Abstract
The aim of this study was to investigate the relationship between the plaque free wall (PFW) measured by optical coherence tomography (OCT) and the plaque burden (PB) measured by intravascular ultrasound (IVUS). We hypothesize that measurement of the PFW could help to estimate the PB, thereby overcoming the limited ability of OCT to visualize the external elastic membrane in the presence of plaque. This could enable selection of the optimal stent-landing zone by OCT, which is traditionally defined by IVUS as a region with a PB < 40 %. PB (IVUS) and PFW angle (OCT and IVUS) were measured in 18 matched IVUS and OCT pullbacks acquired in the same coronary artery. We determined the relationship between OCT measured PFW (PFWOCT) and IVUS PB (PBIVUS) by non-linear regression analysis. An ROC-curve analysis was used to determine the optimal cut-off value of PFW angle for the detection of PB < 40 %. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated. There is a significant correlation between PFWOCT and PBIVUS (r2 = 0.59). The optimal cut-off value of the PFWOCT for the prediction of a PBIVUS < 40 % is ≥220° with a PPV of 78 % and an NPV of 84 %. This study shows that PFWOCT can be considered as a surrogate marker for PBIVUS, which is currently a common criterion to select an optimal stent-landing zone.
Keywords: Optical coherence tomography, Intravascular ultrasound, Plaque free wall, Plaque burden, Stent-landing zone
Introduction
In recent years, intravascular optical coherence tomography (OCT) has emerged as imaging technique for guiding percutaneous coronary intervention (PCI). OCT offers a very high spatial and lateral resolution compared to intravascular ultrasound (IVUS). However, this comes at the expense of penetration depth into the tissue [1]. Based on IVUS studies [2, 3], the plaque burden (PB) is a leading criterion for a stent-landing zone, where areas with PB < 40 % [4] are considered optimal. The ability of OCT to visualize PB is limited by optical attenuation: the signal decreases with depth in tissue. Either a thick layer of fibrous (low-attenuation) plaque or the presence of superficial lipids or dense macrophage accumulations (high-attenuation) [5], may obscure the visibility of the media behind the plaque. This potentially limits the use of OCT to guide stent implantation when it comes to the selection of the optimal stent landing zones within the target artery. More recently, in addition to low plaque burden, the absence of lipid/necrotic core within the landing zone has been advocated as a decision criterion which can very reliably be identified by OCT, in contrast to IVUS [6].
In a previous study [7], we demonstrated a strong inverse linear relationship between PB and the plaque free wall (PFW) angle using IVUS imaging (PBIVUS and PFWIVUS). In the present study we hypothesize that the angle of the PFW measured in OCT (PFWOCT) can likewise reflect the PB. Second, we postulate that regions with a PB < 40 % can be selected for finding an optimal stent-landing zone. When the PFW angle is small (<180°), it might be instantly clear that disease is present with a PB > 40 %, thus forming a suboptimal stent-landing zone. Similarly, sections with a large PFW angle >270° can be easily and reliably identified by OCT as optimal stent-landing zones with a PB < 40 %. However, in the intermediate region between 180°–270° PFW, the interpretation might be more ambiguous. The aim of this study was to investigate the relationship between PFWOCT and PBIVUS and establish the utility (expressed in predictive values for PB < 40 %) of this relationship to detect PB < 40 % for selection of the optimal stent landing zone by OCT.
Materials and methods
Study population
To study the predictive value of the PFWOCT for determination of the PB, we performed a retrospective observational study. NIRS-IVUS (TVC, InfraRedx, Burlington, Massachusetts, USA) and OCT (St. Jude Medical Inc., St. Paul, MN, USA) pullbacks of the same ROI were used from 18 left anterior descending (LAD) coronary arteries of 18 patients with stable or unstable coronary syndrome included in the OC3T study (Erasmus MC, Rotterdam).
The NIRS-IVUS images were acquired using a commercially available hybrid optical/ultrasound catheter with an automated pullback (pullback speed 0.5 mm/s and 16 frames per second). The OCT images were obtained with C7-XR/Illumien and Dragonfly catheter. Automated OCT pullback (pullback speed 20 mm/s) was performed during simultaneous iso-osmolar X-ray contrast medium (Visipaque 320, GE Healthcare, Buckinghamshire, U.K.) delivery through the guide catheter, using a power injector (Medrad ProVis, Bayer HealthCare LLC, NJ, USA; typical flush rate 3.0 ml/s).
Analysis
The OCT and IVUS pullbacks were matched by overlaying the frames with the same side branches. The remainder of the frames was linearly interpolated in between the side branches. Matched NIRS-IVUS and OCT images were analyzed every millimeter over a ROI of the most proximal 20 mm of a pullback. On the IVUS images, the external elastic lamina (vessel area = VA) and lumen area (LA) contours were drawn using QCU-CMS software (version 4.69, Leiden University Medical Centre, LKEB, Division of Image Processing). These contours were used for the calculation of the plaque area (PA = VA − LA) and subsequently the PB (PA/VA × 100 %). PFW angle was defined from the center of the lumen as the arc of the cross-section with a visible, healthy wall having an intima-media thickness of less than 0.5 mm in both imaging modalities. In total, 360 frames were analyzed on both the IVUS and OCT images. The frame data were averaged over 3 mm sections to increase robustness and to compensate for imprecise matching. Images that showed a side branch either on IVUS or OCT, with the wall out of view or a bad flush were excluded. This resulted in 106 matched IVUS and OCT 3 mm sections suitable for analysis. A total of 10 out of the 18 pullbacks of OCT and IVUS were analyzed by two independent expert observers for assessment of the reproducibility.
Statistics
Statistical analysis was performed using SPSS software (version 21.0, SPSS Inc., Chicago, IL, USA). Findings were regarded significant when p < 0.05. The reproducibility of all PFW and PB measurements was assessed by performing an inter-observer agreement analysis to calculate the intraclass correlation coefficient.
Both a linear and a non-linear model were used to assess the relation between PFWIVUS/OCT and PBIVUS. The non-linear model was defined as follows:
and was computed for both PFWOCT and PFWIVUS. For the non-linear regression analysis, the following starting parameters were chosen: a = 0; b = 55; d = −0.2; e = 100. We denote the crossover value between both linear regimes as PFWC = (e − b)/(a − d), resulting from the regression.
To test whether the slope of the relationship between PFWOCT vs PBIVUS and PFWIVUS vs PBIVUS was similar, implying that with PFWOCT and PFWIVUS the same PB is predicted, a student’s t test for two independent samples was used. An ROC-curve analysis was performed to find the optimal cut-off value of PFWOCT to predict a PBIVUS < 40 %. Based on the optimal cut-off value, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of PFWOCT for prediction of PBIVUS < 40 % were calculated.
Results
Relation between PFW and PB in IVUS
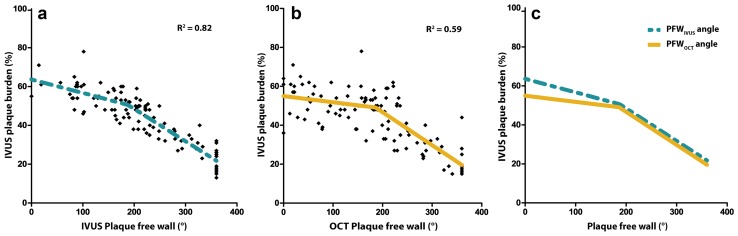
PFWIVUS and PBIVUS were significantly, inversely related (r2 = 0.78) which confirmed the findings of our previous publication [7]. However, a closer fit to the model was found by applying a non-linear regression line (r2 = 0.82) (Fig. 1a), with PFWC = 182°.
Fig. 1.
Relationship between the plaque free wall (PFW) angle and plaque burden (PB). a The PFWIVUS shows a strong inverse, non-linear correlation with the PBIVUS with a crossover point PFWC = 182° PFW. Final parameters: a = −0.069; b = 63.59; d = −0.163; e = 80.74. b A similar relationship is seen for the PFWOCT angle with the PBIVUS with a crossover point PFWC = 186°. Final parameters: a = −0.032; b = 54.98; d = −0.169; e = 80.59. c Overlay of regression lines of (a) and (b). PFWOCT and PFWIVUS perform equally well in predicting PB in the region of PFW angle >186°. In the more diseased regions, the predictive value of the PFWOCT angle is reduced compared to PFWIVUS
Predictive value of PFWOCT
To assess whether the PFWOCT could serve as a surrogate marker for PBIVUS, the PFWOCT values were plotted against the PBIVUS values. Again, a non-linear regression relationship proved significant (r2 = 0.59) (Fig. 1b) with PFWC = 186°. Interestingly, both IVUS and OCT show the same slope (student’s t test for inequality of slopes; p = NS) to predict PB for PFW ≥ 186°. For PFW < 186°, PFWOCT had no clear relationship with the PB, contrasting the PFWIVUS which still showed a weak relationship with the PB (Fig. 1c).
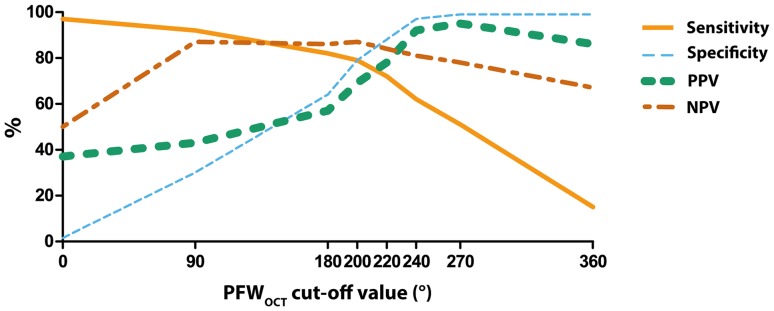
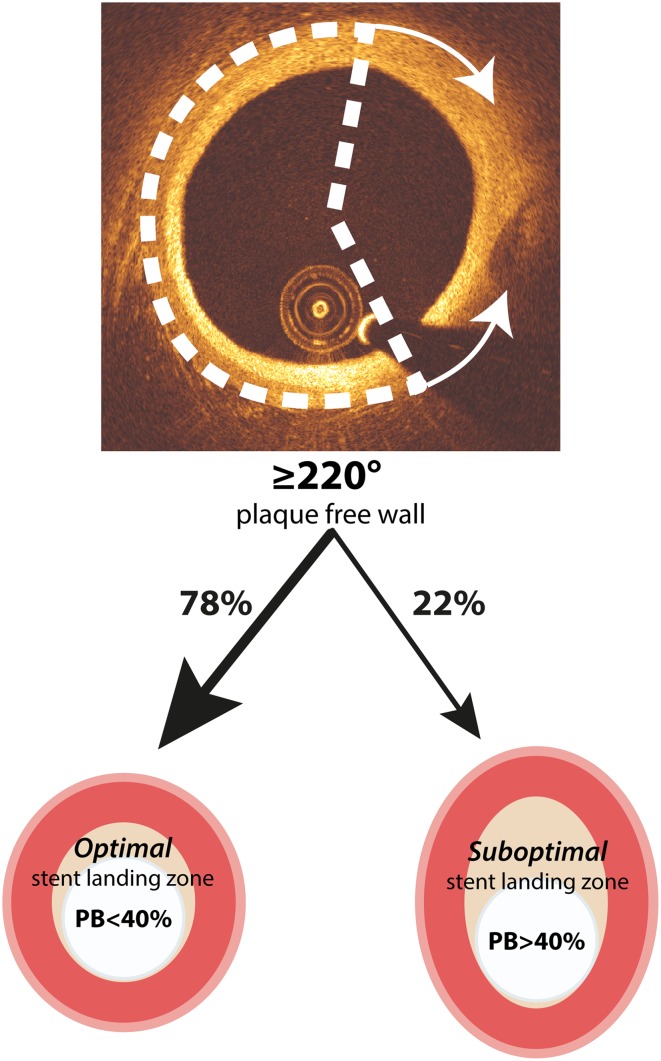
To find the optimal cut-off value of the PFWOCT to predict a PBIVUS < 40 %, an ROC-curve analysis was performed. Optimization of sensitivity, specificity, PPV and NPV resulted in a PFWOCT cut-off value of ≥220° (Fig. 2). With this cut-off value, an optimal stent-landing zone (PB < 40 %) could be predicted correctly in 78 % of the cases (PPV) (Fig. 3). Just as important, the NPV was 84 %, meaning that if the PFW angle is <220°, PB > 40 % and thus forming a suboptimal stent-landing zone in 84 % of the cases. This high negative predictive value will largely prevent stenting too far into a health vessel region (PB < 40 %).
Fig. 2.
Sensitivity, specificity, PPV and NPV values for different PFWOCT angle cut-off points to predict a PB < 40 %. The optimal cut-off point is set at 220° PFWOCT
Fig. 3.
Clinical application of PFWOCT angle detection. When a PFWOCT of ≥220° is detected, in 78 % of the cases this indicates the presence of a PB < 40 %, forming an optimal stent landing zone
As an angle of 220 degrees might be difficult to judge by simple eyeballing in the cathlab, we investigated the predictive value of a PFW > 180 degrees for PB < 40 %. Despite the strong relationship between PFWOCT and PBIVUS, regions with a PFW angle >180° can be regarded as an optimal stent landing zone with a PB < 40 % in only 57 % of the cases. So strikingly, in 43 % of the cases the disease is more severe with a PB > 40 %, even with only half of the circumference being occupied by plaque.
Reproducibility
Inter-observer agreement analyses of the PFW measurements were performed in 189 OCT and 177 IVUS frames and showed high intraclass correlation coefficients of respectively 0.956 (95 % CI 0.941–0.967) and 0.912 (95 % CI 0.884–0.934). The intraclass correlation coefficient of the PBIVUS measurements was equally high at 0.886 (95 % CI 0.847–0.915).
Discussion
This study shows for the first time that PFWOCT can be considered as a surrogate marker for PB, which is currently a common criterion to determine stent-landing zone. In regions with a PFW angle >186°, the PFWOCT has the same predictive value compared to PFWIVUS. The optimized cut-off value to predict optimal stent landing zone (PB < 40 %) is ≥220° PFWOCT.
There is a precarious balance between adequate lesion coverage and using too long stents. When the edge of the stent lands in a plaque area, it poses a risk on plaque disruption and edge dissections on a short-term and in-stent restenosis on a long-term [8]. On the other hand, placing longer stents might prove technically more challenging and might increase the risk on in-stent restenosis and stent thrombosis, even in drug eluting stents [9–11]. For this reason, determining an optimal stent-landing zone is critical for the prevention of future stent-related adverse events.
An optimal stent-landing zone is a region without lipids and a PB < 40 %. In contrast to IVUS, OCT is capable of detecting lipids, but the limited penetration depth hampers PB measurements. OCT can however reliably detect the healthy vessel wall. Therefore, we aimed to investigate if the presence of a normal wall is helpful to predict PB by OCT. This study showed that a measurement of the PFW angle provides a reliable estimate of the PB, without the need to fully visualize the outer wall of the vessel. Together with the fact that OCT is one of the most reliable techniques to structurally assess the presence of a lipid rich plaque, determining the stent-landing zone by OCT becomes even more feasible [12, 13].
The detection of the PFW in OCT can be influenced by the composition of the intima. Plaque components like a lipid/necrotic core and sites of inflammatory activity cause high attenuation and may obscure the vessel wall layers, even in a relatively thin intima. Plaque-free regions (IMT < 0.5 mm) with a superficial layer of macrophages or foam cells (intimal xanthoma) can appear as regions with plaque, since the healthy 3-layered structure of the vessel is not visible. This leads to underestimation of the PFW angle and thus overestimation of the plaque burden. The confounding effect of vessel wall composition may explain why the association between PFWOCT and PB almost vanishes in the more diseased sections. We observe a similar trend in the relation between PFWIVUS and PB, although the detection of the PFW by IVUS is usually not affected by attenuation-inducing plaque components. This might indicate a possible biological phenomenon. In the initial stages of plaque growth, the asymmetric plaque growth results in a simultaneously decreasing PFW angle and increasing PB. Apparently in later stages, the plaque growth becomes more symmetrical, decreasing the PFW angle with only a limited increase in PB and thus weakening the relationship. This explanation should be supported by serial imaging studies.
PCI procedures are always guided by angiography to determine catheter position and lumen narrowing. Currently, it is challenging to identify segments seen on OCT with the corresponding location on angiography which could lead to wrong sizing and positioning of the stent. The recently introduced on-line co-registration of OCT and angiography [14] could greatly increase the utility of OCT and thereby the application of our findings in the clinical work flow. Furthermore, the most predictive cut-off angle of 220° PFW is hard to determine by eye. This might limit direct clinical application of our findings. New software for the automatic detection of the three wall layers and the determination of the PFW angle is under development in our university.
Limitations
Some limitations in our study should be taken into account. Despite the use of side branches for optimal matching of OCT and IVUS pullbacks, errors in matching can occur due to intrinsic differences between the two techniques. These include, amongst others, pullback speed, lateral resolution and frame rate. To minimize the impact of these mismatches on the accuracy of the results, the measurements were averaged over a region of 3 mm, which is a larger than the maximum expected longitudinal mismatch.
Only a limited number of matched pullbacks were available for analysis. Since we analyzed the IVUS images per cross-section and not longitudinally, we regarded the seven data points per pull-back as separate observations. We do not expect that our conclusion would be much different with a larger dataset. However, these results should still be interpreted as being a proof-of-principle study.
Conclusion
In conclusion, this study shows that PFWOCT is a good predictor for the PB in the regions with a PFW angle >186° and gives more insight in the general interpretation of PFWOCT angles. After further clinical validation, this new PB estimation based on OCT could help to identify the optimal landing zone.
Acknowledgments
This study was funded by the European Research Council, grant agreement 310457.
Compliance with ethical standards
The Erasmus MC has a patent licensing agreement with Terumo Corporation. Dr. Van Soest has the rights to receive royalties as part of this agreement. The other authors declare that they have no conflict of interest. Informed consent was obtained from all individual participants included in the study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Footnotes
Evelyn Regar and Jolanda J. Wentzel shared last authorship.
References
- 1.Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 2.Morino Y, Tamiya S, Masuda N, et al. Intravascular ultrasound criteria for determination of optimal longitudinal positioning of sirolimus-eluting stents. Circ J. 2010;74:1609–1616. doi: 10.1253/circj.CJ-10-0025. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi M, Miyazaki S, Myojo M, et al. Impact of the distance from the stent edge to the residual plaque on edge restenosis following everolimus-eluting stent implantation. PLoS One. 2015;10:e0121079. doi: 10.1371/journal.pone.0121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;22:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 5.van Soest G, Goderie T, Regar E et al. Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. J Biomed Opt 15:011105. doi:10.1117/1.3280271 [DOI] [PubMed]
- 6.Gogas BD, Garcia-Garcia HM, Onuma Y, et al. Edge vascular response after percutaneous coronary intervention: an intracoronary ultrasound and optical coherence tomography appraisal: from radioactive platforms to first- and second-generation drug-eluting stents and bioresorbable scaffolds. JACC Cardiovasc Interv. 2013;6:211–221. doi: 10.1016/j.jcin.2013.01.132. [DOI] [PubMed] [Google Scholar]
- 7.Wentzel JJ, Gijsen FJH, van der Giessen R, et al. Positive remodeling at 3year follow up is associated with plaque-free coronary wall segment at baseline: a serial IVUS study. Atherosclerosis. 2014;236:82–90. doi: 10.1016/j.atherosclerosis.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Costa MA, Angiolillo DJ, Tannenbaum M, et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial) Am J Cardiol. 2008;101:1704–1711. doi: 10.1016/j.amjcard.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Teirstein PS. Drug-eluting stent restenosis: an uncommon yet pervasive problem. Circulation. 2010;122:5–7. doi: 10.1161/CIRCULATIONAHA.110.962423. [DOI] [PubMed] [Google Scholar]
- 10.Moreno R, Fernández C, Hernández R, et al. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. 2005;45:954–959. doi: 10.1016/j.jacc.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann R, Mintz GS, Mehran R, et al. Intravascular ultrasound predictors of angiographic restenosis in lesions treated with Palmaz-Schatz stents. J Am Coll Cardiol. 1998;31:43–49. doi: 10.1016/S0735-1097(97)00438-5. [DOI] [PubMed] [Google Scholar]
- 12.Prati F, Romagnoli E, Burzotta F, et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging. 2015;8:1297–1305. doi: 10.1016/j.jcmg.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Kini AS, Motoyama S, Vengrenyuk Y, et al. Multimodality intravascular imaging to predict periprocedural myocardial infarction during percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8:937–945. doi: 10.1016/j.jcin.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Hebsgaard L, Nielsen TM, Tu S, et al. Co-registration of optical coherence tomography and X-ray angiography in percutaneous coronary intervention. The does optical coherence tomography optimize revascularization (DOCTOR) fusion study. Int J Cardiol. 2015;182:272–278. doi: 10.1016/j.ijcard.2014.12.088. [DOI] [PubMed] [Google Scholar]