Abstract
A 57-year-old woman was admitted to National Defense Medical College hospital for treatment of gastric cancer with pyloric stenosis. She had been diagnosed with chronic kidney disease (CKD) 10 years prior, but received no hemodialysis. Because of peritoneal dissemination, a palliative distal gastrectomy was performed. In consideration of renal dysfunction, we decided for chemotherapy with paclitaxel, but not S-1 plus cisplatin regimen which is renal toxic agents. On the 29th postoperative day, chemotherapy using paclitaxel was initiated at a dose of 80 mg/m2. Paclitaxel was administered weekly on days 1, 8, and 15 on a 28-day cycle. The patient tolerated 13 courses of this treatment without any severe adverse effect, such as exacerbation of renal function. Despite the gradual increase in the level of tumor markers, metastases were not detected via radiography during the clinical course. Moreover, renal function was maintained for the duration of the clinical course. To date, standard chemotherapeutic treatment for patients with CKD has not been established. We conclude that weekly paclitaxel is a suitable treatment regimen for patients with renal failure requiring chemotherapy for advanced gastric cancer.
Keywords: Gastric cancer, Paclitaxel, Hemodialysis, Pharmacokinetics
Highlights
-
•
In Japan, the standard therapy for unresectable advanced gastric cancer is S-1 and cisplatin combination chemotherapy. However, the pharmacodynamics of these agents in patients with renal dysfunction remains unclear.
-
•
We herein present a case of chronic kidney disease (CKD) in which the patient was safely given paclitaxel to treat gastric cancer with peritoneal dissemination.
-
•
Weekly paclitaxel therapy is feasible and effective for patients with advanced gastric cancer and renal impairment.
1. Introduction
As the life expectancy of patients with end-stage renal failure has lengthened due to improvements in renal replacement therapy, the need for chemotherapy to treat malignancies in such patients has increased. In Japan, key drugs for gastric cancer is cisplatin and S-1, which is composed of FT (tegafur, the prodrug for 5-FU) and CDHP (5-chloro-2,4-dihydroxypyridine, which inhibits the 5-FU degradation enzyme dihydropyrimidine dehydrogenase) [1]. S-1 and cisplatin combination chemotherapy is the standard regimen for unresectable advanced gastric cancer in Japan [2]. However, the pharmacodynamics of these agents in patients with renal dysfunction remains unclear. Moreover, these drugs are regarded as contraindicated for patients with renal failure due to concerns that adverse events, such as myelotoxicity and non-hematologic toxicities, might be enhanced because of elevated blood metabolite concentrations. We herein present a case of chronic kidney disease (CKD) in which the patient was safely given paclitaxel to treat gastric cancer with peritoneal dissemination. We further discuss the usefulness of a weekly paclitaxel regimen for such patients with advanced gastric cancer. This work was conducted in line with the CARE guide lines for clinical case reporting [3].
2. Case presentation
2.1. Diagnosis
A 57-year-old woman presented at National Defense Medical College hospital complaining of nausea and vomiting. She had been diagnosed with CKD due to chronic nephritis ten years prior. A dialysis shunt had been constructed 2 years prior, but hemodialysis induction had been postponed. An endoscopic examination revealed antral gastric cancer with pyloric stenosis (Fig. 1). A gastrointestinal series also revealed antrum-pylorus narrowing due to cancer (Fig. 2). Computed tomography (CT) scans showed wall thickening of gastric antrum, but no lymph node swelling or distant metastases were evident (Fig. 3). We planned the radical gastrectomy with curative intent. However, upon laparotomy, peritoneal disseminations were encountered. Although a curative intervention was not possible, a palliative distal gastrectomy was performed to resolve the outlet obstruction.
Fig. 1.
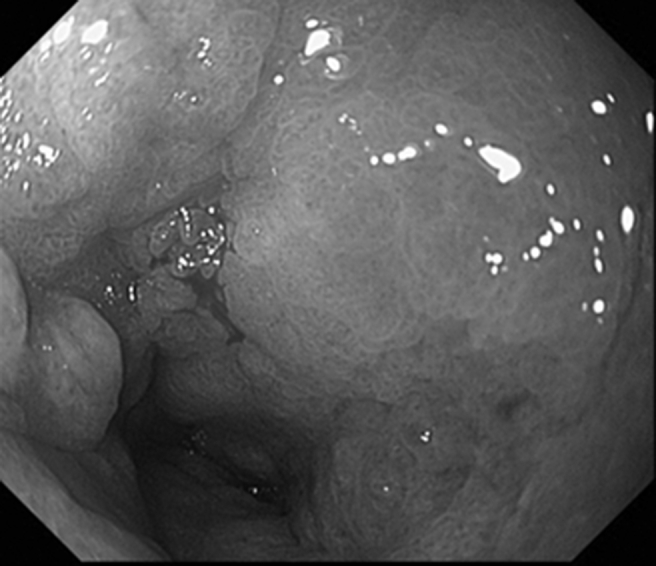
The gastroendoscopic finding showing antral gastric cancer, leading to circumferential pyloric stenosis.
Fig. 2.
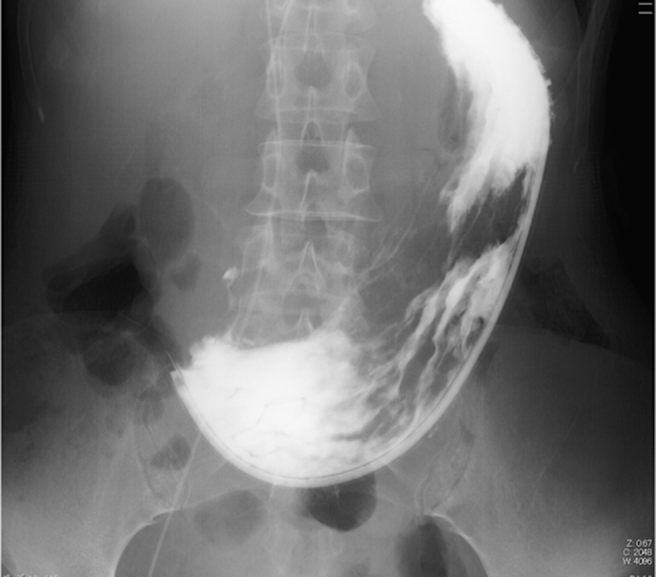
Photofluorography revealing antrum-pylorus narrowing caused by cancer as well as a large amount of residual food in the stomach.
Fig. 3.
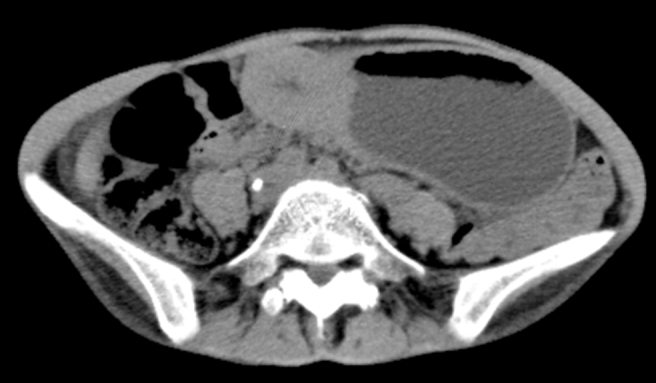
Abdominal CT revealing wall thickening of the antrum of stomach. No lymph node or distant metastases are evident.
2.2. Chemotherapy after surgery
On the 29th postoperative day, chemotherapy using paclitaxel was initiated, for which the patient provided informed consent. Following appropriate premedication, paclitaxel was administered at a dose of 80 mg/m2 as a 90 min intravenous infusion in 250 mL of 5% dextrose solution. Paclitaxel was administered weekly on days 1, 8, and 15 on a 28-day cycle. The patient tolerated 13 courses of this treatment, and no exacerbation of renal function or any other severe adverse effects were observed. During the clinical course, no new lesions were detected by CT scans. Stable disease was maintained for 12 months (Fig. 4) despite a modest increase of CA19-9 and CA125. Unfortunately, uncontrollable ascites and cachexia progressed. The patient died on October 2014, 18 months after her gastrectomy.
Fig. 4.
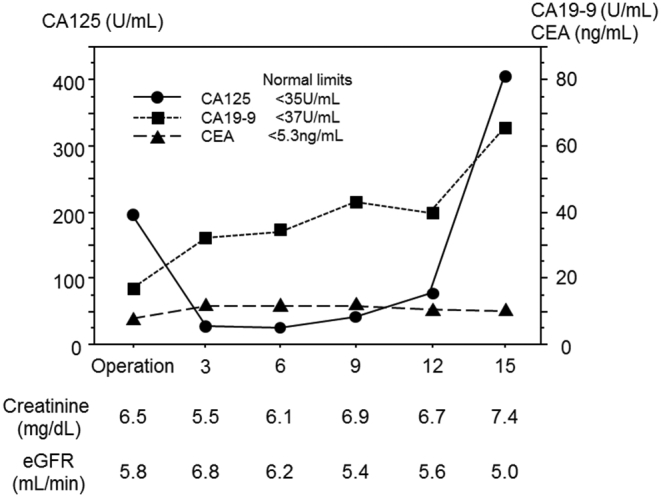
During the clinical course, renal function was not impaired and clinically stable disease (SD) was maintained for 15 months.
3. Discussion
Significant advances in renal replacement therapy have led to prolonged survival in patients with renal impairment, and the overall risk of cancer is increased in such patients [4]. Therefore, oncologists are more likely to encounter patients who suffer from both cancer and renal failure. There are a limited number of reports characterizing chemotherapy for patients with advanced gastric cancer and renal impairment.
Several randomized studies have demonstrated that chemotherapy could improve the prognosis as compared to supportive care in patients with advanced and unresectable gastric cancer. The significance of performing chemotherapy for patients with advanced gastric cancer is now widely recognized [5]. Anticancer drugs such as cisplatin, irinotecan hydrochloride, paclitaxel, 5-FU and its oral prodrugs (S-1 and capecitabine) are effective and have been used to treat gastric cancer. The first-line regimen in Japan for advanced and unresectable gastric cancer is S-1/cisplatin combined chemotherapy, for which safety and efficacy have been characterized in the SPIRITS trial [2]. Nevertheless, both drugs are known to be nephrotoxic and should not be administered to renal failure patients [6]. Recently, oxaliplatin has been used to treat gastric cancer in Japan based on the results of the CLASSIC trial [7]. However, the pharmacokinetics of oxaliplatin are known to be altered in patients with renal impairment; consequently, oxaliplatin also should not to be administered to such patients [8]. Thus, there is no optimal chemotherapy regimen for patients with renal impairment without hemodialysis.
It is known that paclitaxel is extensively metabolized by the liver and secreted in bile, with less than 10% extracted by the kidneys [9]. Kawate et al. reported successful weekly administration of paclitaxel for a patient with unresectable gastric cancer undergoing hemodialysis, and demonstrated that the plasma concentration of paclitaxel is not influenced by hemodialysis [10]. In the present case, the patient had stage 4 CKD but did not require hemodialysis. She did not experience any adverse effects of chemotherapy, including exacerbation of renal function. Thus, weekly paclitaxel therapy should be considered as a safe and feasible treatment for gastric cancer patients with chronic renal failure, both before and after introduction of hemodialysis.
There is currently no consensus on the appropriate regimen for gastric cancer patients with chronic renal failure. Paclitaxel as an anticancer agent for gastric cancer is 2nd-line therapy in Japan and its response rate is relatively low (21%) [11]. Thus, a prospective study of a larger number of patients is necessary to establish a safe and effective regimen for gastric cancer patients with renal failure.
In the present case, we administered weekly paclitaxel therapy under sufficient informed consent and were prepared to administer urgent hemodialysis, if necessary. The patient successfully received chemotherapy without cessation of medication or any change of schedule due to adverse effects or renal toxicity. In conclusion, we believe that weekly paclitaxel therapy is feasible and effective for patients with advanced gastric cancer and renal impairment.
Ethical approval
Ethical approval not required.
Conflicts of interest
All authors have no conflict of interest.
Sources of funding
All authors have no funding of research.
Author contribution
All authors in this manuscript contributed to the interpretation of data, and drafting and writing of this manuscript. KK, HT, HH, NI, KY, SH, ES, and SA were engaged in patient's care in his hospital coarse including surgery under the supervision of HT, KH and JY. All authors have read and approved this manuscript for publication.
Guarantor
Dr. Tsujimoto, who is the Chief of Department of Uppergastrointestinal Surgery, National Defense Medical College, is the Guarantor.
References
- 1.Shirasaka T., Shimamato Y., Ohshimo H. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–557. doi: 10.1097/00001813-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Koizumi W., Narahara H., Hara T. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 3.Gagnier J.J., Kienle G., Altman D.G. The CARE guidelines: consensus-based clinical case reporting guideline development. J. Med. Case Rep. 2013;7:1. doi: 10.1186/1752-1947-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maisonneuve P., Agodoa L., Gellert R. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 5.Glimelius B., Hoffman K., Haglund U. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann. Oncol. 1994;5:189–190. doi: 10.1093/oxfordjournals.annonc.a058778. [DOI] [PubMed] [Google Scholar]
- 6.Tominaga K., Higuchi K., Okazaki H. Safety and efficacy of S-1, a novel oral fluorouracil antitumor drug, for a chronic renal failure patient maintained on hemodialysis. Oncology. 2004;66:358–364. doi: 10.1159/000079483. [DOI] [PubMed] [Google Scholar]
- 7.Noh S.H., Park S.R., Yang H.K. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 8.Takimoto C.H., Graham M.A., Lockwood G. Oxaliplatin pharmacokinetics and pharmacodynamics in adult cancer patients with impaired renal function. Clin. Cancer Res. 2007;13:4832–4839. doi: 10.1158/1078-0432.CCR-07-0475. [DOI] [PubMed] [Google Scholar]
- 9.Wiernik P.H., Schwartz E.L., Strauman J.J. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47:2486–2493. [PubMed] [Google Scholar]
- 10.Kawate S., Takeyoshi I., Morishita Y. Pharmacokinetics of paclitaxel in a hemodialysis patient with advanced gastric cancer: a case report. World J. Gastroenterol. 2006;12:5237–5239. doi: 10.3748/wjg.v12.i32.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hironaka S., Ueda S., Yasui H. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric Cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J. Clin. Oncol. 2013;31:4438–4444. doi: 10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]


