Abstract
Anthocyanins are a class of phytochemicals that have generated considerable interest due to their reported health benefits. It has been proposed that commonly consumed anthocyanins, such as cyandin-3-O-β-glucoside (C3G), confer cellular protection by stimulating biosynthesis of glutathione (GSH), an endogenous antioxidant. Currently, it is unknown whether the health effects of dietary anthocyanins are genetically determined. We therefore tested the hypothesis that anthocyanin-induced alterations in GSH homeostasis vary by genetic background. Mice representing five genetically diverse inbred strains (A/J, 129S1/SvImJ, CAST/EiJ, C57BL/6J, and NOD/ShiLtJ) were assigned to a control or 100 mg/kg C3G diet (n=5/diet/strain) for six weeks. GSH and GSSG levels were quantified in liver, kidney, heart, pancreas, and brain samples using HPLC. The C3G diet promoted an increase in renal GSH concentrations, hepatic GSH/GSSG, and cardiac GSH/GSSG in CAST/EiJ mice. C3G treatment also induced an increase in pancreatic GSH/GSSG in C57BL/6J mice. In contrast, C3G did not affect GSH homeostasis in NOD/ShiLtJ mice. Surprisingly, the C3G-diet caused a decrease in hepatic GSH/GSSG in A/J and 129S1/SvImJ mice compared to controls; C3G-treated 129S1/SvImJ mice also exhibited lower total glutathione in the heart. Overall, we discovered that C3G modulates the GSH system in a strain- and tissue-specific manner. To our knowledge, this study is the first to show that the redox effects of anthocyanins are determined by genetic background.
Abbreviations: GSH, Glutathione; GSSG, Glutathione Disulfide; C3G, Cyandin-3-O-β-Glucoside
Keywords: Oxidative stress, Anthocyanin, Cyandin-3-O-β-glucoside, Polyphenol, Antioxidant, Glutathione
Graphical abstract
Highlights
-
•
Effects of the anthocyanin C3G on the GSH system are genetically determined.
-
•
C3G causes oxidizing shifts in GSH redox potential in A/J and 129S1/SvImJ mice.
-
•
Effects of C3G on GSH were most significant in liver and least potent in brain.
1. Introduction
Anthocyanidins and their glycosides, anthocyanins, are flavonoids that contribute to the blue, purple, and red color of many fruits and vegetables, such as blueberries, blackberries, and purple corn [1]. Integration of foods rich in these compounds appears to modulate chronic disease risk in humans. High dietary anthocyanidin intakes have been associated with lower levels of C-reactive protein (CRP), a circulating predictor of cardiovascular disease (CVD) [2], as well as decreased risk of CVD-related mortality [3], [4]. However, evidence suggests that the relationship between anthocyanidin intake and health outcomes is not entirely consistent. For example, Mursu, et al., found no relationship between anthocyanidin intake and CVD mortality in a cohort of Finnish men [5]. To improve understanding of the relationships between anthocyanidins, their corresponding anthocyanins, and health, factors that contribute to inconsistent epidemiological data must be clarified.
Important insight regarding the relationship between anthocyanin intake and health has been gained from various disease models. In models of diabetes [6], obesity [7], [8], cancer [9], [10], and metabolic syndrome [11], anthocyanins decrease markers of oxidative stress. This effect is attributable, in part, to their strong antioxidant activity. These compounds are potent free radical scavengers [12], [13], [14], [15], [16], and they concurrently increase cellular levels of glutathione (GSH), the most abundant endogenous thiol antioxidant. For example, cyanidin-3-O-β-glucoside (C3G), a commonly consumed anthocyanin, increases hepatic GSH levels nearly threefold in a mouse model of type 2 diabetes (T2D) [6]. Such a significant change in GSH levels is noteworthy, as a more robust GSH system has been associated with stress resistance [17]; conversely, depletion of GSH promotes the onset of morbidities such as impaired glucose tolerance [18], cardiomyopathy [19], and carcinogenesis [20].
Recent studies have demonstrated that, in addition to diet, natural genetic variation regulates tissue GSH levels and redox status (GSH/GSSG) [21], [22]. We predicted that the genetic and dietary regulation of the GSH system intersect, and specifically, that C3G effects on GSH levels and GSH/GSSG would vary by mouse strain. We also expected that these redox effects would be most prominent in organs that metabolize and excrete C3G, notably liver and kidney. To test our hypothesis, mice representing five inbred strains (C57BL/6J, A/J, 129S1/SvImJ, NOD/ShiLtJ, and CAST/EiJ) were fed either control or 100 mg/kg C3G diets for six weeks, and the strains were chosen because of their relative genetic diversity, a characteristic that has been utilized in previous studies and during the generation of novel genetic reference populations [23]. We observed that these strains exhibited divergent responses to C3G; two strains exhibited increases in GSH levels while two others exhibited GSH depletion. Our findings demonstrate that genetic background is a critical determinant of redox effects related to anthocyanin-rich diets. Future studies will clarify the genetic mechanisms that mediate anthocyanin effects, which may inform differential responses to these compounds.
2. Material and methods
2.1. Animals
Female C57BL/6J (B6), A/J (A), 129S1/SvImJ (129), NOD/ShiLtJ (NOD), and CAST/EiJ (CAST) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The mice arrived at our facility at 4–6 weeks of age, depending on availability, and the mice were housed in an animal room on a 12 h light/dark cycle in SPF conditions. At three months of age, five mice from each strain were assigned to the control diet, and an additional five mice were assigned to the C3G diet (100 mg/kg). After six weeks of dietary intervention, the mice were humanely euthanized by cervical dislocation, and tissues were harvested for analyses. Prior to euthanasia, all mice were fasted for four hours. The University of Georgia Institutional Animal Care and Use Committee approved all methods and experimental procedures for this study (AUP # A2013 08-011), and all procedures aligned with the National Institutes of Health guide for the care and use of laboratory animals.
2.2. Diet
Mice were fed a standard purified AIN-93M mouse diet (control) or AIN-93M plus C3G (100 mg/kg). The C3G diet was generated as described previously [6]. Briefly, C3G was obtained from Polyphenols Laboratories AS (Sandnes, Norway), and provided to TestDiet (St. Louis, MO) for diet formulation. Control and C3G diets were pelleted. Dietary interventions were initiated when mice reached three months of age and were sustained for six weeks. During the study period, mice were fed ad libitum and given unrestricted access to water. Diets were stored at −20 °C, and fresh food was provided weekly to maintain optimal stability of dietary C3G. Food intake and weights of the mice were also measured on a weekly basis.
2.3. Assessment of Total Glutathione, GSH, GSSG, and GSH/GSSG Ratios
Liver, kidneys, heart, pancreas, and whole brain were removed, rinsed in ice-cold phosphate-buffered saline (PBS), and flash-frozen in liquid nitrogen. Within 24 h after collection, tissues were homogenized and immediately acidified with perchloric acid. Following centrifugation, acidified supernatants were flash-frozen in liquid nitrogen and stored at −80 °C until analysis. GSH and GSSG were quantified by high performance liquid chromatography (HPLC) coupled with electrochemical detection (Dionex UltiMate 3000, Thermo Scientific, Waltham, MA). The cells were set at 1600 mV with a cleaning potential of 1900 mV between samples. The mobile phase was composed of 4.0% acetonitrile, 0.1% pentafluoropropionic acid, and 0.02% ammonium hydroxide; a flow rate of 0.5 ml/min was set. An injection volume of 2.0 μL was used for liver and kidney samples, while 3.0 μL was used for heart, pancreas, and brain samples. External GSH and GSSG standards were prepared in a 1:1 solution of PBS containing 10 mM DTPA and 10% perchloric acid containing 1 mM DTPA to create the same chemical condition as the samples. After electrochemical detection, data were quantified by the Chromeleon Chromatography Data System Software (Dionex Version 7.2, Thermo Scientific). Total glutathione was calculated as GSH+2GSSG, and glutathione redox status was assessed by the ratio GSH/GSSG. GSH and GSSG concentrations were standardized to total protein, which was quantified by Pierce BCA Protein Assay (Thermo Fisher Scientific, Rockford, IL).
2.4. Assessment of GSH redox potential
GSH redox potentials (Eh) were calculated in liver, kidney, heart, pancreas, and brain samples using the Nernst equation, Eh=Eo+RT/nF ln [disulfide]/([thiol]2). Eo represents the standard potential for the redox couple, R is the gas constant, T is temperature, n is 2 for the number of transferred electrons, F is Faraday's constant, and molar concentrations of GSH and GSSG were used. The standard Eo used for GSH/GSSG was −264 mV (mV) for pH 7.4 [24].
2.5. Assessment of endogenous antioxidant enzyme expression
Total RNA was extracted from flash-frozen liver using TRIzol reagent (Thermo Scientific), and cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Thermo Scientific) according to the manufacturer's instructions. SYBR Green MasterMix (BIO-RAD Life Science Research, Hercules, CA) was used to determine relative gene expression of glutamate-cysteine ligase modifier subunit (Gclm; Forward: CACAATGACCCGAAAGAACTG; Reverse: AGACTTGATGATTCCCCTGCT), glutamate-cysteine ligase catalytic subunit (Gclc; Forward: CCTCCTCCTCCAAACTCAGATA; Reverse: CCACAAATACCACATAGGCAGA), glutathione peroxidase-1 (Gpx-1; Forward: CCCGTGCAATCAGTTC; Reverse: TTCGCACTTCTC AAACAA), and glutathione reductase (Gr; Forward: GGTGGTGGAGAGTCACAAGC; Reverse: ATCGTGCATGAATTCCGAGT) using RT-PCR. SYBR green fluorescence was detected by a LightCycler 480 II (Roche Life Science). Target gene expression was normalized using β-actin (Forward: AGCCATGTACGTAGCCATCC; Reverse: CTCTCAGCTGTGGTGGTGAA) as a reference, and all samples were run in triplicate. Quantitative fold-changes were derived using the ΔΔCt method and are presented as the fold-change relative to the B6 control group.
2.6. Statistical analysis
Statistical analyses were performed using GraphPad Prism (Version 6.0, GraphPad Software Inc., La Jolla, CA, USA) and SPSS Statistics (Version 24, IBM, Armonk, NY, USA). Body weight and food intake analyses were completed using two-way ANOVA with Bonferroni adjustments. Independent t-tests were used to identify which strains exhibited altered total glutathione levels, GSH and GSSG concentrations, GSH/GSSG ratios, GSH redox potentials, and expression of GSH-related enzymes in response to C3G treatment. Correlations between relative hepatic enzyme expression and other GSH phenotypes were separately tested among controls, C3G-fed mice, and all mice pooled together using Pearson's coefficient. Groups were first tested for normality using the Shapiro-Wilk test, and skewed distributions were transformed using the square root (Gclc, Gclm, Gpx-1, and GSSG) or reciprocal square root (Gr) function. The level of statistical significance was defined at P<0.05. Data are reported as means±standard error of mean (SEM).
3. Results
3.1. Food intake and body weight
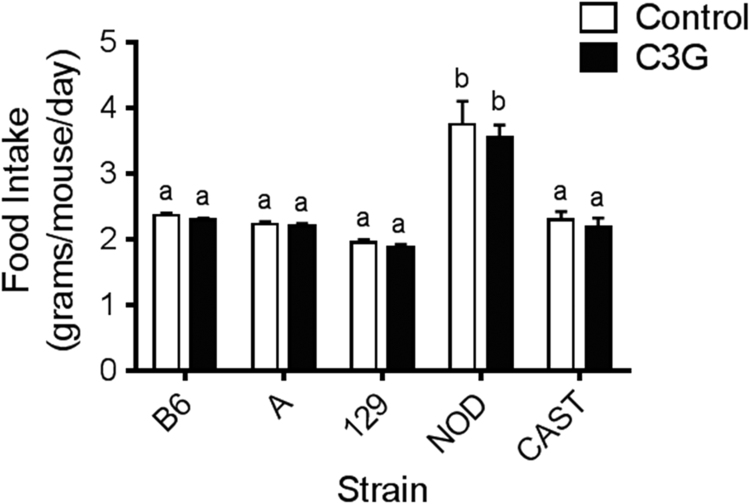
Throughout the six-week duration of the study, there was no significant difference in food intake between C3G-fed mice and their respective controls (Fig. 1). However, a strain effect was observed. NOD mice consumed significantly more food than all other strains, but the NOD treatment groups were not significantly different from one another. Additionally, no differences in body weight changes were observed between groups assigned to the control and C3G diets (Table 1). CAST mice exhibited lower true body weights than the other strains (data not shown). These effects were expected, as CAST mice are wild-derived and are known to be smaller than classical inbred strains.
Fig. 1.
Food intake of mice fed a control or C3G diet for 6 weeks. Data are reported as means±standard error of mean (SEM). Two-way ANOVA with Bonferroni adjustments were used to determine significant differences across all groups. Means without a common letter are statistically different, P<0.05.
Table 1.
Percent initial body weight of mice fed a control or C3G diet for 6 weeks. Data are reported as means±standard error of mean (SEM). Two-way ANOVA with Bonferroni adjustments were used to determine significant differences across all groups at each time point. Means without a common letter are statistically different, P<0.05.
| % Initial body weight | ||||||
|---|---|---|---|---|---|---|
| Group | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 |
| B6 Control | 96.0±1.4a | 96.9±1.2a | 96.7±0.8a | 96.5±2.1ab | 96.8±1.6a | 99.6±0.7a |
| B6 C3G | 97.0±1.9a | 96.8±2.0a | 97.3±1.5a | 97.7±2.2ab | 99.5±3.2a | 99.6±1.6a |
| AJ Control | 101.0±2.6a | 101.9±2.4a | 104.4±2.6a | 108.4±3.2a | 106.9±3.7a | 106.8±3.0a |
| AJ C3G | 101.6±4.0a | 103.1±4.5a | 106.8±4.5a | 106.1±4.7ab | 107.2±4.3a | 105.9±4.1a |
| 129 Control | 96.8±5.9a | 98.8±6.0a | 103.2±5.0a | 102.1±5.6ab | 105.4±5.5a | 108.2±5.6a |
| 129 C3G | 93.9±5.3a | 98.1±5.7a | 99.3±6.0a | 99.5±5.8ab | 101.2±6.1a | 105.7±6.3a |
| NOD Control | 91.9±2.6a | 96.8±2.8a | 99.7±2.5a | 95.5±4.2ab | 95.8±6.2a | 89.5±8.1a |
| NOD C3G | 96.0±2.6a | 100.9±4.2a | 98.8±5.2a | 96.3±7.4ab | 93.4±9.4a | 91.4±10.0a |
| CAST Control | 92.0±0.6a | 91.6±0.4a | 91.3±0.6a | 94.8±1.1ab | 97.9±1.4a | 102.8±1.7a |
| CAST C3G | 90.2±0.9a | 87.8±1.0a | 89.3±1.2a | 87.7±2.2b | 92.9±2.1a | 100.2±2.3a |
3.2. GSH and GSSG levels, total glutathione, redox status, and redox potential
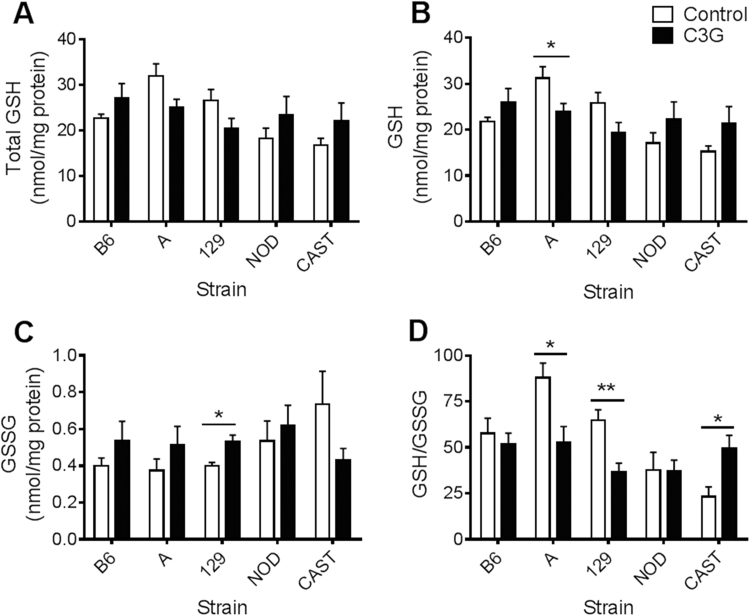
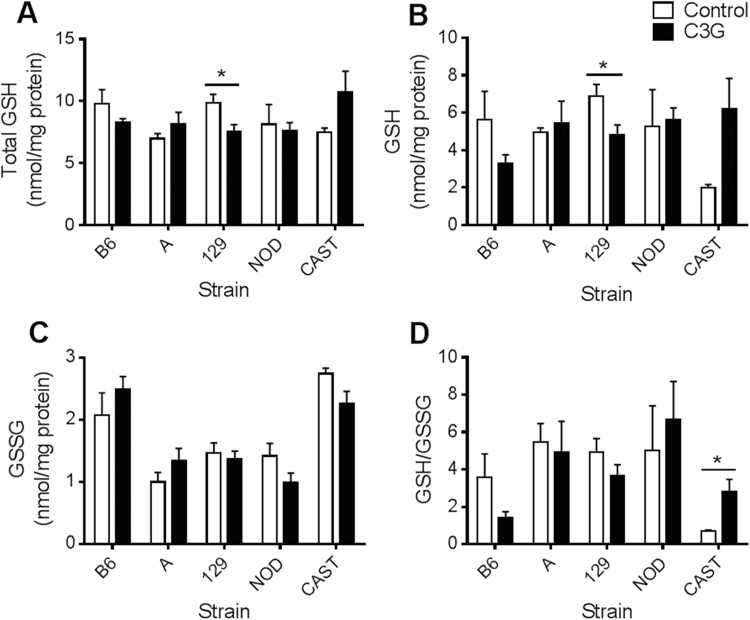
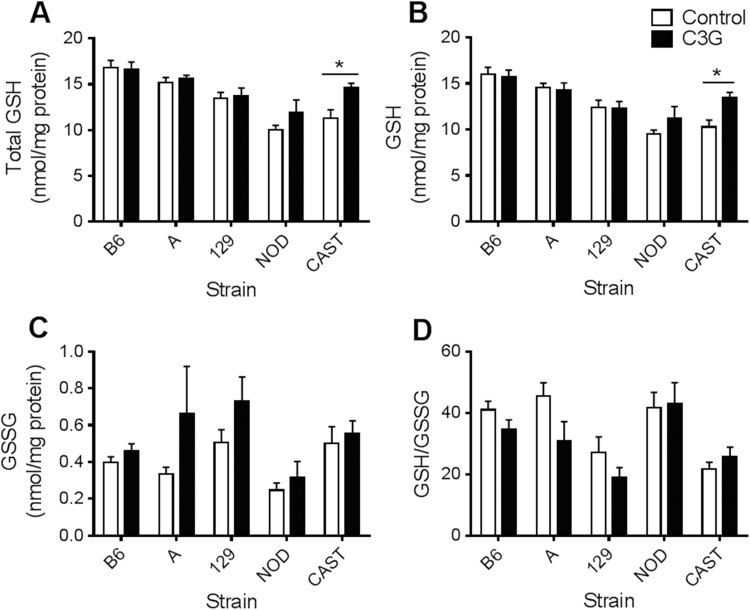
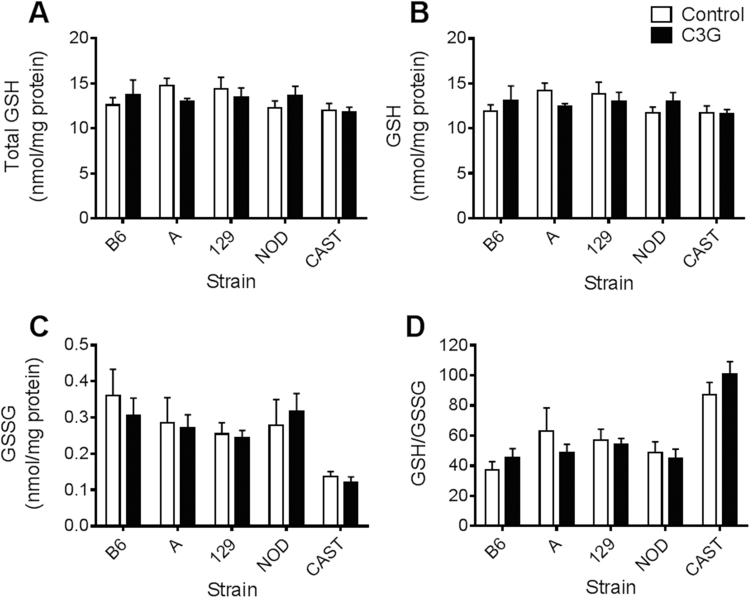
In this study, baseline GSH levels and GSH/GSSG redox statuses reflected the values that have been previously published [21], [25]. In response to C3G treatment, CAST mice showed a twofold increase in hepatic GSH/GSSG (P=0.041; Fig. 2D) and a nearly fourfold increase in cardiac GSH/GSSG (P=0.028; Fig. 3D). Renal total glutathione (P=0.014) and GSH concentrations (P=0.010) increased in C3G-treated CAST mice (Fig. 4A, B), and pancreatic levels of oxidized glutathione, GSSG, were lower in the C3G group compared to control CAST mice (P=0.021) (Fig. 5C). The difference in cardiac GSH concentrations between control and C3G-treated CAST mice approached statistical significance (P=0.056), but did not achieve it.
Fig. 2.
Hepatic glutathione concentrations and redox status in mice fed a control or C3G diet. (A) Liver total glutathione, standardized to total protein; (B) GSH levels; (C) GSSG levels; and (D) GSH/GSSG. Data are reported as means±standard error of mean (SEM). Independent t-tests were conducted to determine significance within each strain in response to C3G treatment, *P<0.05, **P<0.01.
Fig. 3.
Glutathione concentrations and redox status in hearts isolated from mice fed a control or C3G diet. (A) Heart total glutathione, standardized to total protein; (B) GSH levels; (C) GSSG levels; and (D) GSH/GSSG. Data are reported as means±standard error of mean (SEM). Independent t-tests were conducted to determine significance within each strain in response to C3G treatment, *P<0.05.
Fig. 4.
Renal glutathione concentrations and redox status in mice fed a control or C3G diet. (A) Kidney total glutathione, standardized to total protein; (B) GSH levels; (C) GSSG levels; and (D) GSH/GSSG. Data are reported as means±standard error of mean (SEM). Independent t-tests were conducted to determine significance within each strain in response to C3G treatment, *P<0.05.
Fig. 5.
Pancreas glutathione concentrations and redox status in mice fed a control or C3G diet. (A) Pancreas total glutathione, standardized to total protein; (B) GSH levels; (C) GSSG levels; and (D) GSH/GSSG. Data are reported as means±standard error of mean (SEM). Independent t-tests were conducted to determine significance within each strain in response to C3G treatment, *P<0.05.
B6 mice were largely unresponsive to C3G treatment. The exception was B6 pancreas samples, where C3G-fed mice had higher GSH/GSSG than controls (P=0.042; Fig. 5D). However, this effect was modest, only accounting for a 12.5% increase in GSH/GSSG. NOD mice exhibited no phenotypic differences due to C3G treatment, and no significant differences in GSH levels were found within the brains of any of the five strains (Fig. 6).
Fig. 6.
Whole brain glutathione concentrations and redox status in mice fed a control or C3G diet. (A) Whole brain total glutathione, standardized to total protein; (B) GSH levels; (C) GSSG levels; and (D) GSH/GSSG. Data are reported as means±standard error of mean (SEM). Independent t-tests were conducted to determine significance within each strain in response to C3G treatment, *P<0.05.
Surprisingly, the C3G diet caused a 40% decrease in hepatic GSH/GSSG in A mice (P=0.017) and a 43% decrease in 129 mice (P=0.0066) (Fig. 2D). These effects appear to be driven by distinct mechanisms. In A mice, the C3G diet caused a decline in hepatic GSH concentrations (P=0.044) while GSSG levels remained stable (Fig. 2B, C). In contrast, C3G-fed 129 mice displayed increased hepatic GSSG levels (P=0.020) while GSH levels were unaffected (Fig. 2B,C). 129 mice also contained lower cardiac GSH concentrations (P=0.033) and approximately 25% less total glutathione (P=0.034) following the C3G dietary intervention (Fig. 3A, B).
GSH redox potentials were calculated for each tissue (Table 2), and the values were similar to those that have been reported [24], [26]. In these analyses, less negative redox potentials reflect a more oxidizing environment. The C3G diet induced oxidation of the hepatic GSH redox potentials of A mice (P=0.003) and 129 mice (P=0.012). In contrast, the C3G diet promoted a more reducing hepatic redox potential in CAST mice (P=0.031). CAST mice also exhibited more reducing cardiac (P=0.004) and pancreatic (P=0.025) redox potentials on the C3G diet compared to controls. B6 mice exhibited a similar reduction of pancreatic redox potential (P=0.040). No changes were found in redox potential of the kidney or brain as a result of the C3G diet.
Table 2.
GSH redox potentials. Redox potentials (expressed in mV) were calculated in liver, kidney, heart, pancreas, and whole brain samples using the Nernst equation. Data are reported as means±standard error of mean (SEM). Independent t-tests were conducted to determine significance within each strain in response to C3G treatment, *P<0.05, **P<0.01 from control.
| Group | Liver | Kidney | Heart | Pancreas | Brain |
|---|---|---|---|---|---|
| B6 Control | −215.8±2.0 | −204±1.0 | −140.1±10.0 | −188.1±0.7 | −199.5±1.8 |
| B6 C3G | −215.4±1.5 | −201±1.4 | −125.6±4.6 | −190.4±0.7* | −200.6±2.5 |
| AJ Control | −228.7±0.9 | −204±2.0 | −151.8±2.0 | −197.1±1.4 | −204±4.0 |
| AJ C3G | −218.2±2.4** | −196.4±4.7 | −146.9±6.2 | −197±0.7 | −201.7±1.7 |
| 129 Control | −220.1±1.1 | −194.5±3.1 | −152.5±3.4 | −196±1.8 | −204.8±2.1 |
| 129 C3G | −209.4±2.7* | −189.2±2.7 | −146.8±3.6 | −194±1.2 | −203.9±1.7 |
| NOD Control | −207.3±3.6 | −200.6±1.8 | −138.3±10.0 | −192.2±1.8 | −201±2.4 |
| NOD C3G | −211±3.6 | −201.8±2.1 | −154.1±2.9 | −193.6±1.7 | −201.7±1.6 |
| CAST Control | −197.7±1.8 | −187.3±1.2 | −116.3±1.2 | −180.1±0.3 | −208.9±1.5 |
| CAST C3G | −213.3±4.5* | −191.2±2.6 | −141.3±5.3** | −183.8±1.1* | −210.8±1.0 |
3.3. Hepatic expression of GSH-related enzymes
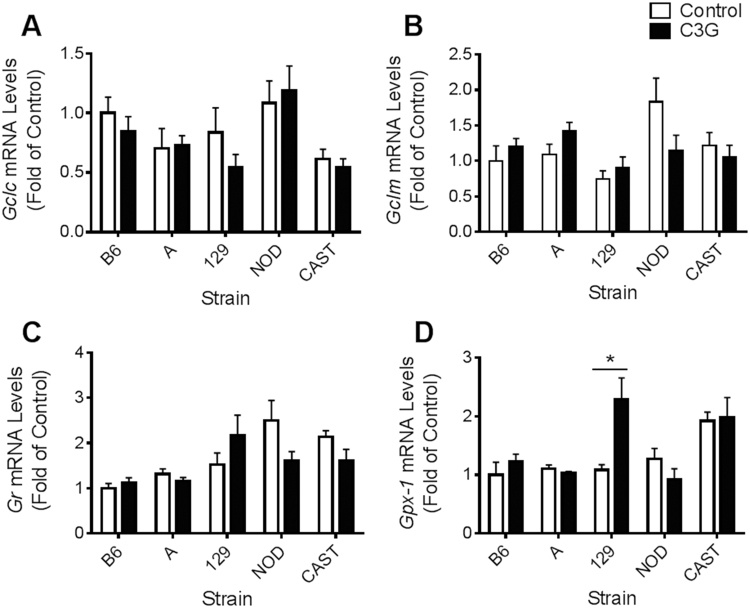
Hepatic expression levels of Gclc, Gclm, and Gr were not significantly different in response to C3G treatment in any of the five strains (Fig. 7A, B, C). Similarly, A, CAST, B6, and NOD did not exhibit altered expression of Gpx-1 in response to C3G supplementation (Fig. 7D). C3G-treated 129 mice did exhibit a twofold increase in Gpx-1 expression compared to controls (P=0.023) (Fig. 7D), which was the only significant change in antioxidant gene expression discovered in this study.
Fig. 7.
Hepatic expression of glutathione enzymes in mice fed a control or high-C3G diet for 6 weeks. (A) Relative Gclc mRNA levels; (B) Gclm mRNA levels; (C) Gr mRNA levels; and (D) Gpx-1 mRNA levels. Data represent fold expression relative to the B6 control group and are reported as means±standard error of mean (SEM). Independent t-tests were conducted to determine significance within each strain in response to C3G treatment, *P<0.05.
3.4. Correlations between expression of GSH-related enzymes and hepatic GSH phenotypes
Relationships between GSH-related enzyme expression levels and GSH phenotypes were tested in the liver (Table 3). After pooling data from control and C3G-treated mice, significant correlations were discovered between Gr and: 1) total glutathione levels (P=0.001); 2) GSH levels (P=<0.001); 3) GSH/GSSG (P=0.003); and 4) GSH redox potential (P=<0.001; Supplemental Figure 1). Significant correlations were also found between Gpx-1 and: 1) total glutathione levels (P=0.042); 2) GSH levels (P=0.037); 3) GSH/GSSG (P=0.049); and 4) GSH redox potential (P=0.014; Supplemental Figure 2). Interestingly, the relationships between Gpx-1 and GSH phenotypes emerged within the control group, yet disappeared in the C3G-treated mice, suggesting that C3G alters the relationship between Gpx-1 expression and GSH homeostasis. In all, higher expression levels of Gr were associated with a more reducing GSH redox potential, while increased expression of Gpx-1 was associated with a more oxidizing redox potential. No significant correlations were present between Gclc or Gclm expression and GSH phenotypes in the pooled data. However, a direct relationship was found between expression of Gclm and GSSG levels in controls (P=0.049), while an inverse relationship was found between Gclm and GSSG levels in C3G-fed mice (P=0.026).
Table 3.
Pearson correlation coefficients for GSH-related enzyme expression and GSH phenotypes in the liver. Correlations were separately tested in controls, C3G-fed mice, and all mice pooled together. The Pearson correlation coefficient is expressed as r, and the P value for the correlation is provided. Statistically significant relationships are indicated in bold, and their corresponding significant p-values are marked with an asterisk.
| Control | C3G | Pooled | ||
|---|---|---|---|---|
| Gclc and Total Glutathione | r | −0.092 | −0.100 | −0.096 |
| p-value | 0.699 | 0.658 | 0.545 | |
| Gclc and GSH | r | −0.081 | −0.102 | −0.090 |
| p-value | 0.735 | 0.650 | 0.572 | |
| Gclc and GSSG | r | −0.190 | −0.025 | −0.132 |
| p-value | 0.422 | 0.911 | 0.404 | |
| Gclc and GSH/GSSG | r | 0.041 | −0.082 | 0.031 |
| p-value | 0.864 | 0.717 | 0.843 | |
| Gclc and GSH Redox Potential | r | −0.017 | 0.161 | 0.043 |
| p-value | 0.942 | 0.474 | 0.786 | |
| Gclm and Total Glutathione | r | −0.228 | −0.182 | −0.209 |
| p-value | 0.334 | 0.417 | 0.185 | |
| Gclm and GSH | r | −0.252 | −0.161 | −0.214 |
| p-value | 0.284 | 0.474 | 0.173 | |
| Gclm and GSSG | r | 0.445 | −0.473 | 0.053 |
| p-value | 0.049* | 0.026* | 0.738 | |
| Gclm and GSH/GSSG | r | −0.365 | 0.388 | −0.102 |
| p-value | 0.113 | 0.075 | 0.519 | |
| Gclm and GSH Redox Potential | r | 0.311 | −0.157 | 0.152 |
| p-value | 0.182 | 0.486 | 0.335 | |
| Gr and Total Glutathione | r | 0.486 | 0.565 | 0.518 |
| p-value | 0.030* | 0.008* | 0.001* | |
| Gr and GSH | r | 0.498 | 0.575 | 0.528 |
| p-value | 0.025* | 0.006* | <0.001* | |
| Gr and GSSG | r | −0.272 | 0.124 | −0.089 |
| p-value | 0.246 | 0.591 | 0.580 | |
| Gr and GSH/GSSG | r | 0.526 | 0.418 | 0.451 |
| p-value | 0.017* | 0.059 | 0.003* | |
| Gr and GSH Redox Potential | r | −0.579 | −0.533 | −0.551 |
| p-value | 0.007* | 0.013* | <0.001* | |
| Gpx−1 and Total Glutathione | r | −0.358 | −0.312 | −0.319 |
| p-value | 0.122 | 0.169 | 0.042* | |
| Gpx−1 and GSH | r | −0.386 | −0.305 | −0.327 |
| p-value | 0.093 | 0.179 | 0.037* | |
| Gpx−1 and GSSG | r | 0.549 | −0.262 | 0.103 |
| p-value | 0.012* | 0.252 | 0.521 | |
| Gpx−1 and GSH/GSSG | r | −0.565 | −0.045 | −0.310 |
| p-value | 0.009* | 0.846 | 0.049* | |
| Gpx−1 and GSH Redox Potential | r | 0.585 | 0.229 | 0.383 |
| p-value | 0.007* | 0.318 | 0.014* | |
4. Discussion
Rationale for the current study was informed by conflicting evidence surrounding chronic disease risk and its relationship with phytochemical intake. For example, total flavonoid intake was found to exhibit an inverse association with stroke incidence [27] and mortality [4], while in other studies, flavonoid intake has exhibited no correlation with risk [28], [29] or mortality [30]. The effect of flavonoid intake on cancer risk has also shown disparate findings. No association was discovered between anthocyanidin consumption and gastric cancer risk in a Korean population [31], but a significant inverse correlation was found in European women [32]. The relationship between T2D and anthocyanidin/anthocyanin intake is similarly uncertain. One study on U.S. adults found an inverse correlation between T2D risk and anthocyanin consumption [33], while a European case-cohort study found no correlation with T2D risk and anthocyanidin intake [34]. Importantly, the inconsistencies highlighted in these studies have been captured in clinical intervention studies as well [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]. In both chronic and acute intervention trials, the effects of anthocyanin consumption on endogenous antioxidant enzyme activity, plasma antioxidant capacity, and DNA damage have been inconsistent [47]. We predict that genetic variation in part drives the variable responses to dietary anthocyanins, and we tested our hypothesis in the current study. We fed mice representing five genetically diverse strains a control or 100 mg/kg C3G diet. Overall food intake did not differ between controls and C3G-fed animals. Furthermore, NOD consumed the largest amounts of C3G diet, yet these mice did not respond in any of the assessments we measured. We therefore concluded that results from the current study were not confounded by strain-dependent differences in food intake.
We tested whether genetic background determines the extent to which C3G regulates GSH levels and redox status. The C3G diet increased pancreatic GSH/GSSG in B6 mice, but this difference represented a relatively minor alteration. The C3G diet exerted no other effects on the GSH system of this strain. Upon initial review, our results appear to conflict with work by Zhu and colleagues, who showed that the same C3G diet increases hepatic GSH synthesis nearly threefold in the same genetic background [6]. However, it must be noted that Zhu, et al., employed db/db mice, which contain a spontaneous mutation on the B6 or C57BLKS/J background that drives a diabetic phenotype. In that study, GSH levels were compared between db/db mice fed control and C3G diets; unstressed wild-type B6 control mice were not included in the design. If unstressed, wild-type B6 mice had been evaluated, as in this study, we predict that a similar lack of effect would have been observed. Taken together, these results suggest that the C3G diet does not alter GSH levels in unstressed B6 mice, and may only rescue GSH levels in stressed, mutant B6 mice. Similarly, the relationship between flavonoid intake and disease risk in some human populations may require a stressor. Cutler, et al., found that flavanone intake was inversely correlated with lung cancer incidence among current and past smokers, but the relationship was not observed among individuals who had never smoked [48].
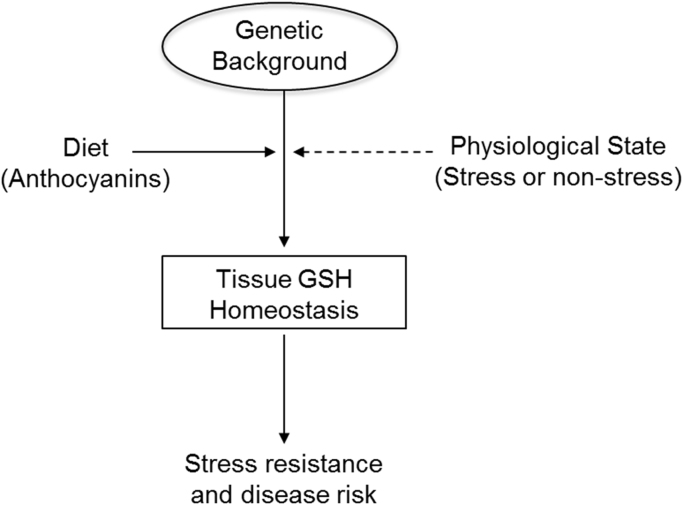
The effects of stress on the C3G-GSH paradigm must be further evaluated in the context of genetic background. Although C3G rescues GSH levels in diabetic B6 mice, the current study showed no effect of this diet on NOD mice, an established model of type 1 diabetes. In contrast, the most potent GSH-inducing effects were observed in CAST mice. CAST is not a model of a specific disease, but these mice appear to exhibit deficiencies within the GSH redox system. Our previous reports identified CAST as having among the lowest GSH levels and GSH/GSSG in a large panel of inbred strains [21]. C3G appears capable of rescuing redox deficiencies in B6 and CAST backgrounds, but it has no effect on the diabetic NOD mice. We predict that genetic background provides a platform on which stress and diet modulate GSH levels (Fig. 8). We initially predicted that these effects are largely independent of gene expression due to the minimal changes in hepatic GSH-related enzyme expression observed here. However, subsequent statistical analyses revealed correlations between GSH phenotypes and expression of GSH-related enzymes, indicating that basal expression levels may play a role in the redox effects outlined in this study. It is important to note that additional factors may have also influenced GSH homeostasis beyond what was assayed, such as glutathione transferase activity, activity of GSH efflux pumps, NAD(P)H supply, as well as composition of the gut microbiome, which could affect C3G metabolism, absorption, and bioactivity.
Fig. 8.
Model of genetic regulation of GSH. Genetic background directly regulates GSH homeostasis and determines the relative effects of diet and physiological stress on this system. Together, these interactions influence disease risk.
This study demonstrated that GSH levels and GSH/GSSG can decrease in response to an established C3G-rich diet [6]. The C3G diet caused apparent disruptions in GSH homeostasis in 129 and A mice, and the effect was most apparent in the liver, suggesting oxidative stress and hepatotoxicity [49], [50], [51], [52]. Several polyphenols are known to exert toxicity at high levels [53], [54], [55], and in the case of epigallocatechin gallate (EGCG), a polyphenol present in green tea, toxicity is determined by genetic background [55]. As use of dietary supplements continues to grow considerably in the United States, it will be critical to further characterize the genetic mechanisms that drive hepatotoxicity attributable to polyphenols such as EGCG and C3G. It will also be important to elucidate whether distinct mechanisms direct toxicity of each compound.
We tested the hypothesis that the redox effects of C3G would be limited to the liver and kidney due to the primary role of these organs in phytochemical metabolism and clearance. Our hypothesis was partially confirmed because the most significant effects were discovered in liver. The kidney, as well as the heart and pancreas, showed fewer and less pronounced effects on GSH homeostasis; the brain exhibited no effects. Overall, our data support a tissue-specific effect of anthocyanins on GSH homeostasis. To our knowledge, this is the first study to show that the redox effects of anthocyanins are determined by genetic background. Our long-term hypothesis predicts that anthocyanins differentially affect humans based on their genetics. If that hypothesis is correct, it may highlight the underlying reason for inconsistent findings in previous epidemiological and clinical studies. Furthermore, such findings would indicate that anthocyanin supplementation may cause toxicity in a highly susceptible subpopulation. Overall, our data will inform future efforts to clarify genetic mechanisms that regulate differential responses to ingested anthocyanins.
Conflict of interest and funding disclosure
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the University of Georgia Office of the Vice President for Research; the College of Family and Consumer Sciences; and NIFA HATCH Grant GEO00735.
Acknowledgments
The authors wish to thank Woo-Kyun Kim and Roshan Adhikary for their help with creating cDNA libraries. We also wish to thank Roger Nilsen for his technical assistance with PCR, Zachary Grunewald for his advice on PCR data analysis, and Erica Coe for her help with designing primers.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.08.014.
Contributor Information
Katie M. Norris, Email: kmn@uga.edu.
Whitney Okie, Email: whitokie@uga.edu.
Claire L. Yakaitis, Email: claireyak@uga.edu.
Robert Pazdro, Email: rpazdro@uga.edu.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.He J., Giusti M.M. Anthocyanins: natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- 2.Chun O.K., Chung S.J., Claycombe K.J., Song W.O. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J. Nutr. 2008;138(4):753–760. doi: 10.1093/jn/138.4.753. [DOI] [PubMed] [Google Scholar]
- 3.Mink P.J., Scrafford C.G., Barraj L.M., Harnack L., Hong C.P., Nettleton J.A., Jacobs D.R., Jr. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007;85(3):895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 4.McCullough M.L., Peterson J.J., Patel R., Jacques P.F., Shah R., Dwyer J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012;95(2):454–464. doi: 10.3945/ajcn.111.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mursu J., Voutilainen S., Nurmi T., Tuomainen T.P., Kurl S., Salonen J.T. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2008;100(4):890–895. doi: 10.1017/S0007114508945694. [DOI] [PubMed] [Google Scholar]
- 6.Zhu W., Jia Q., Wang Y., Zhang Y., Xia M. The anthocyanin cyanidin-3-O-beta-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012;52(2):314–327. doi: 10.1016/j.freeradbiomed.2011.10.483. [DOI] [PubMed] [Google Scholar]
- 7.Kwon S.H., Ahn I.S., Kim S.O., Kong C.S., Chung H.Y., Do M.S., Park K.Y. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J. Med. Food. 2007;10(3):552–556. doi: 10.1089/jmf.2006.147. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi A., Shimizu H., Okazaki Y., Sakaguchi H., Taira T., Suzuki T., Chiji H. Anthocyanin-rich phytochemicals from aronia fruits inhibit visceral fat accumulation and hyperglycemia in high-fat diet-induced dietary obese rats. J. Oleo Sci. 2015;64(12):1243–1250. doi: 10.5650/jos.ess15181. [DOI] [PubMed] [Google Scholar]
- 9.Pratheeshkumar P., Son Y.O., Wang X., Divya S.P., Joseph B., Hitron J.A., Wang L., Kim D., Yin Y., Roy R.V. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-kappaB signaling pathways in SKH-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014;280(1):127–137. doi: 10.1016/j.taap.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorrenti V., Vanella L., Acquaviva R., Cardile V., Giofre S., Di Giacomo C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015;47(4):1303–1310. doi: 10.3892/ijo.2015.3130. [DOI] [PubMed] [Google Scholar]
- 11.Bhaswant M., Fanning K., Netzel M., Mathai M.L., Panchal S.K., Brown L. Cyanidin 3-glucoside improves diet-induced metabolic syndrome in rats. Pharmacol. Res. 2015;102:208–217. doi: 10.1016/j.phrs.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Chorfa N., Savard S., Belkacemi K. An efficient method for high-purity anthocyanin isomers isolation from wild blueberries and their radical scavenging activity. Food Chem. 2016;197(Pt B):1226–1234. doi: 10.1016/j.foodchem.2015.11.076. [DOI] [PubMed] [Google Scholar]
- 13.De Rosso V.V., Moran Vieyra F.E., Mercadante A.Z., Borsarelli C.D. Singlet oxygen quenching by anthocyanin's flavylium cations. Free Radic. Res. 2008;42(10):885–891. doi: 10.1080/10715760802506349. [DOI] [PubMed] [Google Scholar]
- 14.Jang Y.P., Zhou J., Nakanishi K., Sparrow J.R. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem. Photobiol. 2005;81(3):529–536. doi: 10.1562/2004-12-14-RA-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamasaki H., Uefuji H., Sakihama Y. Bleaching of the red anthocyanin induced by superoxide radical. Arch. Biochem. Biophys. 1996;332(1):183–186. doi: 10.1006/abbi.1996.0331. [DOI] [PubMed] [Google Scholar]
- 16.Youdim K.A., Shukitt-Hale B., MacKinnon S., Kalt W., Joseph J.A. Polyphenolics enhance red blood cell resistance to oxidative stress: in vitro and in vivo. Biochim. Biophys. Acta. 2000;1523(1):117–122. doi: 10.1016/s0304-4165(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 17.Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharm. Ther. 2014;141(2):150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Khamaisi M., Kavel O., Rosenstock M., Porat M., Yuli M., Kaiser N., Rudich A. Effect of inhibition of glutathione synthesis on insulin action: in vivo and in vitro studies using buthionine sulfoximine. Biochem J. 2000;349(Pt 2):579–586. doi: 10.1042/0264-6021:3490579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J., Privratsky J.R., Yang X., Dong F., Carlson E.C. Metallothionein alleviates glutathione depletion-induced oxidative cardiomyopathy in murine hearts. Crit. Care Med. 2008;36(7):2106–2116. doi: 10.1097/CCM.0b013e31817bf925. [DOI] [PubMed] [Google Scholar]
- 20.Richie J.P., Jr., Komninou D., Albino A.P. Induction of colon tumorigenesis by glutathione depletion in p53-knock-out mice. Int. J. Oncol. 2007;0(6):1539–1543. [PubMed] [Google Scholar]
- 21.Zhou Y., Harrison D.E., Love-Myers K., Chen Y., Grider A., Wickwire K., Burgess J.R., Stochelski M.A., Pazdro R. Genetic analysis of tissue glutathione concentrations and redox balance. Free Radic. Biol. Med. 2014;71:157–164. doi: 10.1016/j.freeradbiomed.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchiya M., Ji C., Kosyk O., Shymonyak S., Melnyk S., Kono H., Tryndyak V., Muskhelishvili L., Pogribny I.P., Kaplowitz N. Interstrain differences in liver injury and one-carbon metabolism in alcohol-fed mice. Hepatology. 2012;56(1):130–139. doi: 10.1002/hep.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iancu O.D., Darakjian P., Walter N.A., Malmanger B., Oberbeck D., Belknap J., McWeeney S., Hitzemann R. Genetic diversity and striatal gene networks: focus on the heterogeneous stock-collaborative cross (HS-CC) mouse. BMC Genom. 2010;11:585. doi: 10.1186/1471-2164-11-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler T.R., Panoskaltsus-Mortari A., Gu L.H., Jonas C.R., Farrell C.L., Lacey D.L., Jones D.P., Blazar B.R. Regulation of glutathione redox status in lung and liver by conditioning regimens and keratinocyte growth factor in murine allogeneic bone marrow transplantation. Transplantation. 2001;72(8):1354–1362. doi: 10.1097/00007890-200110270-00004. [DOI] [PubMed] [Google Scholar]
- 25.Park H.J., Mah E., Bruno R.S. Validation of high-performance liquid chromatography-boron-doped diamond detection for assessing hepatic glutathione redox status. Anal. Biochem. 2010;407(2):151–159. doi: 10.1016/j.ab.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Benton S.M., Liang Z., Hao L., Liang Y., Hebbar G., Jones D.P., Coopersmith C.M., Ziegler T.R. Differential regulation of tissue thiol-disulfide redox status in a murine model of peritonitis. J. Inflamm. 2012;9(1):36. doi: 10.1186/1476-9255-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keli S.O., Hertog M.G., Feskens E.J., Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch. Intern. Med. 1996;156(6):637–642. [PubMed] [Google Scholar]
- 28.Sesso H.D., Gaziano J.M., Liu S., Buring J.E. Flavonoid intake and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2003;77(6):1400–1408. doi: 10.1093/ajcn/77.6.1400. [DOI] [PubMed] [Google Scholar]
- 29.Cassidy A., Rimm E.B., O'Reilly E.J., Logroscino G., Kay C., Chiuve S.E., Rexrode K.M. Dietary flavonoids and risk of stroke in women. Stroke. 2012;43(4):946–951. doi: 10.1161/STROKEAHA.111.637835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yochum L., Kushi L.H., Meyer K., Folsom A.R. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am. J. Epidemiol. 1999;149(10):943–949. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- 31.Woo H.D., Lee J., Choi I.J., Kim C.G., Lee J.Y., Kwon O., Kim J. Dietary flavonoids and gastric cancer risk in a Korean population. Nutrients. 2014;6(11):4961–4973. doi: 10.3390/nu6114961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamora-Ros R., Agudo A., Lujan-Barroso L., Romieu I., Ferrari P., Knaze V., Bueno-de-Mesquita H.B., Leenders M., Travis R.C., Navarro C. Dietary flavonoid and lignan intake and gastric adenocarcinoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr. 2012;96(6):1398–1408. doi: 10.3945/ajcn.112.037358. [DOI] [PubMed] [Google Scholar]
- 33.Wedick N.M., Pan A., Cassidy A., Rimm E.B., Sampson L., Rosner B., Willett W., Hu F.B., Sun Q., van Dam R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012;95(4):925–933. doi: 10.3945/ajcn.111.028894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamora-Ros R., Forouhi N.G., Sharp S.J., Gonzalez C.A., Buijsse B., Guevara M., van der Schouw Y.T., Amiano P., Boeing H., Bredsdorff L. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: the EPIC-InterAct study. Diabetes Care. 2013;36(12):3961–3970. doi: 10.2337/dc13-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenblat M., Volkova N., Attias J., Mahamid R., Aviram M. Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum's ability to attenuate macrophage cholesterol accumulation. Food Funct. 2010;1(1):99–109. doi: 10.1039/c0fo00011f. [DOI] [PubMed] [Google Scholar]
- 36.Ruel G., Pomerleau S., Couture P., Lamarche B., Couillard C. Changes in plasma antioxidant capacity and oxidized low-density lipoprotein levels in men after short-term cranberry juice consumption. Metabolism. 2005;54(7):856–861. doi: 10.1016/j.metabol.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Ruel G., Pomerleau S., Couture P., Lemieux S., Lamarche B., Couillard C. Low-calorie cranberry juice supplementation reduces plasma oxidized LDL and cell adhesion molecule concentrations in men. Br. J. Nutr. 2008;99(2):352–359. doi: 10.1017/S0007114507811986. [DOI] [PubMed] [Google Scholar]
- 38.Basu A., Du M., Leyva M.J., Sanchez K., Betts N.M., Wu M., Aston C.E., Lyons T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010;140(9):1582–1587. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henning S.M., Seeram N.P., Zhang Y., Li L., Gao K., Lee R.P., Wang D.C., Zerlin A., Karp H., Thames G. Strawberry consumption is associated with increased antioxidant capacity in serum. J. Med. Food. 2010;13(1):116–122. doi: 10.1089/jmf.2009.0048. [DOI] [PubMed] [Google Scholar]
- 40.Lee I.T., Chan Y.C., Lin C.W., Lee W.J., Sheu W.H. Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes. Diabet. Med. 2008;25(12):1473–1477. doi: 10.1111/j.1464-5491.2008.02588.x. [DOI] [PubMed] [Google Scholar]
- 41.Burton-Freeman B., Linares A., Hyson D., Kappagoda T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J. Am. Coll. Nutr. 2010;29(1):46–54. doi: 10.1080/07315724.2010.10719816. [DOI] [PubMed] [Google Scholar]
- 42.Basu A., Wilkinson M., Penugonda K., Simmons B., Betts N.M., Lyons T.J. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: baseline and post intervention effects. Nutr. J. 2009;8:43. doi: 10.1186/1475-2891-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruel G., Lapointe A., Pomerleau S., Couture P., Lemieux S., Lamarche B., Couillard C. Evidence that cranberry juice may improve augmentation index in overweight men. Nutr. Res. 2013;33(1):41–49. doi: 10.1016/j.nutres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez-Suarez J.M., Giampieri F., Tulipani S., Casoli T., Di Stefano G., Gonzalez-Paramas A.M., Santos-Buelga C., Busco F., Quiles J.L., Cordero M.D. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014;25(3):289–294. doi: 10.1016/j.jnutbio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Duthie S.J., Jenkinson A.M., Crozier A., Mullen W., Pirie L., Kyle J., Yap L.S., Christen P., Duthie G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006;45(2):113–122. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 46.Kuntz S., Kunz C., Herrmann J., Borsch C.H., Abel G., Frohling B., Dietrich H., Rudloff S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: results from the randomised, double-blind, placebo-controlled, cross-over ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study. Br. J. Nutr. 2014;112(6):925–936. doi: 10.1017/S0007114514001482. [DOI] [PubMed] [Google Scholar]
- 47.Del C., Martini Bo, D., Porrini M., Klimis-Zacas D., Riso P. Berries and oxidative stress markers: an overview of human intervention studies. Food Funct. 2015;6(9):2890–2917. doi: 10.1039/c5fo00657k. [DOI] [PubMed] [Google Scholar]
- 48.Cutler G.J., Nettleton J.A., Ross J.A., Harnack L.J., Jacobs D.R., Jr., Scrafford C.G., Barraj L.M., Mink P.J., Robien K. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women's Health Study. Int. J. Cancer. 2008;123(3):664–671. doi: 10.1002/ijc.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tien Y.H., Chen B.H., Wang Hsu G.S., Lin W.T., Huang J.H., Lu Y.F. Hepatoprotective and anti-oxidant activities of Glossogyne tenuifolia against acetaminophen-induced hepatotoxicity in mice. Am. J. Chin. Med. 2014;42(6):1385–1398. doi: 10.1142/S0192415X14500876. [DOI] [PubMed] [Google Scholar]
- 50.Noh J.R., Kim Y.H., Hwang J.H., Gang G.T., Kim K.S., Lee I.K., Yun B.S., Lee C.H. Davallialactone protects against acetaminophen overdose-induced liver injuries in mice. Food Chem. Toxicol. 2013;58:14–21. doi: 10.1016/j.fct.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W., Xue J., Ge M., Yu M., Liu L., Zhang Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem. Toxicol. 2013;51:87–92. doi: 10.1016/j.fct.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 52.Sentellas S., Morales-Ibanez O., Zanuy M., Alberti J.J. GSSG/GSH ratios in cryopreserved rat and human hepatocytes as a biomarker for drug induced oxidative stress. Toxicol. Vitr. 2014;28(5):1006–1015. doi: 10.1016/j.tiv.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 53.Dunnick J.K., Hailey J.R. Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundam. Appl. Toxicol. 1992;19(3):423–431. doi: 10.1016/0272-0590(92)90181-g. [DOI] [PubMed] [Google Scholar]
- 54.Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch. Biochem. Biophys. 2014;557:3–10. doi: 10.1016/j.abb.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 55.Church R.J., Gatti D.M., Urban T.J., Long N., Yang X., Shi Q., Eaddy J.S., Mosedale M., Ballard S., Churchill G.A. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol. 2015;76:19–26. doi: 10.1016/j.fct.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material