Abstract
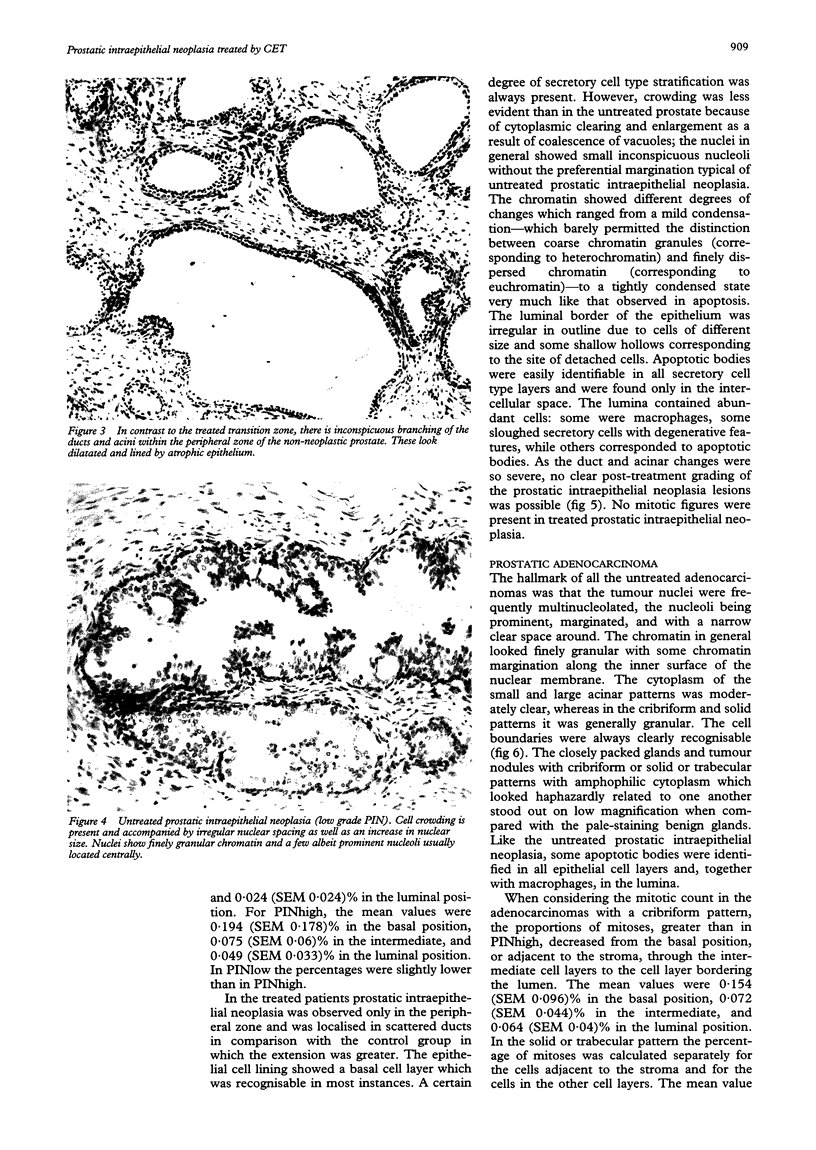
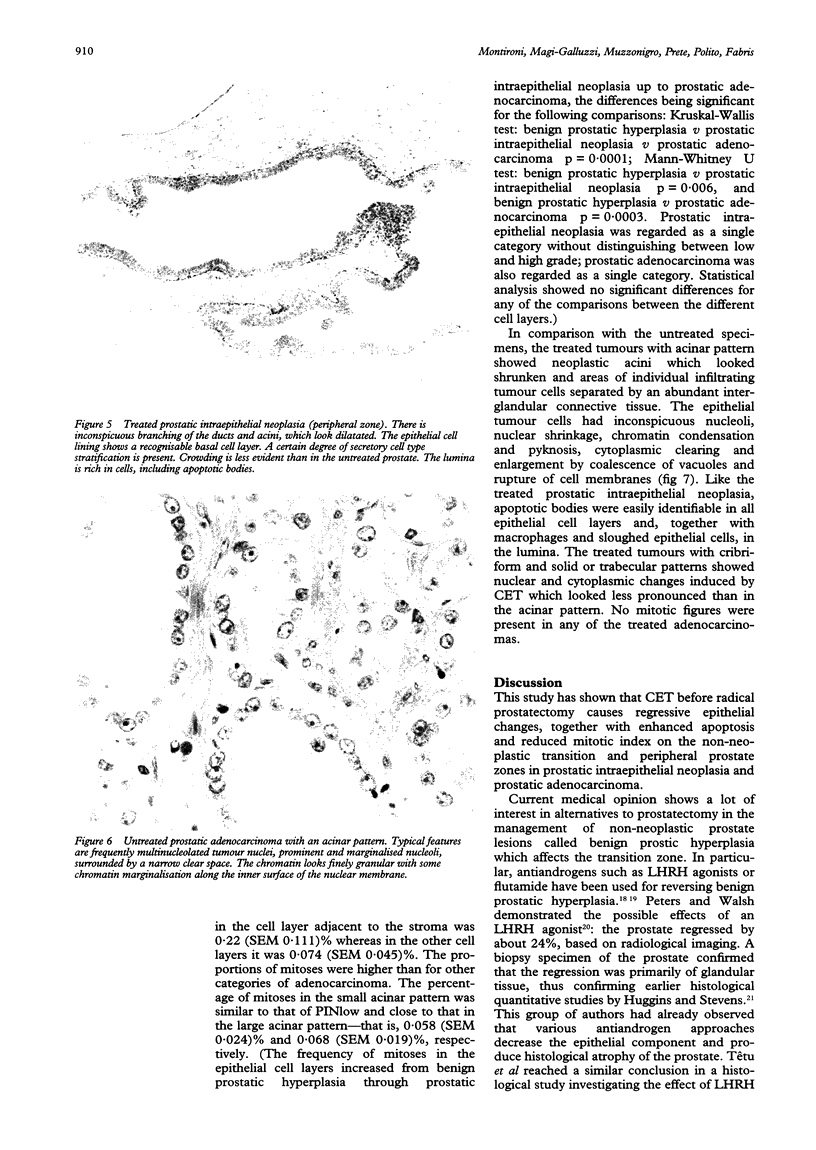
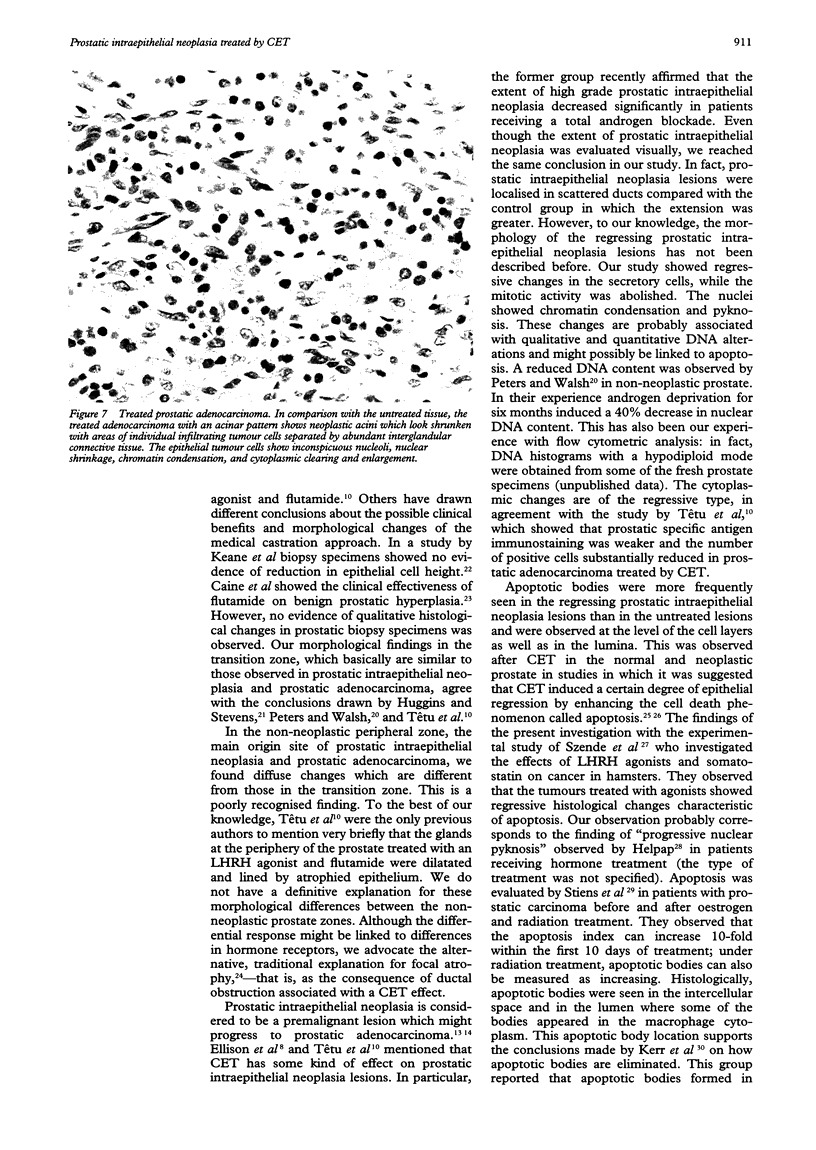
AIMS--To investigate the effect of combination endocrine treatment (CET) or luteinising hormone releasing hormone agonist and flutamide on non-neoplastic prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma. METHODS--The morphology, including the mitotic activity, of 12 radical prostatectomies from patients with prostatic adenocarcinoma pretreated for three months with CET was evaluated in haematoxylin and eosin stained sections and compared with an untreated age and stage matched control group. RESULTS--A differential effect on the non-neoplastic prostate was observed. In fact, the transition zone of the treated prostate showed simplification of the glandular lobules: the ducts and acini were small without undulations of the epithelial border and with a prominent basal cell layer. Within the peripheral zone there was inconspicuous branching of the ducts and acini which looked dilatated and lined by flattened atrophic epithelium. Prostatic intraepithelial neoplasia occurred in scattered ducts and acini in the peripheral zone of 10 of the 12 patients. The epithelial cell lining showed a prominent basal cell layer. A certain degree of secretory cell type stratification was always present. However, crowding was less evident than in the untreated prostate because of cytoplasmic clearing and enlargement as a result of coalescence of vacuoles. The treated adenocarcinomas had neoplastic acini which looked small and shrunken, and areas of individual infiltrating tumour cells separated by abundant interglandular connective tissue. The secretory cells of the nonneoplastic, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma lesions had inconspicuous nucleoli, nuclear shrinkage, chromatin condensation, and cytoplasmic clearing. Apoptotic bodies were easily identifiable in all the cell layers. The lumina were rich in macrophages, sloughed secretory cells with degenerative features, and apoptotic bodies. Mitoses were not observed in any of the treated non-neoplastic prostate, prostatic intraepithelial neoplasia, or prostatic adenocarcinomas, whereas the mitotic frequency increased from non-neoplastic prostate through prostatic intraepithelial neoplasia up to prostatic adenocarcinomas in the untreated specimens. CONCLUSIONS--CET before radical prostatectomy causes regressive epithelial changes together with enhanced apoptosis and blocked mitotic activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akakura K., Bruchovsky N., Goldenberg S. L., Rennie P. S., Buckley A. R., Sullivan L. D. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer. 1993 May 1;71(9):2782–2790. doi: 10.1002/1097-0142(19930501)71:9<2782::aid-cncr2820710916>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bostwick D. G., Brawer M. K. Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer. 1987 Feb 15;59(4):788–794. doi: 10.1002/1097-0142(19870215)59:4<788::aid-cncr2820590421>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Rennie P. S., Coldman A. J., Goldenberg S. L., To M., Lawson D. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990 Apr 15;50(8):2275–2282. [PubMed] [Google Scholar]
- Carroll P. R., Waldman F. M., Rosenau W., Cohen M. B., Vapnek J. M., Fong P., Narayan P., Mayall B. H. Cell proliferation in prostatic adenocarcinoma: in vitro measurement by 5-bromodeoxyuridine incorporation and proliferating cell nuclear antigen expression. J Urol. 1993 Feb;149(2):403–407. doi: 10.1016/s0022-5347(17)36104-9. [DOI] [PubMed] [Google Scholar]
- Crawford E. D., Eisenberger M. A., McLeod D. G., Spaulding J. T., Benson R., Dorr F. A., Blumenstein B. A., Davis M. A., Goodman P. J. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989 Aug 17;321(7):419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- Fair W. R., Aprikian A., Sogani P., Reuter V., Whitmore W. F., Jr The role of neoadjuvant hormonal manipulation in localized prostatic cancer. Cancer. 1993 Feb 1;71(3 Suppl):1031–1038. doi: 10.1002/1097-0142(19930201)71:3+<1031::aid-cncr2820711422>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Geller J. Nonsurgical treatment of prostatic hyperplasia. Cancer. 1992 Jul 1;70(1 Suppl):339–345. [PubMed] [Google Scholar]
- Glashan R. W., Robinson M. R. Cardiovascular complications in the treatment of prostatic carcinoma. Br J Urol. 1981 Dec;53(6):624–627. doi: 10.1111/j.1464-410x.1981.tb03276.x. [DOI] [PubMed] [Google Scholar]
- Jewett H. J. The present status of radical prostatectomy for stages A and B prostatic cancer. Urol Clin North Am. 1975 Feb;2(1):105–124. [PubMed] [Google Scholar]
- Keane P. F., Timoney A. G., Kiely E., Williams G., Stamp G. Response of the benign hypertrophied prostate to treatment with an LHRH analogue. Br J Urol. 1988 Aug;62(2):163–165. doi: 10.1111/j.1464-410x.1988.tb04299.x. [DOI] [PubMed] [Google Scholar]
- Magi Galluzzi C., Montironi R., Giannulis I., Diamanti L., Scarpelli M., Muzzonigro G., Polito M. Prostatic invasive adenocarcinoma. Effect of combination endocrine therapy (LHRH agonist and flutamide) on the expression and location of proliferating cell nuclear antigen (PCNA). Pathol Res Pract. 1993 Dec;189(10):1154–1160. doi: 10.1016/S0344-0338(11)80838-1. [DOI] [PubMed] [Google Scholar]
- McNeal J. E. Normal histology of the prostate. Am J Surg Pathol. 1988 Aug;12(8):619–633. doi: 10.1097/00000478-198808000-00003. [DOI] [PubMed] [Google Scholar]
- Montironi R., Collan Y., Scarpelli M., Sisti S., Barbatelli G., Carnevali A., Pisani E., Mariuzzi G. M. Reproducibility of mitotic counts and identification of mitotic figures in malignant glial tumors. Appl Pathol. 1988;6(4):258–265. [PubMed] [Google Scholar]
- Montironi R., Galluzzi C. M., Diamanti L., Giannulis I., Pisani E., Scarpelli M. Prostatic intra-epithelial neoplasia: expression and location of proliferating cell nuclear antigen in epithelial, endothelial and stromal nuclei. Virchows Arch A Pathol Anat Histopathol. 1993;422(3):185–192. doi: 10.1007/BF01621801. [DOI] [PubMed] [Google Scholar]
- Montironi R., Magi Galluzzi C., Scarpelli M., Giannulis I., Diamanti L. Occurrence of cell death (apoptosis) in prostatic intra-epithelial neoplasia. Virchows Arch A Pathol Anat Histopathol. 1993;423(5):351–357. doi: 10.1007/BF01607147. [DOI] [PubMed] [Google Scholar]
- Murphy W. M., Soloway M. S., Barrows G. H. Pathologic changes associated with androgen deprivation therapy for prostate cancer. Cancer. 1991 Aug 15;68(4):821–828. doi: 10.1002/1097-0142(19910815)68:4<821::aid-cncr2820680426>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Oomens E. H., van Steenbrugge G. J., van der Kwast T. H., Schröder F. H. Application of the monoclonal antibody Ki-67 on prostate biopsies to assess the fraction of human prostatic carcinoma. J Urol. 1991 Jan;145(1):81–85. doi: 10.1016/s0022-5347(17)38253-8. [DOI] [PubMed] [Google Scholar]
- Peters C. A., Walsh P. C. The effect of nafarelin acetate, a luteinizing-hormone-releasing hormone agonist, on benign prostatic hyperplasia. N Engl J Med. 1987 Sep 3;317(10):599–604. doi: 10.1056/NEJM198709033171004. [DOI] [PubMed] [Google Scholar]
- Raymond W. A., Leong A. S., Bolt J. W., Milios J., Jose J. S. Growth fractions in human prostatic carcinoma determined by Ki-67 immunostaining. J Pathol. 1988 Oct;156(2):161–167. doi: 10.1002/path.1711560211. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Tajima M., Sawaki K., Suzuki S., Kudo H., Sassa S., Kuwa K., Sugiura Y., Kasahara N., Nagasawa H. Effects of luteinizing hormone-releasing hormone analogue on DNA synthesis in rat prostate and uterus. In Vivo. 1993 Jan-Feb;7(1):13–16. [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. W., Menon M., Walsh P. C. Hormonal therapy of prostatic cancer. Cancer. 1980 Apr 15;45(7 Suppl):1929–1936. [PubMed] [Google Scholar]
- Sogani P. C., Vagaiwala M. R., Whitmore W. F., Jr Experience with flutamide in patients with advanced prostatic cancer without prior endocrine therapy. Cancer. 1984 Aug 15;54(4):744–750. doi: 10.1002/1097-0142(1984)54:4<744::aid-cncr2820540426>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Szende B., Srkalovic G., Schally A. V., Lapis K., Groot K. Inhibitory effects of analogs of luteinizing hormone-releasing hormone and somatostatin on pancreatic cancers in hamsters. Events that accompany tumor regression. Cancer. 1990 May 15;65(10):2279–2290. doi: 10.1002/1097-0142(19900515)65:10<2279::aid-cncr2820651020>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Têtu B., Srigley J. R., Boivin J. C., Dupont A., Monfette G., Pinault S., Labrie F. Effect of combination endocrine therapy (LHRH agonist and flutamide) on normal prostate and prostatic adenocarcinoma. A histopathologic and immunohistochemical study. Am J Surg Pathol. 1991 Feb;15(2):111–120. doi: 10.1097/00000478-199102000-00002. [DOI] [PubMed] [Google Scholar]
- Visakorpi T. Proliferative activity determined by DNA flow cytometry and proliferating cell nuclear antigen (PCNA) immunohistochemistry as a prognostic factor in prostatic carcinoma. J Pathol. 1992 Sep;168(1):7–13. doi: 10.1002/path.1711680103. [DOI] [PubMed] [Google Scholar]
- Zalatnai A., Paz-Bouza J. I., Redding T. W., Schally A. V. Histologic changes in the rat prostate cancer model after treatment with somatostatin analogs and D-Trp-6-LH-RH. Prostate. 1988;12(1):85–98. doi: 10.1002/pros.2990120111. [DOI] [PubMed] [Google Scholar]
- de Voogt H. J., Rao B. R., Geldof A. A., Gooren L. J., Bouman F. G. Androgen action blockade does not result in reduction in size but changes histology of the normal human prostate. Prostate. 1987;11(4):305–311. doi: 10.1002/pros.2990110403. [DOI] [PubMed] [Google Scholar]