Abstract
Aim
To compare bone regeneration in noncritical rat calvarial bone defects filled with platelet-rich fibrin (PRF), alone or combined with beta-tricalcium phosphate (β-TCP), using micro-computed tomographic (MCT) evaluation.
Animals and methods
Two calvarial bone defects were created in each of 45 male Sprague–Dawley rats (age: 20–22 weeks, weight: 350–450 g), using a dental trephine with an external diameter of 3 mm. The 90 defects were randomly allocated among three groups, each containing 30 unilateral defects in a total of 30 rats. Defects in the control group were allowed to heal spontaneously. Defects in the PRF group received PRF alone. Defects in the PRF/β-TCP group received PRF mixed with β-TCP in a 50⧹50 percentage. Nine animals (three per group) were killed after 1, 2, 3, 4, and 6 postoperative weeks, and 18 calvarial defects from each period were analyzed for new bone formation and bone mineral density using MCT. Results were compared by a one-way Analysis of Variance with the POST HOC Least Significant Difference test.
Results
The volume and mineral density of bone formed in the control group were significantly different from those of the other two groups. Greater bone regeneration was observed in defects receiving PRF with β-TCP compared to defects receiving PRF alone in the first 2 weeks (P < 0.001). However, differences in the volume and density of newly formed bone between the PRF and PRF/β-TCP groups were not significant at 3, 4, and 6 postoperative weeks (P > 0.005).
Conclusion
The addition of β-TCP to PRF significantly improved bone regeneration in the first 2 weeks after surgery. Although the differences between results with and without the addition of β-TCP to PRF were statistically insignificant from weeks 3 to 6, it was nevertheless apparent that the group receiving the combination showed better results. We suggest a synergistic mechanism for this effect.
Keywords: Platelet rich fibrin, BTCP
1. Introduction
Various growth factors are increasingly being used – either alone or together with bone graft materials – in dental and medical applications, especially in maxillofacial surgery for bone augmentation and regeneration. Among these factors are platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) (Yilmaz et al., 2014). PRP has been found to improve bone regeneration due to its high concentration of bioactive proteins (Zimmermann et al., 2001, Zhang et al., 2012). However, studies of the effects of PRP on new bone formation in experimental and human subjects have found inconsistent results (Wiltfang et al., 2003, Gerard et al., 2006, Gerard et al., 2007, Kazakos et al., 2011, Zhang et al., 2012). Some authors have attributed this inconsistency to the extremely short-term effects of grafted PRP, due to the rapidly decreasing concentration of bioactive proteins (Schmitz and Hollinger, 2001, Marx, 2004).
Choukroun’s PRF is a recently developed second-generation platelet concentrate, which is prepared through a simple method that does not require the addition of chemicals to blood samples before centrifugation. The end product is an autologous fibrin matrix containing platelets and leukocyte growth factors, which are obtained from inside the fibrin clot, which may explain the slow release of bioactive proteins (growth factors) from the PRF (Dohan et al., 2006, Dohan Ehrenfest et al., 2009, Zhang et al., 2012). The platelet (thrombocyte) count in PRF is three-to-seven times greater than its normal concentration in blood. Growth factors obtained from PRF include platelet-derived growth factor (PDGF), transforming growth factor (TGF), and insulin-like growth factor (Marx, 2001, Dohan Ehrenfest et al., 2009).
Several clinical studies and case reports have referred to PRF as a promising biomaterial that can be used alone or in combination with other bone graft materials to accelerate bone regeneration (Lee et al., 2012, Mazor et al., 2009, Magremanne et al., 2009, Choukroun et al., 2006). However, studies of the potential synergistic effects of PRF when used in combination with different bone graft materials have found inconsistent results, possibly due to the different bioactive properties of graft materials (Choukroun et al., 2006, Zhang et al., 2012).
Histologic, radiographic, and mechanical analytical methods are available for evaluating the volume and quality of newly formed regenerated bone. For example, advances in radiographic analysis, such as micro-computed tomography (MCT), allow for the three-dimensional reconstruction and measurements of the bone volume (BV) and bone mineral density (BD) of newly formed bone in the study defect (Spicer et al., 2012). Animal models of bone defect healing have been developed in many species, including mice, rats, rabbits, dogs, pigs, sheep, and goats. Much research has focused on rodent models, however, because of their reproducibility and economic considerations (Spicer et al., 2012, Pearce et al., 2007). The generally accepted critical size for calvarial defects is 8 mm, although smaller defects have been used, with a pattern of two defects per animal. This approach allows fewer animals to be killed (Kim et al., 2010, Spicer et al., 2012).
To the best of our knowledge, there have been no clear reports of the application of PRF combined with beta-tricalcium phosphate (β-TCP) in intramembranous bone, the main type of cranio-maxillo-facial bone. Therefore, the aim of the present study was to use histological and MCT evaluations to compare new bone regeneration in noncritical rat calvarial bone defects filled with either PRF alone or a PRF/β-TCP combination.
2. Materials and methods
This study was approved by the College of Dentistry Research Center, King Saud University, Saudi Arabia, duly governed by its “Ethical Consideration for Animals” document, in conformity with National Institute of Health guidelines for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985). Forty-five 20- to 22-week-old male Sprague–Dawley rats (weight: 350–450 g) were used. Rats were individually housed in metallic cages with ease of access to water and food in the Laboratory Animal Center of King Khalid University Hospital, King Saud University, Saudi Arabia, under veterinary supervision.
Rats were anesthetized by intramuscular injection of xylazine (5 mg/kg; Lloyd Laboratories, Shenandoah, IA, USA) and ketamine (20 mg/kg; Sigma–Aldrich, St. Louis, MO, USA). The surgical area was shaved from the bridge of the snout between the eyes to the caudal end of the skull/calvarium. After shaving, an alcohol swab was used to remove hair trimmings, and the area was disinfected with a 10% povidone–iodine solution (Riyadh Pharma, Riyadh, Saudi Arabia) the surgical procedure is illustrated in Fig. 1. The subcutaneous area of the surgical field was injected with half a cartridge of 1.8 mL of 2% lidocaine (Parhawk Laboratories, Inc., Lenexa, KS, USA). An approximately 1.5-cm incision was created, using a #15 blade, down to the periosteum over the scalp from the nasal bone to the caudal of the middle sagittal crest or bregma. The periosteum was sharply incised down the sagittal midline with the same scalpel. Finally, the periosteum was elevated from the cranial bone laterally with a mucoperiosteal elevator.
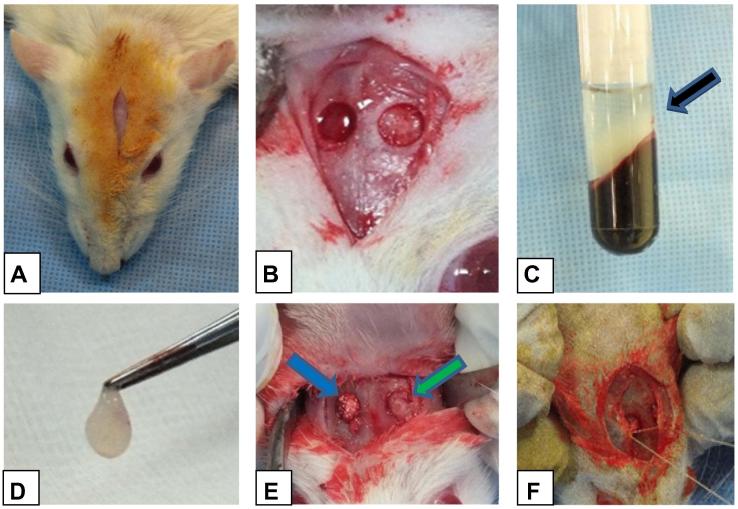
Figure 1.
Clinical intraoperative photographs showing the following; A: incision, B: the bilateral claverial surgical defect, C: PRF inside the glass tube after its preparation (black arrow), D: PRF grasped with a tissue forceps after its separation from the remaining blood clot and components, E: filling one claverial defect with PRF/BTCP (blue arrow) and filling the other one with PRF alone (green arrow), F: closure of the periosteum using 3/0 vicryl.
A 4-mL blood sample was obtained from the orbital sinus of each rat. The blood sample was collected in a plain tube, which was immediately centrifuged at 3000 rpm for 12 min to prepare the PRF.
While blood samples were being centrifuged, two noncritically sized (3-mm diameter) bone defects were created, with one defect in each parietal side of the rat calvarial bone. We followed the protocol of Spicer et al. (2012) for creation of the calvarial defects. Briefly, a dental trephine (Stoma Trephine Bur, 2–3 mm (2 mm inner diameter and 3 mm outer diameter), Dental Latch, Ref: 22349.02; Swallow Dental Supplies Ltd., Silsden, Keighley, West Yorkshire, UK) was used against the superficial aspect of the calvarium, together with a low-speed (1000 rpm) surgical drill under copious saline irrigation. Internal and external diameters of the trephine were 2 and 3 mm, respectively. The bone was not completely penetrated by the trephine to avoid damaging the underlying dural and brain tissues. As the calvarium at the circular border of the defect thinned and became translucent, gentle pressure was exerted with the tip of a small elevator around the inner portion of the defect margins. Then, the trephinated portion of bone was partially displaced downward with slight pressure on one side of the circle. The elevator was used to gently dissect and separate the bone segment from the dura (Fig. 1). The two bone defects were irrigated thoroughly with normal saline.
The 90 defects were randomly allocated among three groups, each containing 30 unilateral defects in a total of 30 rats. In the control group, defects were allowed to heal spontaneously. In the PRF group, defects were treated with PRF. In the PRF/β-TCP group, defects were treated with equal volumes (50⧹50) of PRF mixed with β-TCP (Kasios® TCF; Launaguet, France; particle size: 1000–2000 μm). The periosteum was sutured using 4–0 vicryl suture material, and the surgical site was closed -using 4–0 black silk (Futura Surgicare Pvt. Ltd., Bangalore, India) (Fig. 1).
On the day of surgery, rats were intramuscularly injected twice with the antibiotic amikacin (5 mg/lb). The first injection was given immediately before the surgery and the second one was injected 12 h after the first injection. Rats were given oral amoxicillin (10 mg/lb) twice daily for 5 days, and the anti-inflammatory agent meloxicam (0.5 mg/kg) orally for 3 days.
2.1. MCT analysis of bone defects
At five time points (1, 2, 3, 4, and 6 weeks after surgery), three rats were randomly selected from each group and killed, for a total of nine rats (18 calvarial defects) per period. The calvarial bone of each rat was dissected, fixed in 10% formaldehyde, and wrapped in parafilm (Brand GmbH, Germany) to prevent drying during scanning. Specimens were scanned with a high-resolution in vivo X-ray MCT instrument (SkyScan 1176, Kontich, Belgium) with an aluminum filter (1 mm). Scan settings were as follows: energy of 100 kV, intensity of 343 mA, resolution of 12.39 μm pixels, rotation step size of 0.6°, tomographic rotation of 1800 (1800 projection images were acquired by a tomographic scan over 180-degree sample rotation), and standard camera setting (field width = 1000 pixels). Projection images of cone-beam acquisition and reconstruction were saved as 16-bit TIF files. All scanning and reconstruction parameters were identical for all specimens and calibrations.
We analyzed the MCT scans of specimens using the NRecon reconstruction software, Data Viewer software, and CT-Analyzer program (BRUKER- MICROCT Kontich, Belgium) in a control computer (Windows 7, 64-bit version). Parameters acquired via MCT were the BV (mm3) and BD (mg/cm3) of mineralized tissue within the circular defect area (region of interest, ROI). As a quantitative indicator of newly formed mineralized tissue, BV was defined as the volume of mineralized tissue formed at the defect site during healing. As an indicator of the quality of mineralized tissue, BD was defined as the density of mineralized tissue within the volume of interest (Nooh et al., 2014).
ROIs for BV were identified using the previously mentioned CT analyzer program. The area of newly formed bone was measured in each section of the MCT scan. The product of the measured area and the slice thickness of the scan section yielded the BV in that scan section. The sum of the BV values of each scan section was calculated to determine the total BV of newly formed bone.
To calculate the BD of newly formed bone, four representative sites were selected from each specimen. Each site was selected to represent either the superior or inferior aspect of the proximal and distal portions of the bone cavities, 1 mm from the edges. The mean of the individual BD values obtained from each site was calculated to obtain the total BD of newly formed bone.
2.2. Statistical analysis
Assuming a type 1 error value of 5% (α = 0.05) and power of 0.95, we estimated that we would need a sample size of 90 defects (n = 18/group) distributed evenly among three groups during five examination periods. BV and BD values of the three groups were compared by a one-way Analysis of Variance (ANOVA), followed by the post hoc Least Significant Difference (LSD) test. The factor for ANOVA was the grafted material in the defects (PRF, PRF/β-TCP, or control). P < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS for Windows, version 21 (SPSS, Chicago, IL, USA).
3. Results
All 45 rats showed an uneventful recovery from the anesthesia and surgical procedures. All 90 standardized calvarial bone defects were included in the final analysis. There were no signs of infection, hematoma, or wound dehiscence during the wound healing period. No behavioral changes or signs of paralysis were noted throughout the study period.
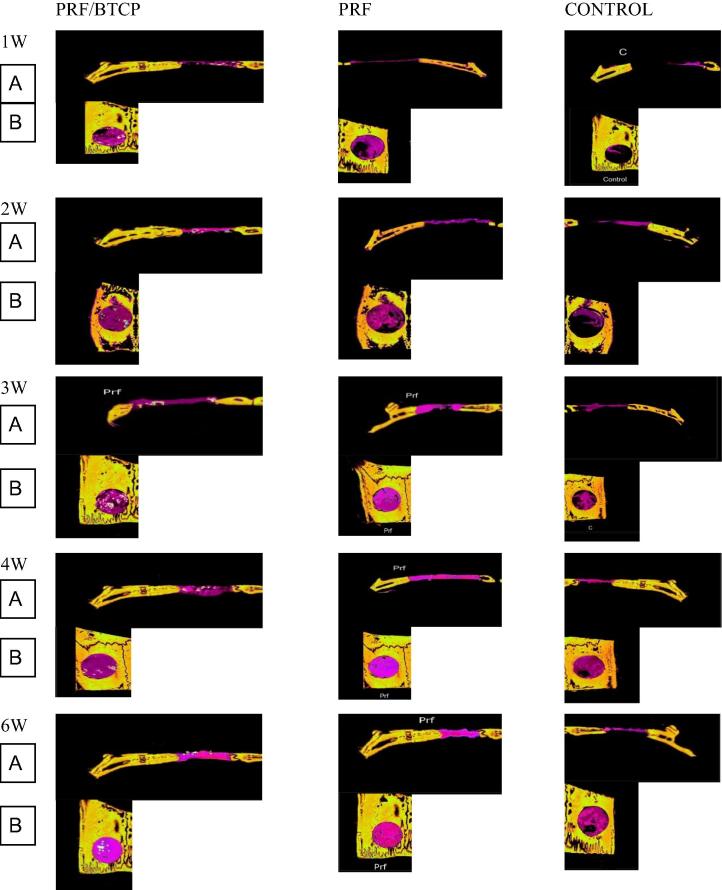
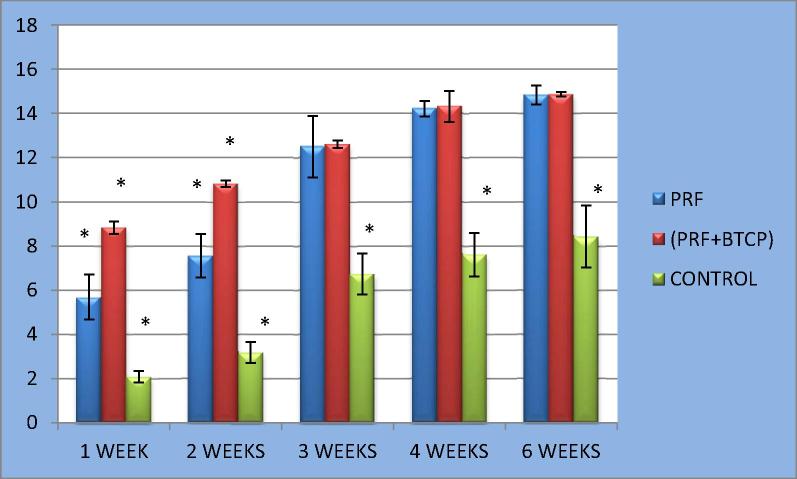
Examination of the specimens after the first postoperative week revealed much smaller volumes of newly formed bone in defects from the control group (n = 6) compared to defects in the PRF group (n = 6) or PRF/β-TCP group (n = 6). The PRF/β-TCP group showed greater BV and BD values compared to the PRF group (Fig. 2, Table 1). After the second postoperative week, the control group specimens showed only a slight increase in the volume of newly formed bone, which was still less than the volumes that had formed after just 1 week in the other two groups. Moreover, the volumes of newly formed bone in the PRF and PRF/β-TCP groups were increased considerably at 2 weeks compared to the volumes at 1 week. The BV in defects of the PRF/β-TCP group remained greater than the BV in the PRF group (Figure 2, Figure 3, Table 1).
Figure 2.
Micro-computed tomography sections of the rat claverial bone showing the three groups all over the study period (A) coronal reconstruction of the defects (B) axial reconstruction of the defects. Old bone was colored with yellow color, while the new bone colored purple.
Table 1.
Showing mean value and slandered deviation of bone volume and bone mineral density in all groups.
| Examination Periods in weeks | Group (n = 6) | Mean ± SD |
|
|---|---|---|---|
| Bone volume (mm3) | Bone density (mg/cm3) | ||
| 1 | Control | 2.079 ± 0.27 | 0.4355 ± 0.047633 |
| PRF | 5.694 ± 1.028741 | 0.5815 ± 0.02362 | |
| PRF+BTCP | 8.834167 ± 0.284476 | 0.6585 ± 0.03084 | |
| 2 | Control | 3.172 ± 0.48 | 0.510333 ± 0.012023 |
| PRF | 7.567 ± 0.983494 | 0.6145 ± 0.018945 | |
| PRF+BTCP | 10.83317 ± 0.146576 | 0.826667 ± 0.038668 | |
| 3 | Control | 6.733 ± 0.92 | 0.602667 ± 0.004853 |
| PRF | 12.501 ± 1.400921 | 0.9095 ± 0.03517 | |
| PRF+BTCP | 12.6145 ± 0.172101 | 0.9245 ± 0.043481 | |
| 4 | Control | 7.608 ± 0.98 | 0.625 ± 0.038575 |
| PRF | 14.214 ± 0.348846 | 0.9415 ± 0.033385 | |
| PRF+BTCP | 14.32335 ± 0.693714 | 0.960167 ± 0.044823 | |
| 6 | Control | 8.429 ± 1.40 | 0.641333 ± 0.034855 |
| PRF | 14.845 ± 0.439037 | 0.991 ± 0.008602 | |
| PRF+BTCP | 14.86883 ± 0.104396 | 0.9955 ± 0.002986 | |
SD = Standard deviation.
Figure 3.
Clustered column chart with error bars showing bone volume (BV) in the 3 groups all over the study period.
After 3, 4, and 6 weeks, all specimens continued to show much smaller volumes of newly formed bone in the control group compared to the other two groups. However, in contrast to the first two periods, the volumes of newly formed bone were very similar in the PRF and PRF/β-TCP groups in these examination periods (Figure 2, Figure 3, Table 1).
3.1. Statistical results
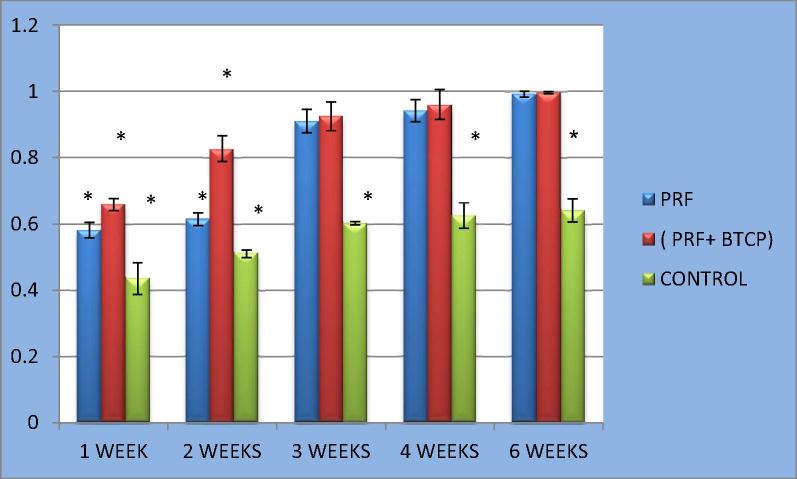
One-way ANOVA with the LSD test for BV and BD revealed significant differences between the control group and the other two groups. The BV and BD values of the PRF/β-TCP group were significantly greater than those of the PRF group in weeks 1 and 2 (P < 0.001). However, these differences were not significant for weeks 3, 4, or 6 (P > 0.05) (Figure 2, Figure 3, Table 2, Table 3).
Table 2.
Showing the results of a one-way ANOVA posthoc statistical analysis of all groups regarding bone volume.
| Examination periods in weeks | Group |
Mean difference (I–J) | P value | |
|---|---|---|---|---|
| I | J | |||
| 1 | Control | PRF | −3.614833⁎ | .000 |
| PRF+BTCP | −6.754833⁎ | .000 | ||
| PRF | Control | 3.614833⁎ | .000 | |
| PRF+BTCP | −3.140000⁎ | .000 | ||
| PRF + BTCP | Control | 6.754833⁎ | .000 | |
| PRF | 3.140000⁎ | .000 | ||
| 2 | Control | PRF | −4.394667⁎ | .000 |
| PRF+BTCP | −7.660333⁎ | .000 | ||
| PRF | Control | 4.394667⁎ | .000 | |
| PRF+BTCP | −3.265667⁎ | .000 | ||
| PRF+BTCP | Control | 7.660333⁎ | .000 | |
| PRF | 3.265667⁎ | .000 | ||
| 3 | Control | PRF | −5.767500⁎ | .000 |
| PRF+BTCP | −5.881000⁎ | .000 | ||
| PRF | Control | 5.767500⁎ | .000 | |
| PRF+BTCP | −.113500 | .856 | ||
| PRF+BTCP | Control | 5.881000⁎ | .000 | |
| PRF | .113500 | .856 | ||
| 4 | Control | PRF | −6.606000⁎ | .000 |
| PRF+BTCP | −6.715017⁎ | .000 | ||
| PRF | Control | 6.606000⁎ | .000 | |
| PRF+BTCP | −.109017 | .814 | ||
| PRF+BTCP | Control | 6.715017⁎ | .000 | |
| PRF | .109017 | .814 | ||
| 6 | Control | PRF | −6.415167⁎ | .000 |
| PRF+BTCP | −6.439000⁎ | .000 | ||
| PRF | Control | 6.415167⁎ | .000 | |
| PRF+BTCP | −.023833 | .965 | ||
| PRF+BTCP | Control | 6.439000⁎ | .000 | |
| PRF | .023833 | .965 | ||
The mean difference is significant at the 0.05 level.
Table 3.
Showing a one-way ANOVA posthoc statistical analysis of all groups regarding the bone mineral density.
| Examination periods in weeks | Group |
Mean difference (I–J) | P value | |
|---|---|---|---|---|
| I | J | |||
| 1 | Control | PRF | −.1460000⁎ | .000 |
| PRF+BTCP | −.2230000⁎ | .000 | ||
| PRF | Control | .1460000⁎ | .000 | |
| PRF+BTCP | −.0770000⁎ | .004 | ||
| PRF+BTCP | Control | .2230000⁎ | .000 | |
| PRF | .770000⁎ | .004 | ||
| 2 | Control | PRF | −.1041667⁎ | .000 |
| PRF+BTCP | −.3163333⁎ | .000 | ||
| PRF | Control | .1041667⁎ | .000 | |
| PRF+BTCP | −.2121667⁎ | .000 | ||
| PRF+BTCP | Control | .3163333⁎ | .000 | |
| PRF | .2121667⁎ | .000 | ||
| 3 | Control | PRF | −.3068333⁎ | .000 |
| PRF+BTCP | −.3218333⁎ | .000 | ||
| PRF | Control | .3068333⁎ | .000 | |
| PRF+BTCP | −.0150000 | .476 | ||
| PRF+BTCP | Control | .3218333⁎ | .000 | |
| PRF | .0150000 | .476 | ||
| 4 | Control | PRF | −.3165000⁎ | .000 |
| PRF+BTCP | −.3351667⁎ | .000 | ||
| PRF | Control | .3165000⁎ | .000 | |
| PRF+BTCP | −.0186667 | .463 | ||
| PRF+BTCP | Control | .3351667⁎ | .000 | |
| PRF | .0186667 | .463 | ||
| 6 | Control | PRF | −.3496667⁎ | .000 |
| PRF+BTCP | −.3541667⁎ | .000 | ||
| PRF | Control | .3496667⁎ | .000 | |
| PRF+BTCP | −.0045000 | .737 | ||
| PRF+BTCP | Control | .3541667⁎ | .000 | |
| PRF | .0045000 | .737 | ||
Significant difference P ≤ 0.05.
4. Discussion
The present study investigated the effect of PRF, either alone or in combination with β-TCP, on bone healing in standardized rat calvarial bone defects. Newly formed bone was evaluated by MCT analysis of the BV and BD values.
Rat calvarial bone defects have been considered a rapid-throughput method for the evaluation of bone regeneration. Rat calvarium allows for a reproducible defect that does not need fixation, as a result of the reduced loading compared to loading in long bones. Calvarial defects can also be used as a model for intramembranous bone formation (Spicer et al., 2012, Pearce et al., 2007, Schmitz and Hollinger, 1986, Takagi and Urist, 1982, Wang et al., 2003).
Our MCT results revealed that the quantity (BV) and density (BD) of newly formed bone were significantly different among the three groups in the first two postoperative weeks. The best results were obtained for defects filled with the PRF/β-TCP combination, followed by PRF alone and then the control group. These results are consistent with those of Yilmaz et al. (2014), who found that adding β-TCP to PRF improved the efficacy of bone formation compared to using either material alone. In another study, Lee et al. (2007) compared autogenous grafts to autogenous graft+PRF combinations for sinus-lifting operations. Based on a histomorphometric analysis, they found a greater amount of bone in the autogenous graft+PRF combination group compared to the group treated with autogenous bone grafts alone (see Fig. 4).
Figure 4.
Clustered column chart with error bars showing bone mineral density (BMD) in the 3 groups all over the study period.
Chazono et al. (2004) and Shirasu et al. (2010) proposed an explanation for the increased bone formation and osteoblast proliferation in the presence of β-TCP. They reported that osteoclastic cells surrounding bone graft particles play a role in the induction of osteoblastic cell migration around bone graft particles of β-TCP by cell-to-cell interactions. These studies are also consistent with the results of Kim et al. (2012), who found that a group receiving PRF mixed with β-TCP showed a larger bone formation area compared to the negative control group and the TCP-alone group.
From the third to the sixth postoperative week, we observed a change in the previous pattern of the first two weeks. Although the PRF/β-TCP group still exhibited a greater improvement in BV and BD compared to the PRF group, this difference was no longer significant. This finding contradicts the results of Yilmaz et al. (2014), who found that the amount of bone was significantly different between the PRF/β-TCP group and the PRF-alone group throughout the study period. Nevertheless, the PRF/β-TCP and PRF groups continued to show highly significant differences in BV and BD compared to the control group.
To explain the findings in the first 14 days of the present study, we refer to similar results from He et al. (2009), which compared the utility of PRF with PRP in osteoblast proliferation. When PRF was used, the levels of released TGF-β1 and PDGF-AB were markedly increased, peaking on day 14 before decreasing slightly. From these results, we can hypothesize that the difference between the PRF/β-TCP and PRF groups in the first 2 weeks had two causes: the bone formation-stimulatory activity of the β-TCP itself (Szabo et al., 2001), and the sustained and gradual increase of growth factor release by PRF. The PRF acts as a biological “glue” to hold the particles together, enabling manipulation of the bone grafting material and holding the graft material in the defect area. Yazawa et al. (2004) and Yilmaz et al. (2014) concluded that this adhesive property had the synergetic effect of accelerating healing of the graft material. After the maximum promoting effect of PRF occurred at day 14, the release of the growth factors started to decrease. Although the presence of β-TCP in combination with PRF still afforded an improvement in bone formation and mineral density over PRF alone, this effect was no longer significant.
Another explanation for the increased bone formation in the PRF/β-TCP group may be the dissolution of β-TCP after grafting. Wang et al. (1998) and Jarcho (1981) suggested that the dissolution of TCP may provide high local concentrations of calcium and phosphate, providing a favorable environment for new bone formation. Conversely, Shirasu et al. (2010) argued that, because calvarial bone has relatively little bone marrow, the number of osteoprogenitor cells at the recipient site is minimal. Thus, for full-thickness defects of intramembranous calvarial bone, β-TCP grafting alone may be insufficient for successful osteoconduction. This fact emphasizes the synergetic effect of the presence of PRF with β-TCP in our study, as the fibrin clot itself may participate in the conduction process.
Lundquist et al. (2008) and He et al. (2009) stated that collagen synthesis creates an extracellular matrix for osteoblasts, which can be used as a skeleton for the deposition of calcium nodules. PRF released the maximum amount of TGF-β1 (which enhances collagen synthesis, possibly through connective tissue growth factor (CTGF), another platelet-derived protein) at 14 days, which may have led to maximum mineralization during this period. According to Uggeri et al. (2007), the fibrin(ogen) content of PRFs may also contribute to their increased mineralization. The lack of a significant difference in our MCT measurements of BV and BD between the PRF/β-TCP and PRF groups may have been affected by the MCT computer program, which we used to subtract from those calculations the β-TCP particles that showed in the MCT cuts. Finally, slow resorption may have retarded the formation of new bone. And this did not affect the PRF group because in the prf group there is no particle at all.
Researchers have explored the potential synergistic effects of PRF combined with various bone graft materials. However, such studies have obtained widely varying results, possibly due to the different bioactive properties of the graft materials (Choukroun et al., 2006, Zhang et al., 2012, Yilmaz et al., 2014, Yazawa et al., 2004, Szabo et al., 2001).
5. Conclusions
The study found improved bone regenerative capacity of defects treated with PRF in combination with β-TCP, compared to defects treated with PRF alone. However, the effect was only statistically significant in the first 2 weeks after surgery. Nevertheless, it was apparent that the group receiving the combination displayed greater bone regeneration. This study has some limitations, and further studies are necessary to strengthen the evidence base and to explore the possible synergistic mechanism of this treatment in intramembranous bone.
Conflict of interest
The author has no conflict of interest to declare.
Footnotes
Peer review under responsibility of King Saud University.
References
- Chazono M., Tanaka T., Komaki H., Fujii K. Bone formation and bioresorption after implantation of injectable beta-tricalcium phosphategranules-hyaluronate complex in rabbit bone defects. J. Biomed. Mater. Res. 2004;70:542–549. doi: 10.1002/jbm.a.30094. [DOI] [PubMed] [Google Scholar]
- Choukroun J., Diss A., Simonpieri A., Girard M.O., Schoeffler C., Dohan S.L. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101:37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Dohan Ehrenfest D.M., Rasmusson L., Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and plateletrich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Gerard D., Carlson E.R., Gotcher J.E., Jacobs M. Effects of platelet-rich plasma on the healing of autologous bone grafted mandibular defects in dogs. J. Oral Maxillofac. Surg. 2006;64:443–451. doi: 10.1016/j.joms.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Gerard D., Carlson E.R., Gotcher J.E., Jacobs M. Effects of platelet-rich plasma at the cellular level on healing of autologous bone-grafted mandibular defects in dogs. J. Oral Maxillofac. Surg. 2007;65:721–727. doi: 10.1016/j.joms.2006.09.025. [DOI] [PubMed] [Google Scholar]
- He L, Lin Y., Hu X., Zhang Y., Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009;108(5):707–713. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Kazakos K., Lyras D.N., Thomaidis V., Agrogiannis G., Botaitis S., Drosos G. Application of PRP gel alone or in combination with guided bone regeneration does not enhance bone healing process: an experimental study in rabbits. J. Craniomaxillofac. Surg. 2011;39:49–53. doi: 10.1016/j.jcms.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Kim K.S., Lee J.Y., Kang Y.M., Kim E., Kim G.H., Rhee S.D. Small intestine submucosa sponge for in vivo support of tissue-engineered bone formation in the presence of rat bone marrow stem cells. Biomaterials. 2010;31:1104–1113. doi: 10.1016/j.biomaterials.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Kim B.J., Kwon T.K., Baek H.S., Hwang D.S., Kim C.H., Chung I.K., Jeong J.S., Shin S.H. A comparative study of the effectiveness of sinus bone grafting with recombinant human bone morphogenetic protein 2-coated tricalcium phosphate and platelet-rich fibrin-mixed tricalcium phosphate in rabbits. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;113(5):583–592. doi: 10.1016/j.tripleo.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Choi B.H., Jung J.H., Zhu S.J., Lee S.H., Huh J.Y., You T.M., Li J. Maxillary sinus floor augmentation using autogenous bone grafts and platelet-enriched fibrin glue with simultaneous implant placement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007;103(3):329–333. doi: 10.1016/j.tripleo.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Lee W.J., Kim S.G., Kim J.Y., Lee Y.C., Choi J.Y., Dragos R., Rotaru H. Restoration of a peri-implant defect by platelet-rich fibrin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2012;113:459–463. doi: 10.1016/j.tripleo.2011.03.043. [DOI] [PubMed] [Google Scholar]
- Lundquist R., Dziegiel M.H., Agren M.S. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Repair Regen. 2008;16:356–363. doi: 10.1111/j.1524-475X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- Magremanne M., Baeyens W., Awada S., Vervaet C. Solitary bone cyst of the mandible and platelet rich fibrin (PRF) Rev. Stomatol. Chir. Maxillofac. 2009;110:105–108. doi: 10.1016/j.stomax.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Marx R.E. Platelet-rich plasma: evidence to support its use. J. Oral Maxillofac. Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Marx R.E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Mazor Z., Horowitz R.A., Del Corso M., Prasad H.S., Rohrer M.D., Ehrenfest D. Sinus floor augmentation with simultaneous implant placement using Choukroun’s platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J. Periodontol. 2009;80:2056–2064. doi: 10.1902/jop.2009.090252. [DOI] [PubMed] [Google Scholar]
- Nooh N., Abdullah W.A., El-Awady Grawish M., Ramalingam S., Hassan G., Javed F., Al-Hezaimi K. Evaluation of bone regenerative capacity following distraction osteogenesis of goat mandibles using two different bone cutting techniques. J. Craniomaxillofac. Surg. 2014;3(42):255–261. doi: 10.1016/j.jcms.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Spicer P.P., Kretlow J.D., Young S., Jansen J.A., Kasper F.K., Mikos A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc. 2012;7(10):1918–1929. doi: 10.1038/nprot.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce A.I., Richards R.G., Milz S., Schneider E., Pearce S.G. Animal models for implant biomaterial research in bone: a review. Eur Cells Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- Schmitz J.P., Hollinger J.O. The biology of platelet-rich plasma. J. Oral Maxillofac. Surg. 2001;59:1119–1121. doi: 10.1053/joms.2001.26801. [DOI] [PubMed] [Google Scholar]
- Schmitz J.P., Hollinger J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986;205:299–308. [PubMed] [Google Scholar]
- Shirasu N., Ueno T., Hirata Y., Hirata A., Kagawa T., Kanou M., Sawaki M., Wakimoto M., Ota K., Matsumura T., Yamada T., Yamachika E., Sano K. Bone formation in a rat calvarial defect model after transplanting autogenous bone marrow with beta-tricalcium phosphate. Acta Histochem. 2010;112:270–277. doi: 10.1016/j.acthis.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Szabo G., Suba Z., Hrabak K., Barbaras J., Nemeth Z. Autogeneous bone versus ß–tricalcium phosphate graft alone for bilateral sinus elevations. Preliminary results. Int. J. Oral Maxillofac. Implants. 2001;16(5):681–692. [PubMed] [Google Scholar]
- Takagi K., Urist M.R. The reaction of the dura to bone morphogenetic protein (BMP) in repair of skull defects. Ann. Surg. 1982;196:100–109. doi: 10.1097/00000658-198207000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggeri J., Belletti S., Guizzardi S., Poli T., Cantarelli S., Scandroglio R., Gatti R. Dose-dependent effects of platelet gel releasate on activities of human osteoblasts. J. Periodontol. 2007;78:1985–1991. doi: 10.1902/jop.2007.070116. [DOI] [PubMed] [Google Scholar]
- Wang J.C. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J. Bone Joint Surg. Am. 2003;85:905–911. doi: 10.2106/00004623-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Wang J., Chen W., Li Y., Fan S., Weng J., Zhang X. Biological evaluation of biphasic calcium phosphate ceramic vertebral laminae. Biomaterials. 1998;19:1387–1392. doi: 10.1016/s0142-9612(98)00014-3. [DOI] [PubMed] [Google Scholar]
- Wiltfang J., Schlegel K.A., Schultze-Mosgau S., Nkenke E., Zimmermann R., Kessler P. Sinus floor augmentation with beta-tricalciumphosphate (beta-TCP): does platelet-rich plasma promote its osseous integration and degradation? Clin. Oral Implants Res. 2003;14:213–218. doi: 10.1034/j.1600-0501.2003.140212.x. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Ogata H., Kimura A., Nakajima T., Mori T., Watanabe N. Basic studies on the bone formation ability by platelet rich plasma in rabbits. J. Craniofac. Surg. 2004;15(3):439–446. doi: 10.1097/00001665-200405000-00019. [DOI] [PubMed] [Google Scholar]
- Yilmaz D., Dogan N., Ozkan A., Sencimen M., Eren B., Mutlu I. Effect of platelet rich fibrin and beta tricalcium phosphate on bone healing. A histological study in pigs. Acta Cirúrgica Bras. 2014;29(1):59–65. doi: 10.1590/S0102-86502014000100009. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Jakubietz R., Jakubietz M., Strasser E., Schlegel A., Wiltfang J. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2001;41:1217–1224. doi: 10.1046/j.1537-2995.2001.41101217.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tangl S., Huber C.D., Lin Y., Qiu L., Rausch-Fan X. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J. Craniomaxillofac. Surg. 2012;40:321–328. doi: 10.1016/j.jcms.2011.04.020. [DOI] [PubMed] [Google Scholar]