Highlights
-
•
Myasthenia gravis (MG) has been reported to correlate with earlier-stage thymoma and theoretically does not accompany thymic carcinoma.
-
•
We encountered two cases of thymic carcinoma with MG.
-
•
Very few reports have described MG associated with thymic carcinoma.
Keywords: Thymic carcinoma; Thymoma; Thymectomy; Myasthenia gravis,
Abstract
Introduction
Myasthenia gravis (MG) has been reported to correlate with earlier-stage thymoma, and theoretically does not accompany thymic carcinoma. However, we encountered two cases of thymic carcinoma with MG.
Presentation of cases
Case 1 involved a 54-year-old man who had been diagnosed with MG based on symptoms and detection of anti-acetylcholine receptor antibody (ARAB). Computed tomography (CT) revealed an anterior mediastinal tumor 30 mm in diameter. Prednisolone (PSL) and tacrolimus were administered without surgery at that time. Six years after diagnosis of MG, he was admitted to our hospital and underwent extended thymectomy. Pathological examination revealed type B2-B3 thymoma according to World Health Organization criteria, comprising 80% of the tumor with small cell carcinoma as 20%. Case 2 involved a 51-year-old woman. She had been diagnosed with MG based on eyelid ptosis and detection of ARAB. Ten years after diagnosis of MG, diaphragm elevation was detected on chest X-ray. CT revealed an anterior mediastinal tumor, 47 mm in diameter. We suspected tumor invasion to the right phrenic nerve, right atrium, and superior vena cava. We therefore performed extended thymectomy after preoperative radiotherapy (40 Gy). Pathological examination revealed squamous cell carcinoma.
Discussion
Most cases of thymic carcinomas appear to arise de novo, but appearance in thymomas has been described. In both our cases, MG was treated with pharmacotherapy alone without extended thymectomy, and thymic carcinoma was considered to have developed from the thymoma during long-term follow-up.
Conclusion
Thymic carcinoma can accompany MG.
1. Introduction
Thymomas are neoplasms arising from or exhibiting differentiation toward thymic epithelial cells, into which immature T cells are mixed in various ratios. Because of the existence of immature T cells, thymomas can be thought of as functional tumors. Thymic carcinomas are malignant epithelial tumors with overt cytologic atypia, and a lack of thymus-like features. This is one of the reasons why myasthenia gravis (MG) can accompany thymoma, but theoretically not thymic carcinoma. However, a retrospective analysis of a Japanese database found that three of 304 patients with thymic carcinomas (1%) had MG preoperatively [1]. Very few reports have described MG associated with thymic carcinoma. The 5-year survival rates for all stages of thymoma and thymic carcinomas are 78% and 40%, respectively [2], [3], [4]. Although MG is very rarely associated with thymic carcinoma, this wide difference in survival rate cannot be ignored. Here, we describe 2 cases of MG associated with thymic carcinoma.
2. Case presentations
2.1. Case 1
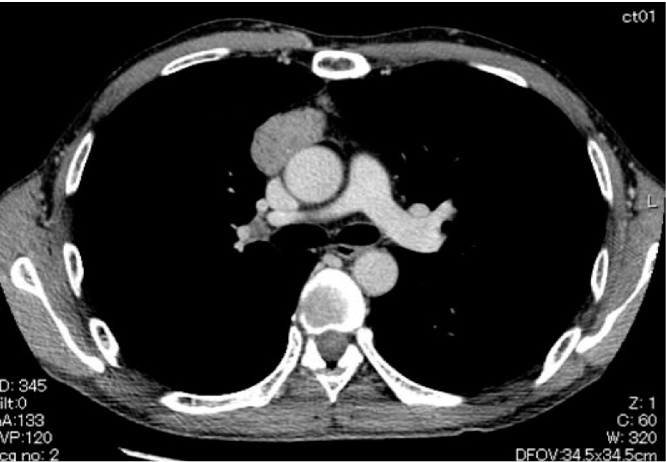
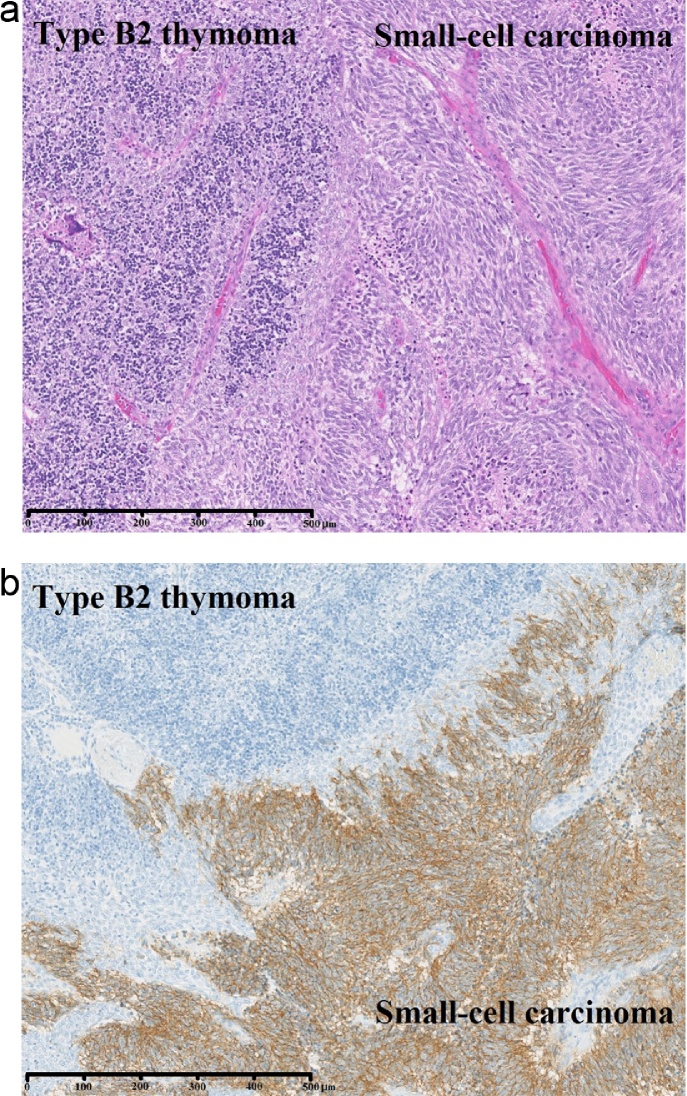
The patient was a 54-year-old man. In 2005, he was diagnosed with MG (Myasthenia Gravis Foundation of America (MGFA) IIA) based on findings of eyelid ptosis, cervical muscle weakness, and detection of anti-acetylcholine receptor antibody (ARAB). Computed tomography (CT) revealed an anterior mediastinal tumor 30 mm in diameter. Treatment at that time comprised prednisolone (PSL) and tacrolimus without surgery. Six years after diagnosis of MG, symptoms were improved and considered stable, and he was therefore admitted to our hospital for surgical treatment of the thymoma. Tumor diameter had grown from 30 mm to 40 mm (Fig. 1). He underwent extended thymectomy and no postoperative complications were seen. Pathological examination of frozen sections showed type B2 thymoma. At 2 weeks after surgery, multiple liver tumors were identified on CT. We therefore performed liver tumor biopsy and diagnosed small cell carcinoma. The final histopathological examination of the previously resected anterior mediastinal tumor revealed type B2-B3 thymoma (according to World Health Organization (WHO) criteria) comprising 80% of the tumor, with small cell carcinoma making up the remaining 20%. Results for neuron-specific enolase (NSE), synaptophysin, and CD56 were positive in the small cell carcinoma and negative in the thymoma on immunohistochemistry (IHC) (Fig. 2).
Fig. 1.
Case 1. Computed tomography (CT) reveals an anterior mediastinal tumor, 40 mm in diameter.
Fig. 2.
(a) Small-cell carcinoma with hematoxylin and eosin (HE) stain. (b) Immunohistochemistry with CD56 shows thymoma comprising 80% of the tumor and small-cell carcinoma comprising 20%.
2.2. Case 2
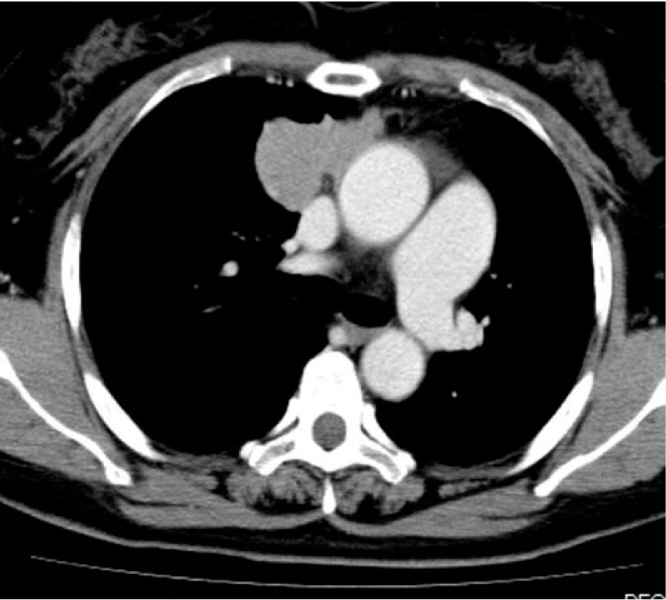
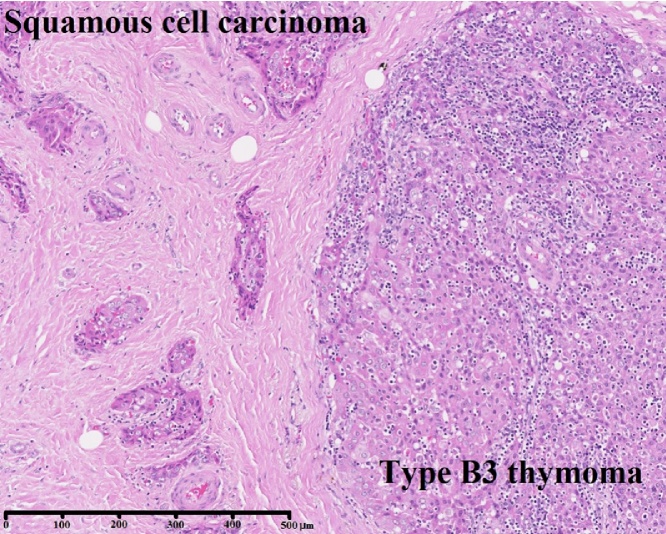
The patient was a 51-year-old woman. In 2004, she had been diagnosed with MG (MGFA I) based on eyelid ptosis and detection of ARAB. Ten years after diagnosis of MG, elevation of the diaphragm was detected on chest X-ray. CT revealed an anterior mediastinal tumor 47 mm in diameter. We suspected tumor invasion to the right phrenic nerve, right atrium, and superior vena cava (Fig. 3), and preoperative radiotherapy (total 40 Gy) was therefore selected. Although the tumor showed a reduction in diameter from 47 mm to 37 mm, invasion to the pericardium remained. At this point, the decision was made to perform surgery. Extended thymectomy and partial resection of the right upper lobe (segments S2 and S3), right phrenic nerve, pericardium, and right pulmonary vein (V2b and V3) were therefore performed. Pathological examination revealed squamous cell carcinoma invading into the pericardium and lung (Masaoka stage III), pT3N0M0 WHO stage III. In this case, the type B3 thymoma was slightly observed within the squamous cell carcinoma (Fig. 4).
Fig. 3.
CT reveals an anterior mediastinal tumor 47 mm in diameter, invading the superior vena cava.
Fig. 4.
Type B3 thymoma within squamous cell carcinoma.
3. Discussion
The relationship between thymoma and MG is well known. Generally, MG has been considered most commonly associated with WHO type A, AB, or B thymoma, but theoretically should not be associated with thymic carcinoma [5], [6], [7], [8], [9]. Most cases of thymic carcinoma appear to arise de novo, but a recent study found that thymic carcinomas can arise in any histological type of thymoma, and thymic carcinoma has been shown to develop in the necrotic tissue of thymoma [10]. The point at which transformation to thymic carcinoma occurs remains unclear. Kim et al. reported three cases of thymic carcinoma with MG. According to that report, all such cases involved type B2 or B3 thymoma in thymic carcinoma [11]. The interval between diagnosis and thymectomy was 10 days, 17 days, and 19 days in these three cases. Malignant transformation of the tumor was considered to have occurred in the long period before tumor detection. Suster et al. reported two cases of thymic squamous cell carcinoma that developed from thymoma after 10 and 14 years [12]. We therefore consider that MG can accompany thymic carcinoma during long-term observation of thymoma. In our two cases, surgery was performed after long-term follow-up of 10 and 6 years. In both cases, we were able to confirm the presence of thymoma in thymic carcinoma. These findings were thus consistent with the reports from Suster et al. [12] and Kim et al. [11].
Since there is a possibility that thymic carcinoma is present in MG thymoma, thymomic tissue with necrosis should be carefully searched during pathological examinations. Even if MG or ARAB has been detected, the possibility of not only thymoma but also thymic carcinoma cases should be considered for anterior mediastinal tumor under long-term observation.
4. Conclusion
Thymic carcinoma can accompany MG. Given the clear difference in 5-year survival rates between thymoma and thymic carcinoma, we should keep in mind the possibility of thymic carcinoma even in cases of MG, especially in cases under long-term observation. Surgery for thymoma should be performed as soon as possible before transformation to carcinoma.
Consent
Written informed consent was obtained from both patients for publication of this case report and the accompanying images.
Conflict of interest
No financial or other potential conflicts of interest exist.
Ethical approval
This study was approved by the ethics committee at Akita University School of Medicine. Both participants provided informed consent and signed a human subject institutional review board consent form.
Funding
None.
Author contribution
Nobuyasu Kurihara: data collection, data analysis, writing the paper, Hajime Saito: data collection, data analysis, Hiroshi Nanjo: data analysis, Hayato Konno: data collection, Maiko Atari: data collection, Yoshitaro Saito: data analysis, Satoshi Fujishima: data collection, Komei Kameyama : data collection, Yoshihiro Minamiya: data collection, data analysis.
Guarantor
Nobuyasu Kurihara.
References
- 1.Nakajima J., Okumura M., Yano M., Date H., Onuki T., Haniuda M. Myasthenia gravis with thymic epithelial tumor: a retrospective analysis of a Japanese database. Eur. J. Carciothorac. Surg. 2016;49:1510–1515. doi: 10.1093/ejcts/ezv380. (pii: ezv380) [DOI] [PubMed] [Google Scholar]
- 2.Zhan P., Xie H., Yu L.K. Response to nab-paclitaxel and nedaplatin in a heavily-metastatic thymic carcinoma: a case report. Oncol. Lett. 2015;9:1715–1718. doi: 10.3892/ol.2015.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo K., Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann. Thorac. Surg. 2003;76:878–884. doi: 10.1016/s0003-4975(03)00555-1. [DOI] [PubMed] [Google Scholar]
- 4.Masaoka A., Yamakawa Y., Niwa H., Fukai I., Saito Y., Tokudome S. Thymectomy and malignancy. Eur. J. Cardiothorac. Surg. 1994;8:251–253. doi: 10.1016/1010-7940(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 5.Marx A., Willcox N., Leite M.I., Chuang W.Y., Schalke B., Nix W. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity. 2010;43:413–427. doi: 10.3109/08916930903555935. [DOI] [PubMed] [Google Scholar]
- 6.Yu L., Zhang X.J., Ma S., Jing Y., Li F., Krasna M.J. Different characteristics of thymomas with and without myasthenia gravis. Ann. Surg. Oncol. 2012;19:94–98. doi: 10.1245/s10434-011-1896-8. [DOI] [PubMed] [Google Scholar]
- 7.Marx A., Ströbel P., Zettl A., Chan J.K., Müller-Hermelink H.K., Harris N.L. Thymomas. In: Travis W.D., Brambilla E., Müller-Hermelink H.K., Harris C.C., editors. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus, and Heart. IARC Press; Lyon: 2004. pp. 152–153. [Google Scholar]
- 8.Ruffini E., Filosso P.L., Mossetti C., Bruna M.C., Novero D., Lista P. Thymoma: inter-relationships among World Health Organization histology, Masaoka staging and myasthenia gravis and their independent prognostic significance: a single-centre experience. Eur. J. Cardiothorac. Surg. 2011;40:146–153. doi: 10.1016/j.ejcts.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa K., Asamura H., Matsuno Y., Suzuki K., Kondo H., Maeshima A. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J. Thorac. Cardiovasc. Surg. 2003;126:1134–1140. doi: 10.1016/s0022-5223(03)00798-0. [DOI] [PubMed] [Google Scholar]
- 10.Kuo T.T., Chan J.K. Thymic carcinoma arising in thymoma is associated with alterations in immunohistochemical profile. Am. J. Surg. Pathol. 1998;22:1474–1481. doi: 10.1097/00000478-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kim Se Hoon, Koh Im Suk, Minn Yang-Ki. Pathologic finding of thymic carcinoma accompanied by myasthenia gravis. J. Clin. Neurol. 2015;11:372–375. doi: 10.3988/jcn.2015.11.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suster S., Moran C.A. Primary thymic epithelial neoplasms showing combined features of thymoma and thymic carcinoma. A clinicopathologic study of 22 cases. Am. J. Surg. Pathol. 1996;20:1469–1480. doi: 10.1097/00000478-199612000-00006. [DOI] [PubMed] [Google Scholar]