Abstract
Stability of the total phenolic content, ascorbic acid, total carotenoids and antioxidant activity in eight fruit beverages was analyzed. The influence of storage temperature (4, 8 and 11 °C) during the product shelf-life (20 days) was evaluated. Pomegranate Juice presented the highest values for antioxidant activity by DPPH• assay (552.93 ± 6.00 GAE μg mL−1), total carotenoids (3.18 ± 0.11 βCE μg mL−1), and total phenolic content (3967.07 ± 2.47 GAE μg mL−1); while Splash Blend recorded the highest levels of ascorbic acid (607.39 ± 2.13 AAE μg mL−1). The antioxidant capacity was stable at 4 and 8 °C for the first 8 days of storage; while carotenoids and ascorbic acid were slightly degraded through the storage time, possibly due to oxidation and/or reactions with other compounds. The results suggest that the observed variation during testing could be related to storage conditions of the final product.
Keywords: Food Science
1. Introduction
Fruits and derived products such as fruit juices are a significant source of many biologically active antioxidant compounds, such as ascorbic acid, tocopherols, carotenoids, and polyphenols (Cilla et al., 2011). These antioxidants found in fruit juices such as pomegranate, apple, orange, guava, peach, and other mixed fruits are thought to contribute towards positive health outcomes in cases of cardiovascular disease, cancer, and age-related degeneration, reason why their consumption in the last years increased at very quick rates (van Poppel and Goldbohm, 1995; Rao and Agarwal, 2000; De Mello-Andrade and Fasolo, 2014).
In this sense, beverage companies are currently making many efforts to formulate juices, which contain bioactive compounds from different fruits and offer value-added products for the consumer (Siró et al., 2008). Nevertheless, usually these products are consumed over a short period set by the manufacturer (expiration date, also called shelf-life). During this time the product maintains the physicochemical characteristics that make it suitable for human consumption; however, currently this criterion does not include the antioxidant properties and how are they affected by one of the principal parameters in the market: the storage (Calderon-Hidalgo, 2007).
Although the activity of antioxidants has been widely studied, the knowledge on the stability of antioxidants is very important in food preservation. More investigation still needed about the effects of storage conditions on the bioactive compounds from different fruits beverages in order to get the optimal conditions of storage, especially for commercial juices, which usually have short-term storage (5–10 days) depending on the industrial processing in the case juices preservers-free (Nicoli et al., 1999). Actually, few studies have addressed the evaluation of changes in the overall antioxidant properties and concentration of bioactive compounds of fruit beverages as affected by storage conditions (Del Caro et al., 2004; Piljac-Zegarac et al., 2009; La Cava and Sgroppo, 2015). Research on antioxidant changes in the food production chain is limited. Consumers show decreasing trust in chemical additives and strongly prefer foods that contain natural antioxidants. Maintaining these compounds during food processing is complicated, as food requires depth investigation about the interactions of these compounds and all other components present in the food and the factors related to the oxidation process (Smith and Charter, 2010). Expanding knowledge in this area should lead to improvements in technological processing methods to produce high-quality food with effective biological activity. In this sense, we hypothesize that storage conditions have strong effects on the bioactive compounds present on fruit beverages.
With the above facts in view, the aim of this study was to determine the effect of storage temperature (4, 8, and 11 °C) in the content of bioactive compounds such as polyphenols, ascorbic acid, total carotenoids, and total antioxidant activity in eight fruit beverage during a period of 20 days.
2. Materials and methods
2.1. Chemicals substances and standards
Specialized reagents as 2,2-Diphenyl-1-picryl-hydrazyl (DPPH•), Folin-Ciocalteu, ethanol, methanol, sodium carbonate, ferric chloride, potassium persulfate, trichloroacetic acid, amberlite XAD-16, acetonitrile, gallic acid, L-ascorbic acid, and others standards were purchased from Sigma Aldrich (Toluca, Mexico) with high purity or UPLC grade, as appropriate.
2.2. Beverages samples
Eight samples namely Vital Joy, Energy-T, Tamarind water, Lemon water, Coconut water, Lemonade, Splash Blend, and Pomegranate juice were manufactured and provided by Food Solutions Company as an innovative commercial project and provide for this study. The composition of these beverages is shown in Table 1. The general description of the manufacturing process was as follows: the concentrates, and pulps of different fruits and vegetables were mixed to regulate the variations of sugar and acids contained in the matrix and obtain a final homogeneous formulation. After mixing, a heat treatment was applied by HTST pasteurization (95 °C for 15 s). The product was taken to the cooling area to reduce its temperature and facilitate handling. Finally, the beverages were packed under aseptic conditions in air-tight polyethylene terephthalate bottles, which were labeled and placed on pallets. To determine the effect of storage conditions and carry out the corresponding analysis, the samples were stored at 3 different temperatures (4, 8 and 11 °C), in order to determinate changes fluctuations on bioactive compounds in beverages according to preliminary experiments. The phenolic content, ascorbic acid content, total carotenoid content, and antioxidant activity were measured during 4, 8, 12, 16 and 20 days in order to represent the conditions of shelf-life.
Table 1.
Composition of the beverages analyzed as indicated on the commercial labels.
| Code | Sample | Composition |
|---|---|---|
| VJ | Vital Joy | Orange juice, carrot juice, apple sauce, mango sauce, and lemon juice |
| SB | Splash Blend | Coconut water, orange juice, peach sauce, apple sauce, mango sauce, and guava sauce |
| ET | Energy-T | Water, sugar, lemon juice, and black tea extract |
| CW | Coconut water | Coconut water |
| LE | Lemonade | Water, sugar, and lemon juice |
| TW | Tamarind water | Water, sugar, and tamarind pulp |
| LC | Lemonade with cucumber | Water, sugar, lemon juice, and cucumber pulp |
| PJ | Pomegranate juice | Pomegranate reconstituted concentrate juice |
2.3. Purification and determination of total phenols
Phenols purification was carried out by column chromatography using Amberlite XAD-16 as stationary phase and absolute ethanol as the mobile phase from the corresponding sample. For purification, 20 mL of juice were added and deposited into a column containing Amberlite XAD-16, washed with water and after with ethanol to elute phenolic compounds.
Total phenol content (TPC) was determined by the Folin-Ciocalteu's Reagent method according to a described procedure (Mena et al., 2012). Briefly, to 250 μL of properly diluted sample (1:5 v/v) were added 250 μL of Folin-Ciocalteu reagent and 250 μL of sodium carbonate (75 g L−1). The obtained mixture was homogenized and incubated at 40 °C for 30 minutes in a water bath. Subsequently were added 2 mL of distilled H2O and the absorbance was recorded at 750 nm. The results were reported as the average of three replicates in gallic acid equivalents expressed as micrograms per milliliter (GAE μg mL−1) according to the calibration curve prepared with the same standard.
2.4. Purification and determination of total carotenoids
To 0.5 mL of each sample were added 25 mL of a solution composed of acetone: hexane: ethanol in a ratio of 1:2:1, respectively. In addition, it contained 0.1% of ascorbic acid to prevent oxidation of carotenoids. The mixture was incubated for 30 minutes at 37 °C according to the methodology previously reported (Herrero et al., 2012). Then the mixture was centrifuged at 10,000 rpm for 5 minutes at 4 °C. The concentration of total carotenoids was recorded at 450 nm using hexane as blank and the results were reported as the average of three replicates and expressed as microequivalents per milliliter of β-carotene (βCE μg mL−1) according to the calibration curve prepared with the same standard.
2.5. Determination of ascorbic acid
The method for extracting and quantifying ascorbic acid used in this study is based on an adaptation (Klimczak and Gliszczynska-Swiglo, 2015). The samples were filtered through 0.2 μm membranes to remove impurities and placed into vials of 1.5 mL for UPLC analysis. Vitamin C detection of each sample was performed on a UPLC ACQUITY (WATERS) with a column ACQUITY UPLC BEH C18 1.7 μm, using as mobile phase a mixture of 25 mM phosphate buffer (pH 3.2) and acetonitrile as a gradient. The conditions tests were: injection flow 0.33 μL min-1 for 2.9 minutes to a PDA detector wavelength of 210 and 245 nm. Results were reported as the average of three repetitions and expressed as micrograms of ascorbic acid per milliliter (AA μg mL−1) according to the calibration curve prepared with the same standard.
2.6. Radical scavenging
Free radical-scavenging capacity was determined using the radical DPPH• according to a methodology previously described (Brand-Williams et al., 1995). Briefly, to 50 mL of properly diluted sample (1: 5 v/v) were added 2950 μL of the solution DPPH• radical, which was prepared in methanol at 60 mM. After a period of incubation in the dark for 30 minutes, the absorbance of the samples was recorded at a wavelength of 517 nm. The ability to inhibit was calculated by the following equation (1) and expressed as percent inhibition of DPPH• radical:
| Inhibition (%) = [(Absorbance control − Absorbance sample)/Absorbance control] * 100 | (1) |
Finally, the results were reported as the average of three replications and reported as gallic acid equivalent expressed in micrograms per milliliter of sample (GAE μg mL−1) according to the calibration curve prepared with the same standard.
2.7. Statistical analysis
All data are presented as mean ± standard deviation (SD) of triplicate analyses of each sample. Data were analyzed by ANOVA and Tukey’s multiple comparison test using the Info-Stat Statistical Software (Cordoba-Argentina, 2009). A probability of 5% or less was accepted as statistically significant.
3. Results and discussions
3.1. Total phenolic content
Table 2 presents the total phenolic content (TPC) for our set of eight fruit juices, over the course of 20 days in storage at 4, 8, and 11 °C. The smallest concentration was found in the Coconut water with 0 ± 0 GAE μg mL−1 (No detection) at 8 days of storage in all temperatures tested. These values are markedly lower than those observed by Elaine-Tanqueco et al. (2007) in coconut water from three varieties of coconuts consumed in Philippines. In their study, the highest phenolic content was 36.90 mg L−1 GAE for Laguna Tall variety which is not comparable to our values for a drink composed only of coconut. Some authors mentioned that this difference could be due to several factors, including ripening stage, harvesting maturity, type of extraction conditions used, same as the yield and bioactivities of the samples (Deng et al., 2014). While, the richest in these compounds was the Pomegranate juice, which contained 3874.42 ± 14.5 GAE μg mL−1 (20 days at 8 °C), and then the Energy-T, Splash Blend, and Vital Joy juices: 657.72 ± 6.29 (20 days at 8 °C), 426.28 ± 17.67 (8 days at 11 °C), and 181.78 ± 16.20 GAE μg mL−1 (20 days at 8 °C), respectively. These juices also exhibited higher mean TPC in comparison to 17 fruit juice and skim milk mixture beverages (608.5 GAE mg L−1) and eight pomegranate juices (1055–1280 GAE μg mL−1) studied by Zulueta et al. (2007) and Sepulveda et al. (2010), respectively.
Table 2.
Evolution on total phenol content and total antioxidant activity obtained by Folin–Ciocalteu's reagent and DPPH&903; radical 2 scavenging assay, respectively, during 20 days of storage at 4, 8, and 11 °C.
| Sample | Storage Temperature | Total phenol content (GAE pg mL−1) |
Total antioxidant activity (GAE pg mL−1) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 days | 8 days | 12 days | 16 days | 20 days | 4 days | 8 days | 12 days | 16 days | 20 days | ||
| SB | 4 °C | 124.43 ± 5.53 | 325.09 ± 16.32 | 98.87 ± 8.29 | 126.78 ± 8.10 | 185.50 ± 10.21 | 213.29 ± 24.77 | 218.46 ± 7.16 | 146.46 ± 3.07 | 184.05 ± 13.08 | 167.42 ± 11.37 |
| 8 °C | 102.61 ± 8.61 | 336.01 ± 10.73 | 92.33 ± 3.12 | 129.81 ± 5.25 | 199.39 ± 9.79 | 223.62 ± 22.54 | 185.77 ± 10.63 | 137.07 ± 14.23 | 194.16 ± 4.44 | 151.25 ± 21.02 | |
| 11 °C | 106.74 ± 11.64 | 426.28 ± 17.67 | 122.46 ± 4.86 | 109.10 ± 3.36 | 168.84 ± 4.55 | 213.29 ± 21.52 | 245.51 ± 17.43 | 184.25 ± 20.95 | 176.83 ± 13.01 | 109.05 ± 17.95 | |
| TW | 4 °C | 32.25 ± 1.32 | 64.98 ± 8.67 | 26.63 ± 3.36 | 35.84 ± 6.02 | 68.53 ± 1.41 | 59.34 ± 7.66 | ND | ND | 8.98 ± 1.73 | 8.10 ± 7.02 |
| 8 °C | 55.85 ± 2.09 | 55.65 ± 4.29 | 36.73 ± 5.76 | 38.03 ± 0.50 | 68.37 ± 1.22 | 54.62 ± 3.34 | ND | ND | 20.82 ± 9.26 | 1.39 ± 2.42 | |
| 11 °C | 53.49 ± 2.69 | 59.82 ± 4.64 | 32.40 ± 3.62 | 39.04 ± 1.33 | 60.97 ± 1.38 | 60.81 ± 2.84 | ND | ND | 9.27 ± 3.60 | ND | |
| CW | 4 °C | 12.36 ± 2.78 | ND | 12.75 ± 1.55 | 38.66 ± 0.94 | 45.81 ± 0.51 | ND | ND | ND | ND | ND |
| 8 °C | 13.21 ± 1.92 | ND | 26.62 ± 0.92 | 29.81 ± 0.61 | 49.89 ± 1.34 | ND | ND | ND | ND | ND | |
| 11 °C | 14.07 ± 2.35 | ND | 8.18 ± 0.44 | 22.81 ± 1.28 | 37.99 ± 0.77 | ND | ND | ND | ND | ND | |
| LE | 4 °C | 12.36 ± 2.78 | ND | 36.20 ± 1.67 | 51.00 ± 2.33 | 74.72 ± 1.64 | ND | ND | ND | ND | ND |
| 8 °C | 13.21 ± 1.92 | ND | 24.26 ± 1.11 | 55.12 ± 2.69 | 60.61 ± 0.51 | ND | ND | ND | ND | ND | |
| 11 °C | 14.07 ± 2.35 | ND | 35.02 ± 3.35 | 17.46 ± 1.63 | 62.65 ± 1.34 | ND | ND | ND | ND | ND | |
| ET | 4 °C | 324.33 ± 7.52 | 180.69 ± 11.79 | 313.79 ± 5.89 | 318.70 ± 3.46 | 550.40 ± 1.34 | 175.17 ± 3.22 | 120.33 ± 3.45 | 69.60 ± 0.47 | 96.96 ± 1.32 | 84.11 ± 0.97 |
| 8 °C | 277.74 ± 1.77 | 64.54 ± 7.85 | 265.26 ± 1.17 | 260.47 ± 10.97 | 657.72 ± 6.29 | 131.48 ± 6.38 | 92.72 ± 7.86 | 89.32 ± 1.34 | 93.28 ± 0.22 | 85.73 ± 1.00 | |
| 11 °C | 373.19 ± 8.56 | 291.09 ± 4.44 | 354.49 ± 3.71 | 216.64 ± 14.13 | 502.78 ± 3.61 | 140.84 ± 8.56 | 132.45 ± 1.02 | 77.67 ± 0.15 | 92.15 ± 2.61 | 85.95 ± 0.85 | |
| VJ | 4 °C | 54.22 ± 0.95 | 39.94 ± 2.56 | 87.98 ± 4.17 | 33.13 ± 5.17 | 178.80 ± 9.02 | 159.56 ± 10.72 | 116.01 ± 10.93 | 129.04 ± 13.68 | 121.50 ± 5.44 | 131.43 ± 20.90 |
| 8 °C | 25.27 ± 0.36 | 42.42 ± 0.91 | 87.59 ± 5.61 | 30.08 ± 1.81 | 181.78 ± 16.20 | 150.51 ± 10.23 | 161.42 ± 23.35 | 128.21 ± 10.90 | 95.89 ± 8.78 | 126.13 ± 16.55 | |
| 11 °C | 21.72 ± 3.08 | 64.79 ± 2.07 | 130.42 ± 7.80 | 88.13 ± 6.32 | 150.73 ± 9.47 | 164.67 ± 13.87 | 153.25 ± 18.43 | 126.29 ± 11.47 | 126.82 ± 12.15 | 138.58 ± 14.21 | |
| LC | 4 °C | 14.55 ± 1.59 | 38.39 ± 0.59 | 7.40 ± 0.0 | 10.42 ± 0.29 | 39.36 ± 1.62 | 37.21 ± 3.53 | 32.82 ± 1.40 | ND | 4.25 ± 4.51 | ND |
| 8 °C | 9.83 ± 3.86 | 44.94 ± 2.72 | 14.29 ± 0.55 | 12.60 ± 0.77 | 41.37 ± 3.74 | 60.22 ± 13.09 | 39.67 ± 1.78 | ND | 0.972 ± 1.68 | ND | |
| 11 °C | 15.88 ± 1.53 | 43.15 ± 3.31 | 4.83 ± 3.73 | 11.43 ± 0.29 | 41.06 ± 3.20 | 39.57 ± 3.57 | 32.00 ± 4.07 | ND | 1.26 ± 2.18 | ND | |
| PJ | 4 °C | 2226.62 ± 17.7 | 3771.49 ± 9.97 | 3767.75 ± 12.8 | 3967.07 ± 2.47 | 3766.33 ± 6.54 | 356.65 ± 1.51 | 552.93 ± 6.00 | 538.49 ± 1.74 | 424.71 ± 7.44 | 539.87 ± 29.16 |
| 8 °C | 1575.54 ± 22.2 | 3589.67 ± 16.6 | 3739.98 ± 4.24 | 2872.19 ± 7.56 | 3874.42 ± 14.5 | 351.79 ± 3.29 | 547.64 ± 5.31 | 541.8 ± 1.16 | 399.46 ± 3.83 | 527.89 ± 24.02 | |
| 11 °C | 2095.86 ± 15.3 | 3734.56 ± 28.0 | 3700.16 ± 24.2 | 3298.76 ± 15.0 | 3166.50 ± 17.3 | 355.68 ± 1.45 | 543.59 ± 3.28 | 537.82 ± 1.53 | 409.37 ± 1.46 | 526.80 ± 19.86 | |
During storage, the TPC values in all samples showed a tendency to increase from day 12, where the samples stored between 8 and 11 °C showed higher polyphenols content than the initial values. Others authors have previously observed this phenomenon and reported a possible increment of polyphenolic compounds associated to the microbial growth or to reactions between oxidized polyphenols and formation of new compounds of antioxidant character during juice storage (Kallithraka et al., 2009; Martinez-Flores et al., 2015). In addition, must be considered the possibility that during juice storage, some compounds could be formed and react with the Folin–Ciocalteu’s reagent and significantly enhance the phenolic content. Usually, Folin-Ciocalteu's method is used for determination of the total phenolic content; however, this reagent is nonspecific, due to this method is affected by the presence of reducing sugars, aromatic amines, sulphur dioxide, ascorbic acid, organic acids and other natural compounds present in fruit juices, making the results often unstable. Finally, our findings suggest that submit the juice samples until 20 days of storage does not decreases the concentration of phenolic compounds, which is in agreement with the observation of Kevers et al. (2007) whom reported that the phenolic compounds of many fruits and vegetables remain stable during storage.
3.2. Total carotenoid content
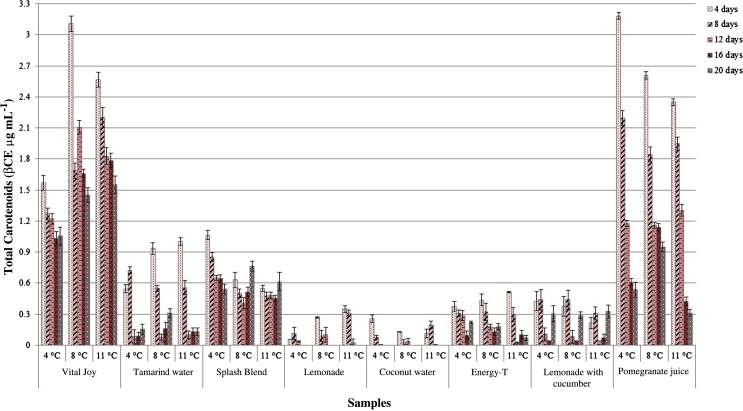
The stability of carotenoid content during storage affects the quality of the product due to different reactions occurring during the storage such as isomerization and oxidation of carotenoids resulting in a loss of many organoleptic characteristics such as color, flavor, among others, and which are the most important attributes related to product quality affecting choice purchase (Baker and Günter, 2004). Fig. 1 shows the change in total carotenoids content of the samples stored at different temperatures for a period of 20 days.
Fig. 1.
Total carotenoids content of eight beverages during 20 days of storage.
At the beginning of storage, the total carotenoid content for Vital Joy was 3.18 ± 0.11 βCE μg mL−1, which is relatively similar to the results found by Stinco et al. (2013) and Abid et al. (2013) for juices formulated with orange and apple juice, respectively. Over the 20 days of storage, the total carotenoids showed a declining trend. A 25% decrease was observed for juices stored at 4, 8, and 11 °C during the first 12 days. Rodriguez-Amaya (1997) mentioned that the cause of instability and consequent degradation of these compounds is due to the susceptibility to oxidation and geometric isomerization of its polyene chain. Oxidation is the main cause of carotenoid loss, which is a spontaneous free-radical chain reaction in the presence of oxygen, light, metals, enzymes and peroxides (Sánchez-Moreno et al., 2003). In addition, in food industry the juice containers are filled with a small gap (headspace) that allow expansion of the food during processing (FAO, 2016). It should be taken into account that there is oxygen present in the headspace of the bottle and it is incorporated into the juice while is manipulated. Without the correct handling of headspace, quality of juices can be change, and if the container is not properly closed, there will be more air and cause the compounds degradation or oxidation.
On the other hand, samples such as Vital Joy and Splash Blend reduced to 50% the initial values until 20 days of storage. According to Klaui and Bauernfeind (1981), this limited degree of degradation may be due to the naturally presence of ascorbic acid in samples acting as protection of carotenoids from oxidation. Choi et al. (2002) reported a lower loss of total carotenoid concentration in ascorbic acid fortified juice compared to control orange juice after 7 weeks of storage. Also, Odriozola-Serrano et al. (2009) evaluated carotenoid stability of orange juices under refrigerated storage. Similar to the effects observed in this work, they reported that the total carotenoid concentration decreased moderately during the exposure time.
3.3. Ascorbic acid content
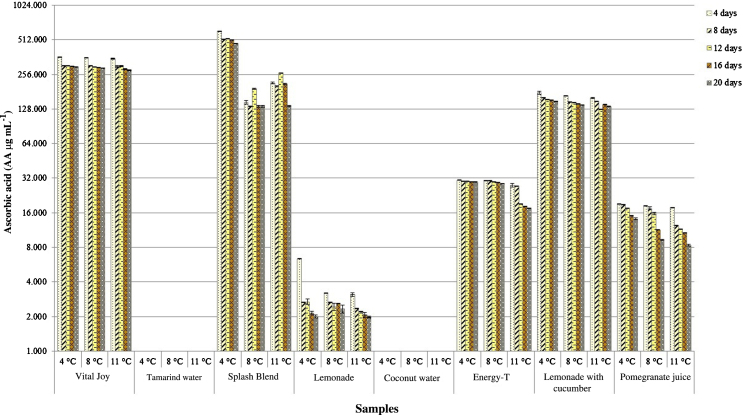
The effect of storage (temperature and time) on the ascorbic acid loss in the juices could be clearly seen in Fig. 2. The mean values of ascorbic acid content over the course of 20 days in storage ranged from 607.39 ± 3.09 to 0 ± 0 (No detected) expressed in AAE μg mL−1 as follows: Splash Blend > Vital Joy > Lemonade with cucumber > Energy-T > Pomegranate juice > Lemonade > Coconut water > Tamarind water. During the storage, despite the technique used for the determination of the ascorbic acid content, in the Tamarind water and Coconut water it was not possible to detect this vitamin under the assay conditions. However, other organic acids and micronutrients, not analyzed in this study, such as tartaric acid, potassium, calcium, phosphorus and other compounds has been reported with an increased presence for tamarind samples (Saideswara-Rao and Mathew, 2012). In contrast, the higher value was recorded by Splash Blend under the following storage conditions: 4 days at 4 °C. Similar values have been reported by Klimczak et al. (2007) in two orange juices stored for 2, 4, and 6 months at 18, 28 and 38 °C were the mean values ranged from 408.570.9 to 361.571.8 AAE mg L−1.
Fig. 2.
Ascorbic acid of eight beverages during 20 days of storage.
By day 8, all juice samples showed a gradual but steady decline in the content of ascorbic acid at all temperatures tested, being the samples stored at 4 °C during the first 4 days which maintained the highest values of this compound. Nevertheless, it is important to consider that the above findings and the fact that the content of ascorbic acid decreases during storage is a dependent factor on the varying conditions, such as temperature, oxygen and light access (Hotz and Gibson, 2007).
3.4. Total antioxidant activity
The composition of fruits, and thus fruit juices, is very diverse, and their antioxidant activity depends on the amount and type of bioactive compounds that occur in them. Several studies reported a high correlation between phenolic content and antioxidant activity; however, other authors suggest that vitamin C is a powerful antioxidant in fruits and can contribute to the antioxidant potential of juices (Kuskoski et al., 2005; Reddy et al., 2010). Therefore, the antioxidant activities in the analyzed fruits cannot be attributed solely to their phenolics contents, but also to the actions of different antioxidant compounds present in the fruits and their interactions.
During storage, a decrease or increase in activity assayed with DPPH&903; test was observed. These fluctuations are shown in Table 2. The initial radical scavenging capacities of juices (first 4 days of storage) ranged from 356.65 ± 1.51 GAE μg mL-1 (Pomegranate juice) to 39.57 ± 3.57 GAE μg mL−1 (Lemonade with cucumber). Pomegranate juice has been previously shown to exhibit the highest antioxidant activity in clarified and cloudy juices, and juices manufactured from different cultivars (Zaouaya et al., 2012; Vegara et al., 2013).
Likewise, Lemonade with cucumber sample recorded considerably higher antioxidant activity than those determined by Gironés-Vilaplana et al. (2012). In their development of a new beverage, the lemon juice presented an antioxidant activity around 13.27 mg mL−1 GAE, which it is three times lower than the activity found in our study. This behavior may be attributed to the presence of cucumber. Mukherjee et al. (2013) reported high concentrations of ascorbic acid and presence of certain terpenes (cucurbitacins) that can be responsible for total antioxidant activity in cucumber extracts.
After the first 8 days in storage, Pomegranate juice showed an increase in total antioxidant activity. According to Pinelo et al. (2004) the increase in this activity may be explained by the strong tendency of polyphenols to undergo polymerization reactions, whereby the resulting oligomers possess larger areas available for charge delocalization. When the degree of polymerization exceeds a critical value, the increased molecular complexity and steric hindrance reduce the availability of hydroxyl groups in reaction with the DPPH radicals, which causes a resultant decrease in the antiradical capacity (Piljac-Zegarac et al., 2009), this may explain the observed increase in antiradical activity in Pomegranate juice.
On the other hand, juice samples like Tamarind water, Vital Joy, Energy-T, and Splash Blend exhibited a significant decrease after de first 4 days of storage in all temperatures tested. Despite good levels of antioxidant activity detected in the assay described above, some authors reported that the observed decrease could occur due to the antioxidant antagonism, which is related to the presence of different bioactive compounds and their interactions, resulting in a decrease in antioxidant activity values (Ferreira-Zielinski et al., 2014). These findings suggest that most of our studied juice samples should be treated as short shelf-life products and that they should be consumed within the first couple of days after opening.
4. Conclusion
The present study demonstrates the importance of storage conditions for the stability of phenolic compounds, ascorbic acid, total carotenoids and total antioxidant activity, and the consideration of this conditions as main quality factors in fruit juices. The results indicate promising perspectives for the exploitation of the fruits juices in the market, and which showed a considerable levels of antioxidants capacity. Natural antioxidants provide an effective alternative to the synthetic antioxidants traditionally used in the food industry. It is well known that antioxidants play a key role in maintaining the flavor and color integrity of food products, but natural antioxidants also help protect the nutritional quality of food. With several different naturally-sourced options available, these products can be useful for consumers, for planning rich antioxidant diets and to nutritionists in estimating the daily intakes of antioxidants and their impact on health.
Although the behavior observed in the content of these compounds and total antioxidant capacity of the analyzed samples is highly variable, it can be concluded that in general these compounds were stable and tended to increase (in some of the assays) during the first 12 days at temperatures of 4 to 8 °C of storage. Some authors report that these conditions may be favorable for the conservation of antioxidants in functional beverages. Pomegranate Juice was the sample that provided the highest concentrations of total polyphenol content and total carotenoids, as well as the highest antioxidant activity in the three assays tested, while the maximum concentration of vitamin C was observed for Splash Blend. This research highlights the need for further investigation in several areas. Firstly, it is important to consider the study of the influence of technological process, packing, matrixes, and the contribution of other antioxidant metabolites to total antioxidant capacity. Further to this, it is important that research aims to provide biologically relevant information on antioxidants by providing data concerning the bioaccessibility and bioavailability of antioxidants in a system.
Declarations
Author contribution statement
Cecilia Castro-López: Performed the experiments; Analyzed and interpreted the data: Wrote the paper.
Ernesto J. Sánchez-Alejo: Conceived and designed the experiments. Saúl Saucedo-Pompa, Romeo Rojas: Analyzed and interpreted the data.
Juana Aranda-Ruiz: Contributed reagents, materials, analysis tools or data.
Guillermo C. Martinez-Avila: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by the Food Solutions Company and to the Mexican Council for Science and Technology (PROINNOVA: 213531 and INFRA 2015: 254178, respectively).
Additional information
No additional information is available for this paper.
Contributor Information
C. Castro-López, Email: caslopcec28@hotmail.com.
E.J. Sánchez-Alejo, Email: ernsanchez@hotmail.com.
S. Saucedo-Pompa, Email: saul.saucedo.p@gmail.com.
R. Rojas, Email: romeo.rojasmln@uanl.edu.mx.
J. Aranda-Ruiz, Email: juany62@hotmail.com.
G.C.G. Martínez-Avila, Email: guillermo.martinezavl@uanl.edu.mx.
References
- Abid M., Jabbar S., Wua T., Hashim M.M., Hua B., Lei S., Zeng X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrasonics Sonochemistry. 2013;21:93–97. doi: 10.1016/j.ultsonch.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Baker R., Günter C. The role of carotenoids in consumer choice and likely benefits from their inclusion into products for human consumption. Trends in Food Science and Technology. 2004;15(10):484–488. [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft und-Technologie. 1995;28:25–30. [Google Scholar]
- Calderon-Hidalgo P.C. (2007). Determination of the antioxidant properties of commercial orange juice under different storage conditions. Available at: http://biblioteca.usac.edu.gt/tesis/06/06_2564.pdf (Accessed 01 Apr. 2016).
- Choi M.H., Kim G.H., Lee H.S. Effects of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Research International. 2002;35:753–759. [Google Scholar]
- Cilla A., Perales S., Lagarda M.J., Barberaa R., Clemente G., Farre, F Influence of storage and in vitro gastrointestinal digestion on total antioxidant capacity of fruit beverages. Journal of Food Composition and Analysis. 2011;24:87–94. [Google Scholar]
- Del Caro A., Piga A., Vacca V., Agabbio M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chemistry. 2004;84:99–105. [Google Scholar]
- De Mello-Andrade, J.M., Fasolo, D. (2014). Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. http://dx.doi.org/10.1016/B978-0-12-398456-2.00020-7.
- Deng Y., Yang G., Yue J., Qian B., Liu Z., Wang D., Zhong Y., Zhao Y. Influences of ripening stages and extracting solvents on the polyphenolic compounds, antimicrobial and antioxidant activities of blueberry leaf extracts. Food Control. 2014;38:184–191. [Google Scholar]
- Elaine-Tanqueco R., Rodríguez F.M.P., Laude R.E., Cueno M. Total Free Sugars, Oil and Total Phenolics Content of Stored Coconut (Cocos nucifera L.) Water. Philippine Journal of Science. 2007;136(2) [Google Scholar]
- FAO (2016). (Online) Available at: http://www.fao.org/Wairdocs/X5403S/x5403s0d.htm (Accessed 25 Jul. 2016).
- Ferreira-Zielinski A.A., Haminiuk C.H.W., I, Alberti A., Nogueira A., Demiate I.M., Granato D. A comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Research International. 2014 http://dx.doi.org/10.1016/j.foodres.2013.09.010 [Google Scholar]
- Gironés-Vilaplana A., Mena P., García-Viguera C., Moreno D.A. Novel beverage rich in antioxidant phenolics: Maqui berry (Aristotelia chilensis) and lemon juice. LWT-Food Science and Technology. 2012;47:279–286. [Google Scholar]
- Herrero M., Cifuentes A., Ibañez A. Extractions techniques for the determinations of carotenoids and vitamins in food. Comprehensive Sampling and Simple Preparation. 2012 [Google Scholar]
- Hotz C., Gibson R.S. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. The Journal of Nutrition. 2007;137:1097–1100. doi: 10.1093/jn/137.4.1097. [DOI] [PubMed] [Google Scholar]
- Kallithraka S., Salacha M.I., Tzourou I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chemistry. 2009;113(2):500–505. [Google Scholar]
- Kevers C., Falkowski M., Tabart J., Defraigne J.O., Dommes J., Pincemail J. Evolution of antioxidant capacity during storage of selected fruits and vegetables. Journal of Agricultural and Food Chemistry. 2007;55:8596–8603. doi: 10.1021/jf071736j. [DOI] [PubMed] [Google Scholar]
- Klaui H., Bauernfeind J.C. Carotenoids as food colors. In: Bauernfeind J.C., editor. Carotenoids as Colorants and Vitamin A precursor. Academic Press; San Diego: 1981. pp. 47–317. [Google Scholar]
- Klimczak I., Gliszczynska-Swiglo A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chemistry. 2015;175:100–105. doi: 10.1016/j.foodchem.2014.11.104. [DOI] [PubMed] [Google Scholar]
- Klimczak I., Małecka M., Szlachta M., Gliszczynska-Swiglo A. Effect of storage on the content of polyphenols: vitamin C and the antioxidant activity of orange juices. Journal of Food Composition and Analysis. 2007;20:313–322. [Google Scholar]
- Kuskoski E.M., Asuero A.G., Troncoso A.M., Mancini-Filho J., Fett R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciência e Tecnologia de Alimentos. 2005;25(4):726–732. [Google Scholar]
- La Cava E.L.M., Sgroppo S.C. Evolution during refrigerated storage of bioactive compounds and quality characteristics of grapefruit [Citrus paradisi (Macf.)] juice treated with UV-C light. LWT-Food Science and Technology. 2015;63:1325–1333. [Google Scholar]
- Martinez-Flores H.E., Garnica-Romo M.G., Bermudez-Aguirre D., Pokhrel P.R., Barbosa-Canovas G.V. Physico-chemical parameters: bioactive compounds and microbial quality of thermo-sonicated carrot juice during storage. Food Chemistry. 2015;172:650–656. doi: 10.1016/j.foodchem.2014.09.072. [DOI] [PubMed] [Google Scholar]
- Mena P., Gironés-Vilaplana A., Marti N., García-Viguera C. Pomegranate varietal wines: Phytochemical composition and quality parameters. Food Chemistry. 2012;133:108–115. [Google Scholar]
- Mukherjee P.K., Nema N.K., Maity N., Sarkar B.K. Phytochemical and therapeutic potential of cucumber. Fitoterapia. 2013;84:227–236. doi: 10.1016/j.fitote.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Nicoli M.C., Anese M., Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends in Food Science and Technology. 1999;10:94–100. [Google Scholar]
- Odriozola-Serrano I., Soliva-Fortuny R., Hernández-Jover T., Martín-Belloso O. Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chemistry. 2009;112:258–266. [Google Scholar]
- Piljac-Zegarac J., Valek L., Martinez S., Belšcak A. Fluctuations in the phenolic content and antioxidant capacity of dark fruit juices in refrigerated storage. Food Chemistry. 2009;113:394–400. [Google Scholar]
- Pinelo M., Manzocco L., Nuñez M.J., Nicoli M.C. Interaction among phenols in food fortification: Negative synergism on antioxidant capacity. Journal of Agricultural and Food Chemistry. 2004;52:1177–1180. doi: 10.1021/jf0350515. [DOI] [PubMed] [Google Scholar]
- Rao A.V., Agarwal S. Role of antioxidant lycopene in cancer and heart disease. Journal of the American College of Nutrition. 2000;19(5):563–569. doi: 10.1080/07315724.2000.10718953. [DOI] [PubMed] [Google Scholar]
- Reddy C.V.K., Sreeramulu D., Raghunath M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Research International. 2010;43(1):285–288. [Google Scholar]
- Rodriguez-Amaya, D.B., 1997. Carotenoids and food preservation: The retention of pro-vitamin A carotenoid in prepared, processed and storage food. Washington, DC: Office of Health and Nutrition, US Agency for International Development. Available from: http://www.mostproject.org/carrots2.pdf.
- Saideswara-Rao Y., Mathew K.M. Woodhead Publishing Limited; 2012. Tamarind. [Google Scholar]
- Sánchez-Moreno C., Plaza L., De Ancos B., Cano P. Vitamin C, pro-vitamin A carotenoid: and other carotenoids in high-pressurized orange juice during refrigerated storage. Journal of Agricultural and Food Chemistry. 2003;51:647–653. doi: 10.1021/jf020795o. [DOI] [PubMed] [Google Scholar]
- Sepulveda E., Saenz C., Pena A., Robert P., Bartolome B., Gómez-Cordoves C. Influence of the genotype on the anthocyanin composition, antioxidant capacity and color of Chilean pomegranate (Punica granatum l.) juices. Chilean Journal of Agricultural Research. 2010;70(1):50–57. [Google Scholar]
- Siró I., Kapolna E., Kapolna B., Lugasi A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite. 2008;51:456–467. doi: 10.1016/j.appet.2008.05.060. [DOI] [PubMed] [Google Scholar]
- Smith J., Charter E. Functional food product development. Functional food science and technology. 2010 ISBN 978-1-4051-7876-1. [Google Scholar]
- Stinco C.M., Fernandez-Vázquez R., Heredia F.J., Melendez-Martinez A.J., Vicario I.M. Bioaccessibility: antioxidant activity and colour of carotenoids in ultrafrozen orange juices: Influence of thawing conditions. LWT-Food Science and Technology. 2013;53:458–463. [Google Scholar]
- van Poppel G., Goldbohm R.A. Epidemiologic evidence for beta-carotene and cancer prevention. The American Journal of Clinical Nutrition. 1995;62(6):1393S–1402S. doi: 10.1093/ajcn/62.6.1393S. [DOI] [PubMed] [Google Scholar]
- Vegara S., Mena P., Martí N., Saura D., Valero M. Approaches to understanding the contribution of anthocyanins to the antioxidant capacity of pasteurized pomegranate juices. Food Chemistry. 2013;141:1630–1636. doi: 10.1016/j.foodchem.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Zaouaya F., Mena P., García-Viguera C., Marsa M. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Industrial Crops and Products. 2012;40:81–89. [Google Scholar]
- Zulueta A., Esteve M.J., Frasquet I., Frígola A. Vitamin C, vitamin A: phenolic compounds and total antioxidant capacity of new fruit juice and skim milk mixture beverages marketed in Spain. Food Chemistry. 2007;103:1365–1374. [Google Scholar]