) |
| Induction chemotherapy (n = 43) | 20 (47) |
| Palliative chemotherapy (n = 63) | 40 (63) |
Table 4.
Multivariate analysis of hypomagnesemia.
| Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Initial Mg (mg/dl) | 161.988 | 9.436–2780.895 | <0.001 |
| Initial Ca (mg/dl) | 0.717 | 0.293–1.752 | 0.465 |
| Initial CCr (ml/min) | 0.748–8.726 | 0.134 | |
| ≥60 (n = 104) | Referent | ||
| <60 (n = 27) | 2.556 | ||
| Age (year) | 0.363–2.295 | 0.849 | |
| <60 (n = 54) | Referent | ||
| ≥60 (n = 77) | 0.914 | ||
| Nutrition risk index | 0.623–4.104 | 0.329 | |
| ≥100 (n = 83) | Referent | ||
| <100 (n = 48) | 1.599 | ||
| Cmab cycle, n | 1.16–13.98 | 0.02 | |
| <7 (n = 35) | Referent | ||
| ≥7 (n = 96) | 3.556 | ||
| Concurrent CDDP | 5.219–107.574 | <0.001 | |
| No (n = 104) | Referent | ||
| Yes (n = 27) | 23.695 | ||
| Concurrent CBDCA | 1.831–16.439 | 0.002 | |
| No (n = 71) | Referent | ||
| Yes (n = 60) | 5.487 | ||
| Platinum history | 0.729–5.009 | 0.188 | |
| No (n = 80) | Referent | ||
| Yes (n = 51) | 1.911 | ||
| Rash (grade) | 0.468–3.187 | 0.683 | |
| <2 (n = 89) | Referent | ||
| ≥2 (n = 42) | 1.221 |
CCr, creatinine clearance.
Table 5.
Serum Mg change according to risk classification.
| High-risk group n = 66 |
Low-risk group n = 15 |
P-value | Odds ratio | |
|---|---|---|---|---|
| Cmab cycle ≥7 and concurrent platinum (+) | Cmab cycle <7 and concurrent platinum (−) | |||
| Median initial Mg (range) | 2.2 (1.8–2.6) | 2.2 (1.8–2.6) | – | – |
| ΔMg (mg/dl)c (range) | −0.5 (0 to −1.2) | −0.3 (0 to −0.8) | 0.001a | – |
| ΔMg%d (range) | −21 (0 to −67) | −13 (0 to −31) | 0.001a | – |
| Hypomagnesemiae | 44 (66.6%) | 1 (6.6%) | <0.001b | 28.0 |
aMann–Whitney U test.
bFisher’s exact test.
cMinimum Mg − initial Mg.
dΔMg/initial Mg.
eAll grades.
Status after Appearance of Hypomagnesemia
Among the 66 patients who developed hypomagnesemia, 61 (94%) continued with Cmab-containing treatment, of whom 52 received intravenous (IV) Mg supplementation. These 61 patients with hypomagnesemia received a cumulative total of 533 Cmab administrations. Intravenous Mg supplementation during treatment was given at 16.6 meq/cycle. Thirteen (25%) of the 52 supplemented patients received oral low-dose Mg sulfate temporarily (median dose 3 g/day). No patient solely discontinued Cmab-containing treatment due to intolerable hypomagnesemia only. In those patients with hypomagnesemia at the time of Cmab discontinuation (n = 49), median time to serum Mg recovery to pretreatment levels was 35 days (range 6–408 days), and all patients eventually recovered (Figure 2). Minimum serum Mg level during Cmab-containing treatment, concurrent platinum administration, and number of Cmab administrations were not associated with a longer recovery period (≥35 days).
Figure 2.
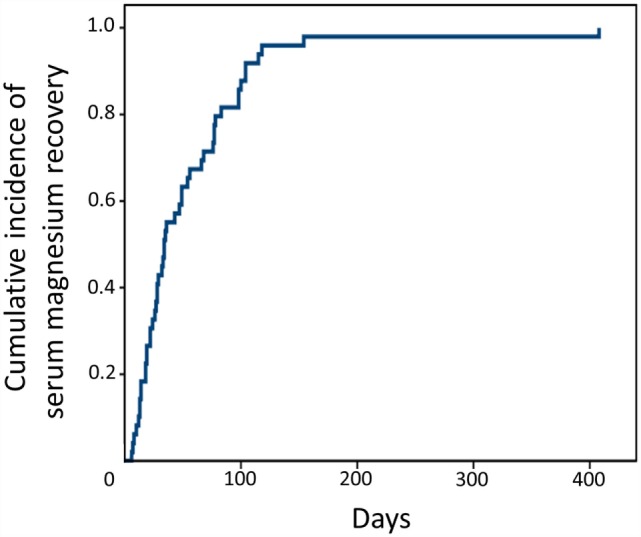
Time to recovery from hypomagnesemia after stopping Cmab administration.
Discussion
In this study, we found that Cmab is associated with a significant risk of hypomagnesemia in patients with head and neck cancer who were receiving concurrent platinum therapy for an extended period. Risk varied with treatment form. These findings indicate the importance of early monitoring of serum Mg level during Cmab-based therapy and suggest that although prophylactic Mg supplementation is not necessary, special attention should be given to high-risk patients.
In healthy individuals, serum Mg concentrations are tightly controlled between intestinal absorption and renal excretion and vary between 0.77 mmol/l (1.82 mg/dl) and 1.10 mmol/l (2.33 mg/dl) (9). Eighty percent of serum Mg is filtered in the glomeruli, with 95% being reabsorbed in the nephron. Patients with advanced cancer might develop hypomagnesemia for any of several reasons, besides anti-EGFR therapy, including decreased oral intake, surgery, platinum agents, and diarrhea (10, 11). Hypomagnesemia during anti-EGFR therapy is mainly caused by a decrease in Mg reabsorption in the kidney. The Mg channel transient receptor potential cation channel, sub-family M, member 6 (TRPM6) is involved in the active reabsorption of Mg in the limb of the loop of Henle and distal convoluted tubule. Activation of renal EGFR located at the basolateral membrane is necessary to prevent renal Mg wasting by stimulation of the epithelial Mg channel TRPM6 (12, 13). Tejpar et al. found clear evidence of defective renal Mg handling, namely, an inappropriately high fractional excretion of Mg in the urine in the setting of serum hypomagnesemia (14). In our study, almost all patients showed a progressive decrease in serum Mg concentration, and the overall incidence of hypomagnesemia was comparable with that in a previous report, which mainly examined patients with colorectal cancer (15). However, the incidence of clinically important grade 3/4 hypomagnesemia was relatively low (0.8%) in comparison with a previously reported meta-analysis, in which grade 3/4 hypomagnesemia occurred in 4.8–5.6% of patients receiving Cmab-based therapy (16, 17). The authors suggested that it might be reasonable to start proactive Mg supplement when grade 1 hypomagnesemia occurred. Patients with higher baseline Mg levels tend to have more pronounced Mg wasting, as previously reported (14).
Our multivariate analysis to assess potential variables affecting the development of hypomagnesemia found that the only baseline characteristic, which predicted hypomagnesemia was low baseline Mg level, irrespective of patient age, baseline creatinine clearance, nutrition status, or history of platinum administration. Concurrent platinum administration was also detected as a risk factor for hypomagnesemia. CDDP directly causes cytotoxic damage to the proximal tubule and tubular reabsorption defects, resulting in hypomagnesemia (18). In addition, a recent study showed that CDDP treatment might also downregulate EGF and TRPM6 in the rat kidney, causing renal Mg loss (19). Moreover, CDDP itself has been associated with shifts in erythrocyte cellular-to-plasma Mg ratios (20). Hypomagnesemia is observed in up to 50% of patients treated with CDDP-containing regimens (18, 21). Carboplatin nephrotoxicity is similar in nature to CDDP nephrotoxicity but occurs less frequently and is usually much less severe. Carboplatin also causes tubular damage which leads to hypomagnesemia but is often reversible (22). Mild glomerular impairment and hypomagnesemia have each been reported in up to 25% of children (23–25).
A retrospective study of 114 colorectal cancer patients from the Roswell Park Cancer Institute suggested a direct relationship between the duration of Cmab exposure and hypomagnesemia (26). Tejpar et al. reported similar findings in a prospective study of 98 patients treated with EGFR-targeting monoclonal antibodies with or without chemotherapy (14). Both studies showed that less than 3 months of Cmab exposure was associated with a lower incidence of hypomagnesemia. This finding is important, given that many patients with head and neck cancer receive Cmab for long periods as maintenance. Together, these findings clearly explain the difference in incidence between our present bioradiation group and palliative group. Generally, patients treated with bioradiation receive fewer administrations of Cmab than those receiving palliative chemotherapy and without concurrent platinum agents.
Although an optimal replacement strategy has yet to be determined, Mg replacement should be considered for patients with grade 2 hypomagnesemia with risk factors (elderly, cardiac disease history) and should be offered to patients with grade 3/4 hypomagnesemia from the viewpoint of safety (27). Further, the biologic relationship between Mg and cancer progression is unclear, and there is no definite evidence to suggest that Mg supplementation reverses the anti-tumor effects of EGFR inhibition. This suggests that proactive Mg replacement should be recommended. In our study, after the appearance of hypomagnesemia, we successfully continued Cmab administration mainly under intravenous Mg supplementation, indicating its effectiveness and tolerance. With respect to supplemental method, many patients with metastatic colorectal cancer receiving Cmab developed severe and refractory hypomagnesemia and poorly tolerated oral Mg supplementation due to diarrhea (28). On the other hand, rapid elevation of plasma Mg concentration may inhibit Mg reabsorption, leading to hypermagnesiuria (29, 30). In addition, one-quarter of our present patients who continued Cmab administration after the appearance of hypomagnesemia received oral Mg sulfate in combination with intravenous Mg supplementation without severe diarrhea. The optimum method of Mg supplementation warrants further evaluation. In a previous study (14), hypomagnesemia was reversible and complete recovery was seen once the anti-EGFR targeted agent was discontinued. In our present report, serum Mg levels corrected within 4–6 weeks of stopping Cmab. A stop-and-go approach to Cmab administration is an alternative for patients with severe and refractory hypomagnesemia and without a large tumor burden. Several retrospective studies have examined the role of early hypomagnesemia induced by Cmab as a predictor of efficacy and outcome in colorectal cancer (31, 32). However, the role of hypomagnesemia as predictive of outcome in head and neck patients treated with Cmab has not been clearly established, and we were also unable to assess this because of the heterogeneity of patient characteristics and treatment forms.
Several limitations of the study warrant mention. First, due to a lack of precise information, we did not evaluate other potential variables, such as use of diuretics, diarrhea, or oral intake during treatment. As an example, CDDP treatment also produces gastrointestinal side effects and requires diuretics, which might lead to greater Mg depletion. Second, we did not assess symptoms related to hypomagnesemia with a validated questionnaire. This might have underestimated the symptomatic impact of Cmab-induced hypomagnesemia. Prospective observational studies will provide further evidence to guide practice.
Conclusion
In this study, we showed that Cmab is associated with a significant risk of hypomagnesemia in patients with head and neck cancer who were receiving longer term administration and concurrent platinum therapy. This risk varies with the treatment form. Early monitoring of serum Mg level is important when Cmab-based therapy is performed, especially in high-risk patients. Evaluation of timing, dose, and route of administration of Mg supplementation, as well as nutritional education, in this population requires further study.
Author Contributions
TE participated in the study concept and design, interpreted the data, and drafted the manuscript. SS, TY, and TW participated in the study concept and design and interpreted the data. MT extracted, managed, and analyzed the data. All authors provided critical revisions and approved the final manuscript.
Conflict of Interest Statement
MT receives honoraria from Merck Serono. The remaining co-authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer NK declared a past co-authorship with one of the authors (MT) to the handling Editor, who ensured that the process met the standards of a fair and objective review.
References
- 1.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med (2006) 354(6):567–78. 10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- 2.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med (2007) 359(11):1116–27. 10.1056/NEJMoa0802656 [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol (2007) 25(16):2171–7. 10.1200/JCO.2006.06.7447 [DOI] [PubMed] [Google Scholar]
- 4.Qi WX, Sun YJ, Shen Z, Yao Y. Risk of anti-EGFR monoclonal antibody-related skin rash: an up-to-date meta-analysis of 25 randomized controlled trials. J Chemother (2014) 26(6):359–68. 10.1179/1973947813Y.0000000155 [DOI] [PubMed] [Google Scholar]
- 5.Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol (2012) 67(3):400–8. 10.1016/j.jaad.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 6.Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Barni S. Risk of anti-EGFR monoclonal antibody-related hypomagnesemia: systematic review and pooled analysis of randomized studies. Expert Opin Drug Saf (2012) 11(Suppl 1):S9–19. 10.1517/14740338.2011.606213 [DOI] [PubMed] [Google Scholar]
- 7.Schrag D, Chung KY, Flombaum C, Saltz L. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst (2005) 97(16):1221–4. 10.1093/jnci/dji242 [DOI] [PubMed] [Google Scholar]
- 8.The veterans affairs total parenteral nutrition cooperative study group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med (1991) 325:525–32. 10.1056/NEJM199108223250801 [DOI] [PubMed] [Google Scholar]
- 9.Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res (2010) 23(4):S194–8. 10.1684/mrh.2010.0213 [DOI] [PubMed] [Google Scholar]
- 10.Deheinzelin D, Negri EM, Tucci MR, Salem MZ, da Cruz VM, Oliveira RM, et al. Hypomagnesemia in critically ill cancer patients: a prospective study of predictive factors. Braz J Med Biol Res (2000) 33(12):1443–8. 10.1590/S0100-879X2000001200007 [DOI] [PubMed] [Google Scholar]
- 11.Kaplinsky C, Alon US. Magnesium homeostasis and hypomagnesemia in children with malignancy. Pediatr Blood Cancer (2013) 60(5):734–40. 10.1002/pbc.24460 [DOI] [PubMed] [Google Scholar]
- 12.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem (2004) 279(1):19–25. 10.1074/jbc.M311201200 [DOI] [PubMed] [Google Scholar]
- 13.Groenestege WM, Thebault S, van der Wijst J, van den Berg D, Janssen R, Tejpar S, et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest (2007) 117(8):2260–7. 10.1172/JCI31680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tejpar S, Piessevaux H, Claes K, Piront P, Hoenderop JG, Verslype C, et al. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol (2007) 8(5):387–94. 10.1016/S1470-2045(07)70108-0 [DOI] [PubMed] [Google Scholar]
- 15.Maliakal P, Ledford A. Electrolyte and protein imbalance following anti-EGFR therapy in cancer patients: a comparative study. Exp Ther Med (2010) 1(2):307–11. 10.3892/etm_00000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Qi Y, Zhang D, Gong C, Yao A, Xiao Y, et al. Electrolyte disorders assessment in solid tumor patients treated with anti-EGFR monoclonal antibodies: a pooled analysis of 25 randomized clinical trials. Tumour Biol (2015) 36(5):3471–82. 10.1007/s13277-014-2983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Liao C, Tan A, Liu L, Gao F. Meta-analysis of incidence and risk of hypomagnesemia with cetuximab for advanced cancer. Chemotherapy (2010) 56(6):459–65. 10.1159/000321011 [DOI] [PubMed] [Google Scholar]
- 18.Sutton RA, Walker VR, Halabe A, Swenerton K, Coppin CM. Chronic hypomagnesemia caused by cisplatin: effect of calcitriol. J Lab Clin Med (1991) 117(1):40–3. [PubMed] [Google Scholar]
- 19.Ledeganck KJ, Boulet GA, Bogers JJ, Verpooten GA, De Winter BY. The TRPM6/EGF pathway is downregulated in a rat model of cisplatin nephrotoxicity. PLoS One (2013) 8(2):e57016. 10.1371/journal.pone.0057016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sartori S, Nielsen I, Tassinari D, Rigolin F, Arcudi D, Abbasciano V. Changes in intracellular magnesium concentrations during cisplatin chemotherapy. Oncology (1993) 50(4):230–4. 10.1159/000227185 [DOI] [PubMed] [Google Scholar]
- 21.Lam M, Adelstein DJ. Hypomagnesemia and renal magnesium wasting in patients treated with cisplatin. Am J Kidney Dis (1986) 8:164–9. 10.1016/S0272-6386(86)80020-8 [DOI] [PubMed] [Google Scholar]
- 22.Foster BJ, Clagett-Carr K, Leyland-Jones B, Hoth D. Results of NCI-sponsored phase I trials with carboplatin. Cancer Treat Rev (1985) 12(Suppl A):43–9. 10.1016/0305-7372(85)90017-9 [DOI] [PubMed] [Google Scholar]
- 23.Pinkerton CR, Broadbent V, Horwich A, Levitt J, McElwain TJ, Meller ST, et al. ‘JEB’ – a carboplatin based regimen for malignant germ cell tumours in children. Br J Cancer (1990) 62(2):257–62. 10.1038/bjc.1990.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt LJ, Broadbent V. Nephrotoxicity following carboplatin use in children: is routine monitoring of renal function necessary? Med Pediatr Oncol (1993) 21(1):31–5. 10.1002/mpo.2950210107 [DOI] [PubMed] [Google Scholar]
- 25.Bergeron C, Dubourg L, Chastagner P, Mechinaud F, Plouvier E, Desfachelles AS, et al. Long-term renal and hearing toxicity of carboplatin in infants treated for localized and unresectable neuroblastoma: results of the SFOP NBL90 study. Pediatr Blood Cancer (2005) 45(1):32–6. 10.1002/pbc.20379 [DOI] [PubMed] [Google Scholar]
- 26.Fakih MG, Wilding G, Lombardo J. Cetuximab-induced hypomagnesemia in patients with colorectal cancer. Clin Colorectal Cancer (2006) 6(2):152–6. 10.3816/CCC.2006.n.033 [DOI] [PubMed] [Google Scholar]
- 27.Saif MW. Management of hypomagnesemia in cancer patients receiving chemotherapy. J Support Oncol (2008) 6:243–8. [PubMed] [Google Scholar]
- 28.Fakih M. Management of anti-EGFR-targeting monoclonal antibody-induced hypomagnesemia. Oncology (Williston Park) (2008) 22(1):74–6. [PubMed] [Google Scholar]
- 29.al-Ghamdi SM, Cameron EC, Sutton RA. Magnesium deficiency: pathophysiologic and clinical overview. Am J Kidney Dis (1994) 24(5):737–52. 10.1016/S0272-6386(12)80667-6 [DOI] [PubMed] [Google Scholar]
- 30.Quamme GA. Renal magnesium handling: new insights in understanding old problems. Kidney Int (1997) 52(5):1180–95. 10.1038/ki.1997.443 [DOI] [PubMed] [Google Scholar]
- 31.Vincenzi B, Santini D, Galluzzo S, Russo A, Fulfaro F, Silletta M, et al. Early magnesium reduction in advanced colorectal cancer patients treated with cetuximab plus irinotecan as predictive factor of efficacy and outcome. Clin Cancer Res (2008) 14(13):4219–24. 10.1158/1078-0432.CCR-08-0077 [DOI] [PubMed] [Google Scholar]
- 32.Vincenzi B, Galluzzo S, Santini D, Rocci L, Loupakis F, Correale P, et al. Early magnesium modifications as a surrogate marker of efficacy of cetuximab-based anticancer treatment in KRAS wild-type advanced colorectal cancer patients. Ann Oncol (2011) 22(5):1141–6. 10.1093/annonc/mdq550 [DOI] [PubMed] [Google Scholar]



