Abstract
PURPOSE
Liver metastases from renal cell carcinoma (RCC) are not uncommon in the course of disease. However, data about tumor response to intraarterial therapy (IAT) are scarce. This study assessed whether changes of enhancing tumor volume using quantitative European Association for the Study of the Liver (qEASL) on magnetic resonance imaging (MRI) and computed tomography (CT) can evaluate tumor response and predict overall survival (OS) early after therapy.
METHODS AND MATERIALS
Fourteen patients with liver metastatic RCC treated with IAT (transarterial chemoembolization: n= 9 and yttrium-90: n= 5) were retrospectively included. All patients underwent contrast-enhanced imaging (MRI: n= 10 and CT: n= 4) 3 to 4 weeks pre- and posttreatment. Response to treatment was evaluated on the arterial phase using Response Evaluation Criteria in Solid Tumors (RECIST), World Health Organization, modified RECIST, EASL, tumor volume, and qEASL. Paired t test was used to compare measurements pre- and post-IAT. Patients were stratified into responders (≥65% decrease in qEASL) and nonresponders (<65% decrease in qEASL). OS was evaluated using Kaplan-Meier curves with log-rank test and the Cox proportional hazard model.
RESULTS
Mean qEASL (cm3) decreased from 93.5 to 67.2 cm3 (P= .004) and mean qEASL (%) from 63.1% to 35.6% (P= .001). No significant changes were observed using other response criteria. qEASL was the only significant predictor of OS when used to stratify patients into responders and nonresponders with median OS of 31.9 versus 11.1 months (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.19-0.97; P= .042) for qEASL (cm3) and 29.9 versus 10.2 months (HR, 0.09; 95% CI, 0.01-0.74; P= .025) for qEASL (%).
CONCLUSION
Three-dimensional (3D) quantitative tumor analysis is a reliable predictor of OS when assessing treatment response after IAT in patients with RCC metastatic to the liver. qEASL outperforms conventional non-3D methods and can be used as a surrogate marker for OS early after therapy.
Introduction
Renal cell carcinoma (RCC) is the most common primary malignancy of the kidney and accounts for 2% to 4% of all new cancer cases in developed countries per year [1], [2]. Five-year survival rates for local tumor stage are high; however, metastases are not uncommon in the course of disease [3]. In fact, 20% of patients show metastatic spread at the initial diagnosis, and of all patients who have undergone curative nephrectomy, 20% to 40% will develop RCC metastases [4], [5]. The liver is one of the most common metastatic sites causing a dramatic drop of the 5-year survival rate to 20% [4], [6]. The poor prognosis of the disease, once it shows metastatic spread, is due to the ineffectiveness of conventional therapies such as systemic chemotherapy, radiation, or hormone therapy [7], [8], [9]. Improved understanding of the underlying biology of renal cell cancer has led to new treatment approaches including targeted therapies such as antiangiogenic agents or tyrosine kinase inhibitors [7], [8]. Beyond that, some selected patients may benefit from metastasectomy, yet a majority of patients are not amendable for surgery [10], [11]. For those patients, image-guided intraarterial therapies (IATs) such as transarterial chemoembolization (TACE) as well as yttrium-90 (Y-90) radioembolization have been used and evaluated in the past [12], [13].
The role of radiological assessment of solid tumors especially for tumor treatment response cannot be overestimated, and the evaluation of tumor response to therapy is crucial to the course of treatment [14]. For this purpose, over the last decades, several radiological response criteria were established. The World Health Organization (WHO) introduced an evaluation system for solid liver tumors that is based on the sum of the product of two-dimensional (2D) diameters of tumor lesions [15]. The Response Evaluation Criteria in Solid Tumors (RECIST) was introduced in 2000 (revised in 2009) to address some of the limitations of the WHO criteria. RECIST measurements are based on the sum of the one-dimensional (1D) longest diameters [15], [16]. However, in terms of IATs, both criteria are greatly limited because most IATs cause tumor necrosis without immediate changes on overall lesion size rather than a decrease of viable tumor tissue [17]. The European Association for the Study of the Liver (EASL) therefore recommended measuring tumor response by changes of the uptake of contrast medium by the tumor tissue [18]. The American Association for the Study of Liver Disease shared the same approach by suggesting measuring changes in tumor enhancement as a biomarker of tumor viability and introduced the modified RECIST (mRECIST) criteria [19]. Yet, all 1D and 2D evaluation methods are flawed with a limited reproducibility and an essential inaccuracy when assessing the entity of necrotic and heterogenic tumor lesions [20], [21]. These clinically relevant limitations have led to the development of three-dimensional (3D) quantitative image analysis techniques that are able to achieve a reproducible, biologically accurate, and clinically practicable tumor evaluation [17], [22], [23]. The purpose of this study is to assess whether changes of enhancing tumor volume using quantitative EASL (qEASL) on magnetic resonance imaging (MRI) and computed tomography (CT) can evaluate tumor response and predict survival after one session of IAT.
Methods and Materials
Study Design and Cohort
The study was compliant with the Health Insurance Portability and Accountability Act and approved by the Institutional Review Board. This two-center study consisted of patients with liver metastases from RCC treated with TACE or Y-90 procedures at the Johns Hopkins Hospital, Baltimore and the Northwestern Memorial Hospital, Chicago from 2000 to 2014. A review of the prospectively collected imaging databases at both institutions identified 24 patients [23].
Patients that were included into the final survival analysis fulfilled the following criteria: 1) diagnosis of RCC liver metastases confirmed by pathological criteria; 2) patients were IAT naive; and 3) patients underwent dynamic contrast-enhanced MR or CT imaging before and after the first IAT. Seven patients were excluded for the following reasons: previous IAT (n= 3) and absence of baseline and/or follow-up MRI (n= 7). On the basis of these criteria, the final study population included 14 patients.
IAT Protocol
TACE
All TACE procedures were performed by the same interventional radiologist (Jean-Francois Geschwind) with 18 years of experience in hepatic interventions by using a consistent approach as reported previously [24]. Briefly, angiographic steps were performed with contrast runs from the celiac trunk and the superior mesenteric artery to define the hepatic arterial anatomy, to determine portal venous patency, and to evaluate tumor vascularity. The contrast agent used was Oxilan (Guerbet, France). Patients were treated with selective (lobar or segmental) and super-selective injections. A solution containing 50 mg of doxorubicin and 10 mg of mitomycin-C in a 1:1 mixture with Lipiodol (Guerbet, France) was infused and followed by administration of 100- to 300-μm–diameter microspheres (Embospheres; Merit Medical, South Jordan, UT). Substantial arterial flow reduction to the tumor was defined as the technical end point of embolization.
Y-90
All Y-90 procedures were performed by one interventional radiologist (Riad Salem) with over 15 years of experience in hepatic interventions. Patients were treated based on previously published recommendations and guidelines using a selective segmental or lobar treatment with glass microspheres loaded with Y-90 (TheraSphere; Nordion, Ottawa, Canada) [25], [26]. Prior to treatment, an angiogram was performed in order to embolize parasitizing arteries and optimize catheter tip positioning. Furthermore, 99technetium-macroaggregated albumin was infused to estimate pulmonary and gastrointestinal shunting.
MR and CT Imaging Protocol
All patients underwent dynamic contrast-enhanced MR or CT imaging at baseline and approximately 3 to 4 weeks after IAT. MRIs were obtained on a 1.5-T MRI scanner (Magnetom Avanto; Siemens Medical Solutions, Forchheim, Germany) using a phased array torso coil. Standard MR liver protocol included the following: axial T2-weighted fast spin-echo images, axial single-shot breath-hold gradient-echo diffusion-weighted echo-planar images, and axial breath-hold unenhanced and contrast-enhanced (0.1 mmol/kg of intravenous gadodiamide [Omniscan; Amersham, Princeton, NJ]) T1-weighted 3D fat-suppressed spoiled gradient-echo. CT scans were obtained on a Lightspeed scanner (GE Healthcare, Princeton, NJ). A triphasic contrast-enhanced CT liver protocol was employed that used a standard power injector (Medrad, Indianola, PA) connected to an intravenous catheter to inject 125 ml of Omnipaque 350 (GE Healthcare) at a rate of 4 to 5 ml/sec. All contrast-enhanced images were acquired in the arterial, portal venous, and delayed phases (20, 70, and 180 seconds after intravenous contrast administration, respectively).
Image Data Analysis
Conventional Response Criteria
Two independent radiological readers (one board-certified radiologist (Rüdiger Egbert Schernthaner) with 8 years of experience in abdominal imaging and a radiological reader (Florian Nima Fleckenstein) with 1 year of experience) conducted non-3D tumor response assessment during the same reading session to ensure careful comparison of pre- and posttreatment findings. Readers were blinded to patient survival and clinical data. Any discrepancy was resolved by consensus. All measurements were done using standardized electronic calipers by using Digital Imaging in Communications and Medicine files.
Volumetric Response Criteria
Semiautomatic quantitative 3D-image analysis was done by a radiological reader (Florian Nima Fleckenstein) in training with 1 year of experience in the field of volumetric image analysis closely supervised by a board-certified radiologist (Rüdiger Egbert Schernthaner) with 8 years of experience in abdominal MRI.
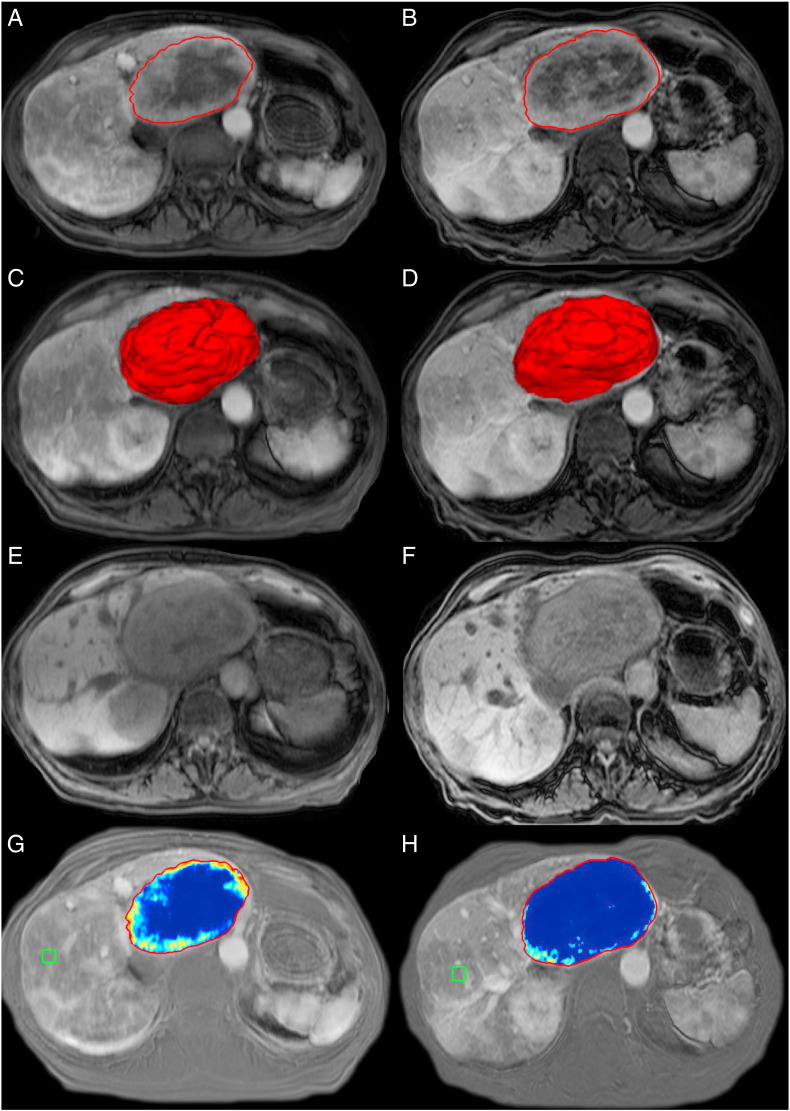
Quantitative volumetric tumor analysis of the target lesions was done using a semiautomatic 3D segmentation software (Medisys; Philips Research, Suresnes, France) as previously described [22]. Briefly, a segmentation mask was semiautomatically created for every tumor lesion on the arterial phase of the contrast-enhanced baseline and follow-up MR and CT images (Figure 1, A and B). The arterial phase was chosen due to the hypervascular character of RCC metastases. Overall tumor volume defined as volumetric RECIST (vRECIST) was expressed in cubic centimeters (cm3) directly calculated from the generated segmentation masks. Figure 1, C and D illustrates the corresponding 3D models. These 3D masks were then used for quantitative analysis of the tumor enhancement (qEASL). In order to remove any background signal, the precontrast scan (Figure 1, E and F) was subtracted from the arterial-phase scan (Figure 1, A and B), resulting in images that only show effective contrast uptake during the arterial phase. In the next step, a region of interest (ROI) formed by 1 cm3 was placed in an area of extratumoral liver parenchyma as a reference in order to calculate the relative contrast enhancement (Figure 1, G and H). Quantitative volumetric tumor enhancement was expressed in cubic centimeter for each lesion qEASL (cm3) and percentage of enhancing tumor volume qEASL (%). The software automatically generated a color map in order to visualize the enhancement pattern (blue representing nonenhancing necrotic tissue and red representing viable, enhancing tumor tissue; Figure 1, G and H). The accuracy, reader-independent reproducibility of semiautomatic tumor segmentation, as well as the radiological-pathological validation of 3D quantitative tumor enhancement analysis have been reported previously [17], [21], [27].
Figure 1.
Quantitative volumetric contrast-enhanced MRI assessment technique (qEASL). The left column represents baseline MRI, and the right column represents the follow-up MRI after IAT. (A and B) Semiautomated tumor segmentation on the arterial phase of a contrast-enhanced MRI. (C and D) The corresponding volume of the segmented tumor in a 3D model. (E and F) The precontrast MR sequence to demonstrate baseline background signal intensity of the tumors. (G and H) The qEASL color maps of the tumor on the subtracted MRI scan (the scan before contrast material [E and F] was subtracted from the arterial phase scan [A and B] to remove any background signal intensity). Color maps: Red represents maximum enhancement, and blue represents no enhancement, normalized by the ROI. Green box: 3D ROI used as the reference background of image intensity.
Tumor Response Assessment
Tumor response to IAT procedures was evaluated according to the WHO, RECIST, EASL, mRECIST, vRECIST, and qEASL criteria, respectively. Due to the fact that no guidelines for volumetric tumor response criteria exist and for the purpose of a unified and simplified response assessment in a clinical setting, we selected cutoff values that are based on the currently used RECIST and mRECIST criteria for vRECIST and qEASL thresholds. A decrease of 30% was defined as partial response (PR) using the 1D evaluation criteria RECIST and mRECIST. According to the formula volume = 4/3πr3, this corresponds to a decrease of 65% of tumor volume. Complete response (CR) was defined as the disappearance of the target lesion. Both CR and PR were considered objective tumor response. Patients with objective response were classified as responders. Patients with stable disease (SD, no change in tumor size) and progressive disease (PD, tumor growth) were classified as nonresponders. The accuracy and value of these thresholds were described in previous works [22], [28]. Table 1 provides an overview of the different cutoffs.
Table 1.
Response Criteria
| WHO | RECIST | EASL | mRECIST | vRECIST | qEASL (cm3) | qEASL (%) | |
|---|---|---|---|---|---|---|---|
| CR | Disappearance of all target lesions | Disappearance of all target lesions | Disappearance of all enhancing tissue in all target lesions | Disappearance of all enhancing tissue in all target lesions | Disappearance of all target lesions | Disappearance of all enhancing tissue in all target lesions | Disappearance of all enhancing tissue in all target lesions |
| PR | ≥50% decrease in the sum of the product of bidimensional diameter of the target lesions | ≥30% decrease in the sum of the longest diameter of the target lesions | ≥50% decrease in the sum of the product of bidimensional diameter of enhancing tissue of the lesions | ≥30% decrease in the sum of the longest enhancing diameter of the target lesions | ≥65% decrease in the sum of the volume of the target lesions | ≥65% decrease in the sum of enhancing tissue volume of the lesions | ≥65% decrease in the sum of percentage of enhancing tissue of the lesions |
| PD | ≥25% increase in the sum of the product of bidimensional diameter of the target lesions | ≥20% increase in the sum of the longest diameter of the target lesions | ≥25% increase in the sum of the product of bidimensional diameter of the lesions | ≥20% increase in the sum of the longest enhancing diameter of the target lesions | ≥73% increase in the sum of the volume of the target lesions | ≥73% increase in the sum of enhancing tissue volume of the lesions | ≥73% increase in the sum of percentage of enhancing tissue of the lesions |
| SD | Any case that does not qualify for CR, PR, or PD | Any case that does not qualify for CR, PR, or PD | Any case that does not qualify for CR, PR, or PD | Any case that does not qualify for CR, PR, or PD | Any case that does not qualify for CR, PR, or PD | Any case that does not qualify for CR, PR, or PD | Any case that does not qualify for CR, PR, or PD |
Note: RECIST mRECIST are calculated by measuring the longest diameter of the enhancing tumor in the axial plane. WHO is calculated by measuring the longest diameter of the tumor in the axial plane and by drawing a line perpendicular to it. EASL is calculated by measuring the longest diameter of the enhancing tumor in the axial plane and by drawing a line perpendicular to it. qEASL (cm3) is calculated by measuring the volume of enhancing tumor. qEASL (%) is calculated by measuring the percentage of enhancing tumor in the lesion volume.
Statistical Analysis and Survival Assessment
Descriptive statistics were used to summarize the data. Mean and range were used for continuous variables; and frequency and percent, for nominal data. Reader agreement of non-3D measurements was assessed using intraclass correlation coefficient. Significance levels and confidence intervals (CIs) were calculated. Student's t test was used to compare tumor size, volume, and enhancement prior and after IAT in order to evaluate tumor response to treatment. P values ≤ .05 were defined as statistically significant. Overall survival (OS) was defined as the time from the date of treatment to the date of death. Patients lost in follow-up or alive at the end-of-observation date (November 5, 2014) were censored. Kaplan-Meier survival curves were plotted for each method using the described thresholds. Median OS and the 95% CI were calculated. The predictive value of each method was assessed by Cox proportional hazard modeling (HR). All statistical analyses were performed using the statistical software R (R Foundation for Statistical Computing, Version 3.1.2, Vienna, Austria, 2014) and SPSS (IBM, Version 22, Armonk, NY).
Results
Patient Characteristics, Treatment, and Interreader Agreement
Table 2 summarizes baseline data of the patient cohort. Mean lesion size was 7.20 ± 2.75 cm. Mean follow-up period was 24.8 ± 28.4 months (range, 2.1-114.7). Mean time from RCC diagnosis to liver metastasis was 53.9 ± 62.1 months (range, 0-203.2). Mean time from baseline MRI to IAT was 2.0 ± 1.7 weeks (range, 0-6) and from IAT to follow-up MRI 3.9 ± 1.4 weeks (range, 3-8). A mean of 2.1 ± 1.2 (range, 1-4) IAT procedures was performed per patient for a total of 29 treatments. For the first round of IAT treatments, a total number of 5 (36%) Y-90 and 9 (64%) TACE procedures were performed. The targeted liver lobe was in 8 (64%) cases the right and in 5 (36%) cases the left liver lobe. All IAT procedures were technically successful, and no relevant complications or toxicities were noted. Furthermore, there were no significant changes in pre- and posttreatment Eastern Cooperative Oncology Group (ECOG) performance score. After the first treatment, 6 (43%) patients were assigned an ECOG performance score of 0, and 8 (57%) patients were assigned an ECOG performance score of 1. Of note, interreader agreement was excellent with intraclass correlation coefficients for RECIST, WHO, mRECIST, and EASL measurements of 0.968, 0.945, 0.966, and 0.959.
Table 2.
Baseline Patient Characteristics
| Parameter | N (%) |
|---|---|
| Demographics | |
| Number of patients | 14 (100) |
| Age | |
| Mean (SD) | 65.6 (9.0) |
| <65 years | 6 (43) |
| ≥65 years | 8 (57) |
| Sex | |
| Female | 6 (43) |
| Male | 8 (57) |
| Ethnicity | |
| Caucasian | 11 (79) |
| Asian | 2 (14) |
| Afro-American | 1 (7) |
| Patient and liver assessment | |
| ECOG status | |
| 0 | 9 (64) |
| 1 | 5 (36) |
| Child-Pugh class | |
| A | 12 (86) |
| B | 2 (14) |
| Treatment | |
| Kidney | |
| Nephrectomy | 14 (100) |
| Liver | |
| TACE | 22 (75) |
| Y90 | 7 (25) |
| Number of IATs | |
| 1 | 6 (43) |
| 2 | 4 (29) |
| 3 | 1 (7) |
| 4 | 3 (21) |
| Tumor characteristics | |
| Number of lesions | 29 |
| Mean lesions per patient (SD) | 2.07 (1.17) |
| Extrahepatic disease | 4 (29) |
Image Analysis
Table 3 summarizes pre- and posttreatment values as measured by conventional response criteria such as RECIST, WHO, mRECIST, and EASL as well as by volumetric assessment methods such as vRECIST, qEASL (%), and qEASL (cm3). None of the conventional response criteria or vRECIST could show significant changes after IAT. There was a significant (P= .004) decrease of enhancing tumor volume (qEASL [cm3]) after IAT as well as a significant decrease of percentage of enhancing tumor (qEASL [%]) (P= .001) after IAT.
Table 3.
Tumor Changes in Target Lesions after IAT According to Conventional and Volumetric Criteria
| Mean Baseline Value (SD) | Mean Follow-Up Value (SD) | P Value | |
|---|---|---|---|
| Conventional response criteria | |||
| RECIST (cm) | 7.20 (2.75) | 7.74 (3.16) | .067 |
| WHO (cm2) | 419.36 (250.39) | 478.06 (291.72) | .134 |
| mRECIST (cm) | 5.51 (1.81) | 5.07 (2.68) | .389 |
| EASL (cm2) | 240.24 (158.19) | 209.46 (178.07) | .421 |
| Volumetric response criteria | |||
| vRECIST (cm3) | 170.13 (149.12) | 178.30 (162.16) | .596 |
| qEASL (cm3) | 93.45 (94.23) | 67.15 (98.21) | .004 |
| qEASL (%) | 63.07 (27.23) | 35.63 (29.83) | .001 |
Tumor Response Assessment
Conventional Response Criteria
According to the RECIST criteria, 12 (86%) patients showed SD and 2 (14%) PD. The WHO criteria classified 9 (64%) as SD and 5 (36%) patients as PD. Stratification following the mRECIST criteria classified 1 (7%) patient as CR, 4 (29%) patients as PR, 8 (57%) patients as SD, and 1 (7%) patient as PD. When using EASL guideline criteria, 1 (7%) patient showed CR, 3 (21%) patients PR, 7 (50%) patients SD, and 3 (21%) patients PD.
Volumetric Response Criteria
According to vRECIST, 13 (93%) patients showed SD and 1 (7%) patient PD. When using qEASL (cm3), 4 (29%) patients were classified as PR and 10 (71%) patients as SD. According to the qEASL (%) criteria, 6 (43%) patients showed PR and 8 (57%) patients showed SD.
Survival Data
By the end-of-observation date, a total of 11 patients (79%) were deceased. The median OS of the study cohort was 11.6 months (95% CI, 5.9-39.5). According to the anatomic criteria RECIST, WHO, and vRECIST, all patients were classified as nonresponder. Hence, stratification was not possible and no survival analysis was conducted. Figure 2 shows plotted Kaplan-Meier curves for stratification according to the remaining assessment criteria. qEASLs (cm3 and %) were the only methods to show a significant stratification of patients into responders and nonresponders. Median OS for qEASL (cm3) was 11.1 months for nonresponders versus 31.9 months for responders (HR, 0.43; 95% CI, 0.19-0.97; P= .042). For qEASL (%) assessment, the median OS was 10.2 months for nonresponders and 29.9 months for responders (HR, 0.09; 95% CI, 0.01-0.74; P= .025).
Figure 2.
Kaplan-Meier analysis based on target lesion response. (A–D) Survival analysis according to tumor response criteria (mRECIST, EASL, qEASL [cm3], and qEASL [%]). All patients were nonresponders using WHO, RECIST, and vRECIST criteria; hence, calculation of survival data was not possible.
Discussion
The main finding of this study is that quantitative volumetric changes of tumor enhancement (qEASL) reliably reflects therapy response and predicts survival in patients with metastatic RCC early after the first IAT procedure.
Metastatic RCC is one of the most treatment-resistant malignancies, and the prognosis is highly dependent on tumor progression in the liver [4], [7], [29]. Therefore, it is crucial to assess tumor response early after treatment to guide the course of therapy and prevent loss of time.
This study showed that conventional anatomical response assessment methods (RECIST and WHO) fail to differentiate between responders and nonresponders. Although mRECIST and EASL are able to identify response to treatment in some patients, both fail to reach statistical significance. qEASL is the only set of criteria that reliably predicts patient survival and can therefore be seen as the ideal assessment method to evaluate early tumor response to IAT.
One reason uni- and bidimensional tumor response criteria fail to predict survival might be the wrong assumption that tumor diameter (RECIST and WHO) and enhancing tumor diameter (mRECIST and EASL) are directly correlated with the volume of viable tumor. In fact, most tumors show asymmetrical growth and heterogeneous patterns of necrosis after treatment, resulting in misleading findings when using diameter- and enhancing diameter–based methods [30]. Taking these tumor characteristics into account, the intellectual approach behind qEASL is a 3D quantitative technique that allows to accurately assess viable tumor volume. Time efficiency and feasibility in a clinical setting as well as histopathological correlation could be shown in recent studies [17], [31].
Although not uncommon, literature on liver metastases from RCC and especially on response to IAT is scarce. The largest prospective study included 22 patients that were treated repeatedly with TACE [12]. Treatment response was evaluated with the RECIST criteria 4 weeks after treatment. Partial response was achieved in 13.7%. However, RECIST failed to predict patient survival, which corresponds with our findings. In contrast, when tumor response is evaluated with qEASL, our study is able to reliably identify responders and nonresponders after IAT and predict survival.
Another retrospective study looked at safety and efficacy of Y-90 radioembolization in six patients with metastatic RCC to the liver [13]. Tumor response of these patients was evaluated using the mRECIST and EASL criteria. The authors only reported the best response on any of the follow-up images acquired 2 to 3 months after IAT and every 3 months thereafter. Three patients showed CR and one patient PR. However, in terms of survival prediction, a major drawback of this study is the long time frame needed until treatment success or failure was reliably evaluated. The advantage of qEASL is not only the reliable prediction of patient survival but also to do so at an early time point, approximately 3 to 4 weeks after treatment.
There were several limitations to this study. First, the study cohort is relatively small. However, patients with liver-dominant metastatic RCC are rare, and it is very unlikely to find a bigger cohort of patients without endangering the consistency of treatment protocols. Our study is a two-center study that includes patients that were treated with TACE and Y-90. Both techniques form a mainstay of interventional oncology. The fact that qEASL works regardless of the used treatment technique is clearly a strength of this study. Second, for all TACE treatments, only patients with pre- and posttreatment MRI were included. This might have caused a bias. However, the measurement of enhancement on CT scans after TACE is limited by the accumulation of iodized oil in the target region. For patients with Y-90 treatments, this limitation does not apply, and response to treatment was measured on MRI as well as on CT scans, respectively. This is another big advantage of qEASL because it can be used with both MRI as well as CT scans as long as baseline imaging and follow-up imaging are consistent. Third, the study lacks histopathological validation of radiological measurements. Yet, it is quite unrealistic to acquire this validation because patients not considered amendable for resection are referred to IAT treatment.
In conclusion, the presented results show that quantitative volumetric tumor enhancement (qEASL) is an early surrogate biomarker of survival in patients with RCC metastatic to the liver after one session of IAT.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Levi F, Ferlay J, Galeone C. The changing pattern of kidney cancer incidence and mortality in Europe. BJU Int. 2008;101:949–958. doi: 10.1111/j.1464-410X.2008.07451.x. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society; 2015. Cancer Facts & Figs. 2015. [ http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Available via, Accessed January 5th, 2015] [Google Scholar]
- 4.McKay RR, Kroeger N, Xie W. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol. 2014;65:577–584. doi: 10.1016/j.eururo.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leibovich BC, Blute ML, Cheville JC. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–1671. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi M, Sun M, Jeldres C. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973–980. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 7.de Reijke TM, Bellmunt J, van Poppel H, Marreaud S, Aapro M. EORTC-GU group expert opinion on metastatic renal cell cancer. Eur J Cancer. 2009;45:765–773. doi: 10.1016/j.ejca.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Coppin C, Le L, Porzsolt F, Wilt T. Targeted therapy for advanced renal cell carcinoma. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD006017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Negrier S, Escudier B, Gomez F. Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d'Immunotherapie. Ann Oncol. 2002;13:1460–1468. doi: 10.1093/annonc/mdf257. [DOI] [PubMed] [Google Scholar]
- 10.Breau RH, Blute ML. Surgery for renal cell carcinoma metastases. Curr Opin Urol. 2010;20:375–381. doi: 10.1097/MOU.0b013e32833c7ada. [DOI] [PubMed] [Google Scholar]
- 11.Staehler MD, Kruse J, Haseke N. Liver resection for metastatic disease prolongs survival in renal cell carcinoma: 12-year results from a retrospective comparative analysis. World J Urol. 2010;28:543–547. doi: 10.1007/s00345-010-0560-4. [DOI] [PubMed] [Google Scholar]
- 12.Nabil M, Gruber T, Yakoub D, Ackermann H, Zangos S, Vogl TJ. Repetitive transarterial chemoembolization (TACE) of liver metastases from renal cell carcinoma: local control and survival results. Eur Radiol. 2008;18:1456–1463. doi: 10.1007/s00330-008-0887-z. [DOI] [PubMed] [Google Scholar]
- 13.Abdelmaksoud MH, Louie JD, Hwang GL, Kothary N, Minor DR, Sze DY. Yttrium-90 radioembolization of renal cell carcinoma metastatic to the liver. J Vasc Interv Radiol. 2012;23:323–330. doi: 10.1016/j.jvir.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Lewandowski RJ, Geschwind JF, Liapi E, Salem R. Transcatheter intraarterial therapies: rationale and overview. Radiology. 2011;259:641–657. doi: 10.1148/radiol.11081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Chapiro J, Wood LD, Lin M. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted mr imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014;273:746–758. doi: 10.1148/radiol.14140033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M, Llovet JM. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 19.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riaz A, Memon K, Miller FH. Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic-pathologic correlation. J Hepatol. 2011;54:695–704. doi: 10.1016/j.jhep.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonekamp D, Bonekamp S, Halappa VG. Interobserver agreement of semi-automated and manual measurements of functional MRI metrics of treatment response in hepatocellular carcinoma. Eur J Radiol. 2014;83:487–496. doi: 10.1016/j.ejrad.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duran R, Chapiro J, Frangakis C. Uveal melanoma metastatic to the liver: the role of quantitative volumetric contrast-enhanced MR imaging in the assessment of early tumor response after transarterial chemoembolization. Transl Oncol. 2014;7:447–455. doi: 10.1016/j.tranon.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleckenstein FN, Schernthaner RE, Duran R. 3D quantitative tumour burden analysis in patients with hepatocellular carcinoma before TACE: comparing single-lesion vs. multi-lesion imaging biomarkers as predictors of patient survival. Eur Radiol. 2016 doi: 10.1007/s00330-015-4168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liapi E, Geschwind JF. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovasc Intervent Radiol. 2011;34:37–49. doi: 10.1007/s00270-010-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 26.Salem R, Thurston KG. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 2: special topics. J Vasc Interv Radiol. 2006;17:1425–1439. doi: 10.1097/01.RVI.0000235779.88652.53. [DOI] [PubMed] [Google Scholar]
- 27.Chockalingam A, Duran R, Sohn JH. Radiologic-pathologic analysis of quantitative 3D tumour enhancement on contrast-enhanced MR imaging: a study of ROI placement. Eur Radiol. 2016;26:103–113. doi: 10.1007/s00330-015-3812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapiro J, Duran R, Lin M. Identifying staging markers for hepatocellular carcinoma before transarterial chemoembolization: comparison of three-dimensional quantitative versus non–three-dimensional imaging markers. Radiology. 2014 doi: 10.1148/radiol.14141180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aloia TA, Adam R, Azoulay D, Bismuth H, Castaing D. Outcome following hepatic resection of metastatic renal tumors: the Paul Brousse Hospital experience. HPB. 2006;8:100–105. doi: 10.1080/13651820500496266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Guindalini FD, Botelho MP, Harmath CB. Assessment of liver tumor response to therapy: role of quantitative imaging. Radiographics. 2013;33:1781–1800. doi: 10.1148/rg.336135511. [DOI] [PubMed] [Google Scholar]
- 31.Lin M, Pellerin O, Bhagat N. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2012;23:1629–1637. doi: 10.1016/j.jvir.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]