Abstract
We present a 71-year old woman treated with 14 days of 5 mg intraventricular caspofungin for Scedosporium apiospermum complex meningoencephalitis diagnosed after spinal fusion and instrumentation. Cerebrospinal fluid studies improved during therapy and intraventricular administration was well tolerated. Within weeks of discontinuation, the patient experienced clinical deterioration with disease progression. There are sparse data on the efficacy and safety of administering intraventricular caspofungin. While apparently safe, intraventricular caspofungin was insufficient for disease control in this case.
Keywords: Intraventricular, Caspofungin, Scedosporium apiospermum complex, Scedosporium apiospermum
1. Introduction
Scedosporium apiospermum complex, Scedosporium apiospermum and Scedosporium minutisporum, is a ubiquitous saprophytic fungus found in soil, sewage, and polluted waters [1]. Exposure through cutaneous breach or inhalation can result in skin and soft tissue infection or sinopulmonary disease, although Scedosporium apiospermum complex can disseminate widely, including to the central nervous system (CNS) [1], [2]. Most disseminated infections occur in association with immunocompromised states, such as malignancy, use of immunosuppressive agents, or advanced HIV, although this is not prerequisite for disease [1], [2], [3]. We describe a case of Scedosporium apiospermum complex infection in an immunocompetent woman who developed meningoencephalitis following spinal surgery. After failure of conventional therapies, intraventricular administration of caspofungin was attempted as part of a salvage treatment regimen.
2. Case
A fully independent 71-year old female with a history of encephalomalacia from remote traumatic brain injury presented to clinic with progressive neurogenic claudication and radiculopathy associated with magnetic resonance imaging (MRI) findings of lateral recess stenosis at L2–L3, L3–L4, and L4–L5 and central canal stenosis from L4 to L5 anterolisthesis. She underwent elective surgical decompression and instrumentation from L2 to L5 with placement of an allograft interbody spacer and multi-level autograft (day 0). The procedure took place sterilely using an institutional protocol for prophylactic antibiotics including perioperative cefazolin and powdered vancomycin administered to the wound cavity before closure. Her post-surgical course was complicated by displacement of the L4–L5 interbody spacer and ongoing L4 radiculopathy. The surgical site was re-explored and the interbody spacer replaced (day 22). The construct was augmented with bone morphogenic protein.
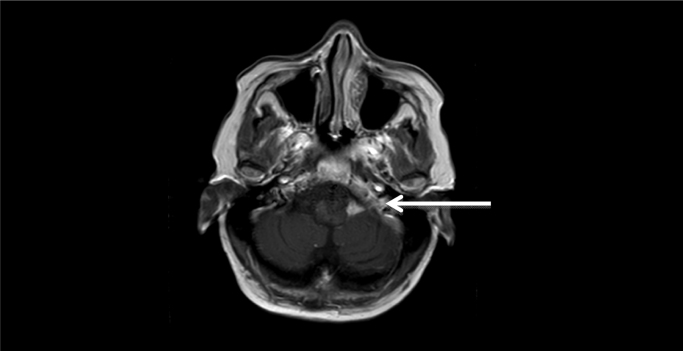
The patient had resolution of radicular symptoms in the month following the second surgery, but began to experience cognitive decline, decreased mobility, and incontinence over several weeks. She was readmitted after a ground level fall (day 92). During this admission, she developed bradykinesia, right-sided hemiparesis, hypophonia, and disorientation. An MRI revealed diffuse ventriculomegaly with possible hydrocephalus as well as two areas of nodular enhancement in the region of the left cerebellopontine angle (CPA) [Fig. 1]. A right frontal extraventricular drain (EVD) was placed (day 96) and her mental status improved.
Fig. 1.
MRI showing enhancing lesion at the left cerebellopontine angle before biopsy confirmation of Scedosporium apiospermum complex infection.
During this hospitalization, an extensive infectious work-up including cerebrospinal fluid (CSF) bacterial, fungal, AFB, and Nocardia stains and cultures; CSF Mycobacterium tuberculosis, non-tuberculous Mycobacterium, Mycoplasma, Tropheryma whipplei, bacterial, and fungal polymerase chain reaction (PCR) studies; CSF cryptococcal antigen; CSF herpes simplex virus, cytomegalovirus and Epstein-barr virus PCRs; serum Coccidioides, Blastomyces, and Brucella antibody testing; Leptospira titer, Lyme serology, and HIV testing was unrevealing. She had an elevated angiotensin converting enzyme (ACE) level and was treated empirically with high-dose steroids for possible neurosarcoidosis but continued to decline clinically. She ultimately underwent bilateral suboccipital craniectomy for biopsy of the CPA mass with placement of a right ventriculoperitoneal shunt (VPS) for hydrocephalus (day 137). Specimens were sent for frozen section and permanent section pathology and microbiological studies, including bacterial, fungal, and mycobacterial stains and culture and broad-range fungal PCR. The frozen section pathology showed tissue necrosis, neutrophilic infiltrate and focal giant cell reaction. Multiple tissue fungal cultures and fungal PCR were positive for Scedosporium apiospermum complex with the following susceptibilities reported by the University of Texas San Antonio Department of Pathology: amphotericin minimum inhibitory concentration (MIC) 16 mcg/mL, posaconazole MIC 2 mcg/mL, voriconazole MIC 1 mcg/mL, caspofungin MIC 1 mcg/mL, and terbinafine MIC >2 mcg/mL. Of note, molecular testing was not conducted to differentiate between Scedosporium apiospermum or Scedosporium minutisporum.
Intravenous voriconazole, often considered first-line therapy, was initiated day 139 with gradual clinical improvement [4]. After two weeks, however, the patient developed shock-like sensations down her upper extremities and progressively worsening bilateral weakness. Repeat MRI showed progression of the initial CPA lesion further into the basal cisterns, diffuse leptomeningeal enhancement, and enhancement of the cervical and superior thoracic CSF space. Given disease progression, intravenous terbinafine was added to her regimen at 250 mg PO daily (day 154), as synergy between terbinafine and azoles may inhibit the growth of Scedosporium isolates [5]. She had a percutaneous endoscopic gastrostomy (PEG) tube placed for feeding and at discharge had only limited residual motor function of her left extremities.
Following discharge home with home health care, she remained on intravenous voriconazole and terbinafine with plan to continue for an undefined course given severity of the infection, lack of experience base to guide treatment duration, and anticipation of difficulty definitively eradicating the infection with retention of the VPS. Serum voriconazole level was maintained in a therapeutic range (1.0 mg/mL and 5.5 mg/mL) apart from a single subtherapeutic concentration of 0.7 mg/mL 6 months into the treatment course.
Eight months into therapy, she was readmitted for two days of increasing somnolence and lethargy (day 366). Her VPS was malpositioned with the distal portion seen intrahepatically on imaging and she underwent shunt adjustment (day 229). After re-positioning, the VPS ceased functioning and a second VPS was placed in the right 4th ventricle (day 367). Voriconazole was transitioned to tablet form through PEG tube and terbinafine was discontinued during admission (day 367). Following shunt adjustment, her mental status condition improved and she was discharged (day 381). After discharge, fungal CSF cultures from day 370 returned positive for Scedosporium apiospermum complex (day 399). Fungal susceptibility testing was not performed and no changes were made to her antifungal regimen.
The patient remained on voriconazole for over two months before being readmitted for unresponsiveness and fevers (day 467). Both of her shunts were exchanged for EVDs. Specimens from the removed shunt systems grew Scedosporium apiospermum complex. She was restarted on terbinafine 250 mg twice daily by PEG tube with continued voriconazole treatment. Scedosporium apiospermum complex susceptibilities were re-tested with the following results: amphotericin MIC 16 mcg/mL, posaconazole MIC 4 mcg/mL, voriconazole MIC 2 mcg/mL, caspofungin MIC 2 mcg/mL, terbinafine MIC >2 mcg/mL, and isavuconazole MIC >16 mcg/mL.
Given the protracted failure of conventional therapy, more aggressive treatment approaches were considered. A successful case of intraventricular caspofungin administration in curing Scedosporium apiospermum complex meningoencephalitis was identified in the literature [6]. In this reported case of a 2-year old near-drowning victim, intraventricular caspofungin in combination with terbinafine and voriconazole was successful in treating multiple Scedosporium apiospermum complex intracerebral abscesses. Although other clinical data on efficacy were lacking, intraventricular caspofungin was considered a viable option for the patient given her disease progression on usual therapy and the increasing MIC for voriconazole. The patient and her family were counseled on the treatment, its risks and potential benefits, available alternatives, and the experimental nature of the plan and consented to treatment.
A preparation of 5 mg/mL caspofungin was prepared for daily administration, using CANCIDAS® (Merck and Co.) which contains a sterile, lyophilized product of caspofungin acetate (50 mg). Following CANCIDAS® package insert instructions, we aseptically added 10.8 mL of preservative-free 0.9% sodium chloride injection into a CANCIDAS® 50 mg vial, reconstituting to a final concentration of 5 mg/mL [7]. The vial was mixed gently until a clear solution was obtained. We then aseptically transferred 1 mL of reconstituted CANCIDAS®, equivalent to a 5 mg dose, into a syringe. This 5 mg caspofungin dose was administered intraventricularly by the neurosurgeon.
Administration was carried out as follows: The proximal port of the patient's right frontal EVD was prepped with betadine each day. Five mL of sterile, preservative-free normal saline was injected into the port as a primer, followed by 1 mL of the 5 mg/mL caspofungin preparation, which was then followed by an additional 5 mL of sterile, preservative-free normal saline. Both the right frontal and right occipital EVDs were then occluded proximally for approximately 30 min to allow for dissemination and absorption of the drug more directly into the CNS. The patient was also given intravenous anti-emetics and narcotic pain medication to help with symptoms from increased intracranial pressure and potential drug-related cerebritis. This routine was continued daily for 14 days total (days 480–493). CSF samples were sent roughly every other day to monitor for trends in cell counts.
Intraventricular caspofungin was well-tolerated. The patient experienced some nausea and headaches temporally associated with intraventricular drug administration and EVD clamping, but these symptoms were well-controlled with IV pain medications and anti-emetics. The patient also experienced transient somnolence during administration, but remained easily arousable with no changes from the pre-admission neurological exam. Repeat computed tomography showed no new lesions or signs of cerebral edema. Her intracranial pressures remained stable on ICP monitoring throughout the caspofungin treatment course. Semi-daily CSF studies showed an oscillatory damping pattern over the course of intraventricular treatment with a decrease in inflammatory cells, although a relative increase in percent eosinophils was noted [Table 1]. The patient's shunts were re-internalized (day 497) and she was discharged on voriconazole and terbinafine through her PEG tube.
Table 1.
Cerebrospinal fluid profile during intraventricular caspofungin therapy.a
| Day intraventricular caspofungin | 2 | 3 | 5 | 7 | 9 | 11 | 13 |
|---|---|---|---|---|---|---|---|
| CSF WBC (cells/μL) | 39 | 40 | 14 | 32 | 10 | 29 | 14 |
| % neutrophils | 39 | 16 | 14 | 4 | 4 | 0 | 0 |
| % lymphocytes | 26 | 14 | 13 | 13 | 17 | 13 | 28 |
| % macrophages | 30 | 67 | 62 | 70 | 57 | 74 | 43 |
| % eosinophils | 5 | 3 | 11 | 13 | 22 | 13 | 29 |
| RBC (cells/μL) | 653 | 475 | 41 | 132 | 205 | 93 | 152 |
| Protein (mg/dL) | 29 | 22 | 28 | 32 | 37 | 28 | 31 |
| Glucose (mg/dL) | 88 | 73 | 80 | 75 | 74 | 86 | 79 |
Values from first sample if multiple cerebrospinal fluid samples were collected on the same day.
Unfortunately, the patient's condition deteriorated five weeks after discharge with development of sixth and seventh cranial nerve palsies (day 530). An MRI of the brain and cervical spine were obtained demonstrated interval development of a new right frontal lobe focus of enhancement measuring 1.0 cm×0.4 cm, along with thickened enhancement of the right occipital horn and increased diffuse pachymeningeal enhancement concerning for progression of intracranial infection. Paradoxically, CSF obtained at the time was within normal limits in its nucleated cell count, glucose, and protein measures. In addition, cultures from that sample and the distal catheter did not grow bacteria or fungi after 4 weeks, and a CSF fungal PCR was negative. The patient and her family did not want additional aggressive treatment maneuvers and she was discharged home to continue voriconazole and terbinafine. The patient ultimately expired within 4 months of discharge (day 648).
3. Discussion
We describe the case of a woman with post-surgical Scedosporium apiospermum complex infection that failed to resolve with usual antifungal therapies. Scedosporium apiospermum complex characteristically exhibits excellent in vitro susceptibility to voriconazole, which is typically selected as initial therapy [8]. After our patient failed voriconazole therapy, terbinafine was added to her regimen. While MICs of terbinafine for Scedosporium apiospermum complex are characteristically high, terbinafine exhibits in vitro synergy with other antifungal agents used to treat Scedosporium and has been used in combination regimens to successfully treat CNS disease [6], [9], [10]. Unfortunately, our patient did not respond to combination treatment options.
After treatment failure on voriconazole and terbinafine, we hoped to obtain high antifungal concentrations in the CNS in order to improve the chance of complete fungal eradication after externalization of both CNS shunts. Decades of experience exist in the use of intraventricular amphotericin and, to a lesser extent, miconazole, for the treatment of serious CNS fungal diseases [11]. The MIC of amphotericin for the initial Scedosporium apiospermum complex isolate was characteristically high (MIC 16 mcg/mL) and we instead opted to use intraventricular caspofungin administered through a clamped EVD. Only 1 case report describes intraventricular administration of an echinocandin for treatment of CNS fungal disease [6]. A 2-year old boy with multiple Scedosporium apiospermum complex brain abscesses after a near-drowning event was treated for 3 weeks with a combination of intravenous voriconazole, terbinafine, and caspofungin. Intraventricular caspofungin was added at a dose of 1–2 mg daily for 20 days after a brain MRI demonstrated new intraventricular, ependymal reaction. Following intraventricular caspofungin therapy, MRI revealed diminution of the brain abscesses but also showed massive cerebral edema and ventriculitis. It is unclear whether these latter findings were a result of treatment or instead related to the underlying disease. The patient ultimately survived, albeit with intellectual deficiency. Our case provides further insight into the safety and clinical and laboratory response to caspofungin administered intraventricularly for the treatment of serious CNS Scedosporium apiospermum complex infections.
Caspofungin is a semisynthetic compound derived from a fermentation product of Glarea lozoyensis. It is a water soluble lipopeptide with a molecular weight of 1213 kDa that is highly protein bound in plasma (approximately 96%) [12]. It is an echinocandin antifungal that inhibits the synthesis of β (1,3)-D-glucan. Mechanistically, disruption of cell wall permeability results in osmotic stress, lysis and cell death. The clinical role of caspofungin in CNS infections is somewhat limited due to its large molecular weight, high plasma protein binding, and water solubility, precluding its ability to penetrate across the blood brain barrier and achieve therapeutic CSF concentration during conventional intravenous therapy [12]. In principle, intraventricular or intrathecal administration of caspofungin can overcome this problem by enhancing distribution of the drug into brain parenchymal tissue [6].
While the single published case suggested that intraventricular caspofungin could be effective treatment for CNS Scedosporium apiospermum complex infection [6], results of use for our patient were mixed. Treatment was well-tolerated, did not cause apparent ventriculitis, and led to initial CSF culture clearance in our patient, but clinical and radiographic evidence of disease progression emerged in the post-treatment phase. It is not clear if intrathecal antifungal therapy produced high drug concentrations in brain parenchyma. In future applications of intraventricular caspofungin to treat Scedosporium apiospermum complex-related meningoencephalitis, it is worth considering higher doses or longer courses as typical for management of invasive fungal disease. Ultimately, alternative antifungal agents with an acceptable CNS safety profile and better CNS penetration may be needed.
Conflict of interest
All authors report no potential conflicts of interest.
Acknowledgments
We thank MM (our patient's husband) for his extremely well-organized data and timeline of the patient's medical course, and David Fredricks and Jill Gersh for manuscript review.
References
- 1.Cortez K.J., Roilides E., Quiroz-Telles F., Meletiadis J., Antachopoulos C., Knudsen T. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 2008;21(1):157–197. doi: 10.1128/CMR.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nesky M.A., McDougal E.C., Peacock J.E., Jr Scedosporium apiospermum complex brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin. Infect. Dis. 2000;31(3):673–677. doi: 10.1086/314042. [DOI] [PubMed] [Google Scholar]
- 3.Castiglioni B., Sutton D.A., Rinaldi M.G., Fung J., Kusne S. Scedosporium apiospermum complex (Anamorph Scedosporium apiospermum). Infection in solid organ transplant recipients in a tertiary medical center and review of the literature. Medicine. 2002;81(5):333–348. doi: 10.1097/00005792-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Troke P., Aguirrebengoa K., Arteaga C., Ellis D., Heath C.H., Lutsar I. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob. Agents Chemother. 2008;52(5):1743–1750. doi: 10.1128/AAC.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meletiadis J., Mouton J.W., Meis J.F., Verweij P.E. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 2003;47(1):106–117. doi: 10.1128/AAC.47.1.106-117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mursch K., Trnovec S., Ratz H., Hammer D., Horré R., Klinghammer A. Successful treatment of multiple Scedosporium apiospermum complex brain abscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode. Childs Nerv. Syst. 2006;22(2):189–192. doi: 10.1007/s00381-005-1151-3. [DOI] [PubMed] [Google Scholar]
- 7.Cancidas(R), [package insert]. Whitehouse Station NMCI, 2001.
- 8.Lackner M., de Hoog G.S., Verweij P.E., Najafzadeh M.J., Curfs-Breuker I., Klaassen C.H. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob. Agents Chemother. 2012;56(5):2635–2642. doi: 10.1128/AAC.05910-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henao-Martínez A.F., Castillo-Mancilla J.R., Barron M.A., Nichol A.C. Combination antifungal therapy in the treatment of Scedosporium apiospermum central nervous system infections. Case Rep. Infect. Dis. 2013:589490. doi: 10.1155/2013/589490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meletiadis J., Meis J.F., Mouton J.W., Rodriquez-Tudela J.L., Donnelly J.P., Verweij P.E. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 2002;46(1):62–68. doi: 10.1128/AAC.46.1.62-68.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen D.Y., Bottini A.G., Hall W.A., Haines S.J. Infections in neurologic surgery. The intraventricular use of antibiotics. Neurosurg. Clin. N. Am. 1992;3(2):343–354. [PubMed] [Google Scholar]
- 12.Dodds Ashley E.S., Lewis R., Martin C., Andes D. Pharmacology of Systemic Antifungal Agents. 2006;43:S28–S39. osaaCID. [Google Scholar]