Abstract
Objective
The objective of this study was to perform a hip structure analysis (HSA) of teriparatide (TPTD) treatment in women with postmenopausal osteoporosis.
Methods
The study included 96 patients with postmenopausal osteoporosis and received 20 μg TPTD daily. HSA was performed by dual-energy X-ray absorptiometry.
Results
The percent changes from baseline for the cross-sectional moment of inertia, section modulus, buckling ratio, and femoral strength index based on HSA results were 9.8% (p < 0.01), 10.7%, 3.3%, and 14.9% (p < 0.01), respectively, at 24 months.
Conclusion
Based on the HSA results obtained with DXA, TPTD was effective for hip structures.
Keywords: Dual-energy X-ray absorptiometry, Hip structure analysis, Osteoporosis, Rheumatoid arthritis, Teriparatide
1. Introduction
Due to the aging population in Japan, the prevention and treatment of osteoporosis and the prevention of bone fragility fractures are of great medical and societal importance. The incidence of osteoporosis and concomitant vertebral and femoral neck fractures has continued to increase. Bone fragility fractures negatively influence a patient's life expectancy; the relative risk of death has been reported to be 8.6 times and 6.7 times higher due to vertebral or femoral neck fractures, respectively.1 Rheumatoid arthritis (RA), which is a disorder associated with secondary osteoporosis, also presents a high risk of osteoporotic fractures.2
To date, the most common treatment for osteoporosis has been a bisphosphonate (BP), and many reports support its efficacy,3, 4, 5 including in Japanese patients.6, 7, 8 On the other hand, BP use is associated with a small risk of jaw osteonecrosis or an atypical femoral fracture.9 Though long-term administration is known to play a role in these side effects, no consensus has been reached as to the optimal duration of treatment.10
Where existing medication fails to achieve the desired effects, or in cases of severe osteoporosis, the early introduction of an anabolic anti-osteoporotic drug has been considered desirable. Parathyroid hormone (PTH), administered intermittently, serves as an osteogenic promoter and exhibits osteo-anabolic behavior.11, 12 Although the precise mechanism of the drug's action is not yet clear, a comparison of intermittent and continuous administration to rodents shows contrasting effects on osteoblasts and osteoclasts. Osteogenesis is promoted by stimulating osteoblasts through intermittent administration, whereas continuous administration acts instead on osteoclasts.13 Teriparatide (TPTD, or recombinant human PTH 1–34) has been approved for use as an osteogenic promoter since October 2010 in Japan, although its actual clinical efficacy, especially on bone strength in the hip, is not clear in many respects.
Dual-energy X-ray absorptiometry (DXA) and quantitative computed tomography (QCT) are common tools for measuring bone mineral density (BMD). QCT is considered the standard tool for obtaining structural measurements of the proximal femur, while DXA measurements make it possible to estimate reproducibly the strength of the femoral neck based on its resistance to bending.14 In elderly women, the cross-sectional moment of inertia (CSMI), cross-sectional area (CSA), section modulus (SM), and the femoral neck axis length and width as obtained through a hip structure analysis (HSA) by DXA correlate highly to QCT results.15
The purpose of this study was to clarify bone strength of the hip as assessed by DXA in women with postmenopausal osteoporosis and to examine the effects of TPTD on lumbar spine, total hip, and hand BMD.
2. Materials and methods
All patients were women with postmenopausal osteoporosis with or without RA and were at least 60 years old. The patients had a lumbar spine or total hip BMD T-score of at least 2.5 standard deviations (SDs) below the corresponding average BMD for normal young adult women, as assessed by DXA. The subjects received once-daily, self-administered 20-μg subcutaneous injections of TPTD. The study was conducted with approval from the Institutional Review Board of Kamagaya General Hospital, and all patients provided informed consent.
The HSA of the left hip was measured with DXA at baseline (at the time that TPTD administration began) and at 3, 6, 12, 18, and 24 months. The main structural parameters derived by HSA were CSMI, CSA, SM, buckling ratio (BR), cortical width (CW), and the femoral strength index (FSI). The CSMI is a parameter of resistance to bending and is obtained by multiplying the integral of the area by the distance from the center of mass. The CSA is a parameter of resistance to axial compressive load and is obtained by calculating and is obtained by calculating BMD from DXA. SM is a parameter of resistance to bending and is obtained by calculating from the values of CSMI and the maximum distance between the center of the mass and the outer cortex. BR is a parameter of cortical stability and is obtained by calculating from the values of CSMI, SM, and cortical width neck. The BMD of the lumbar spine, total hip, femoral neck, femoral shaft, and hand were measured at baseline and 3, 6, 12, 18, and 24 months. The DXA and HSA by DXA devices were part of the PROGIDY system (GE Healthcare, Madison, WI, USA).
The main outcomes of interest from HSA by DXA were the estimated CSMI, CSA, SM, BR, neck CW, shaft CW, and FSI as well as the percent change from baseline for these variables at 3, 6, 12, 18, and 24 months. Secondary outcomes were the estimated BMD of the lumbar spine, total hip, femoral neck, femoral shaft, and hand as well as the percent change from baseline for these variables at 3, 6, 12, 18, and 24 months. BMD was expressed as a percentage relative to 100%. Patients were included in the analysis of outcomes if they had undergone HSA and BMD at 3 months, based on the intention-to-treat (ITT) principle. Missing data were imputed by carrying forward the last observation. Further, we analyzed the data per protocol (PP).
All statistical analyses were performed on data from patients who had evaluable observations at specific time points. Differences in HSA and BMD from baseline to each measurement point were tested by the paired t-test. Significance was established at p < 0.05.
3. Results
3.1. Background characteristics
A total of 96 patients underwent HSA and BMD analyses. There were 65 patients with postmenopausal osteoporosis without RA (group without RA) and 31 patients with postmenopausal with RA (RA group). Characteristics (age, body weight, body mass index, prevalent fragility fractures, lumbar spine T score, total hip T score, lumbar BMD, total hip BMD, femoral neck BMD, femoral shaft BMD, hand BMD, CSMI, SM, BR, neck CW, shaft CW, and FSI) of the patients overall and by RA are shown in Table 1.
Table 1.
Baseline demographics and clinical characteristics for all patients.
| All patients (n = 96) | Group without RA (n = 65) | RA group (n = 31) | |
|---|---|---|---|
| Age (years), mean (SD) | 74.1 (7.8) | 75.8 (7.0) | 70.5 (8.2) |
| Body weight (kg), mean (SD) | 46.0 (7.8) | 46.2 (7.3) | 45.4 (6.4) |
| Body mass index (kg/m2), mean (SD) | 21.0 (2.7) | 21.3 (2.8) | 20.5 (2.4) |
| Presence of previous fragility fractures, n (%) | 51 (53.1) | 38 (58.5) | 13 (41.9) |
| Lumbar spine T score, mean (SD) | −3.2 (1.4) | −3.2 (1.4) | −3.1 (1.3) |
| Total hip T score, mean (SD) | −2.6 (1.2) | −2.7 (1.0) | −2.4 (1.6) |
| Lumbar spine BMD (L2–L4, g/cm2), mean (SD) | 0.739 (0.166) | 0.737 (0.172) | 0.744 (0.156) |
| Total hip BMD (g/cm2), mean (SD) | 0.604 (0.114) | 0.604 (0.118) | 0.605 (0.108) |
| Femoral neck BMD (g/cm2), mean (SD) | 0.598 (0.107) | 0.594 (0.112) | 0.606 (0.097) |
| Femoral shaft BMD (g/cm2), mean (SD) | 0.707 (0.144) | 0.710 (0.147) | 0.702 (0.138) |
| Hand BMD (g/cm2), mean (SD) | 0.255 (0.048) | 0.263 (0.048) | 0.238 (0.046) |
| CSMI (mm3), mean (SD) | 5440.4 (1710.7) | 5435.0 (1797.1) | 5451.7 (1541.6) |
| CSA (mm2), mean (SD) | 84.1 (16.0) | 83.8 (16.6) | 84.9 (14.8) |
| SM (cm3), mean (SD) | 332.1 (90.7) | 331.0 (95.7) | 334.4 (80.7) |
| BR, mean (SD) | 5.0 (1.8) | 5.1 (2.0) | 4.7 (1.3) |
| Neck CW (mm), mean (SD) | 3.7 (1.4) | 3.8 (1.5) | 3.7 (1.1) |
| Shaft CW (mm), mean (SD) | 3.6 (1.4) | 3.7 (1.7) | 3.4 (0.6) |
| FSI, mean (SD) | 1.4 (0.4) | 1.3 (0.3) | 1.5 (0.5) |
RA: rheumatoid arthritis; SD: standard deviation; BMD: bone mineral density; CSMI: cross-sectional moment of inertia; CSA: cross-sectional area; SM: section modulus, BR: buckling ratio; CW: cortical width; FSI: femoral strength index.
The persistence rate was 94.8% at 6 months, 87.5% at 12 months, 83.3% at 18 months, and 72.9% at 24 months.
3.2. Efficacy on HSA based on ITT
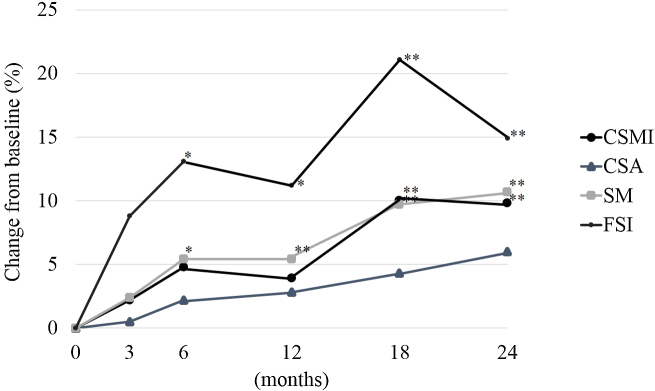
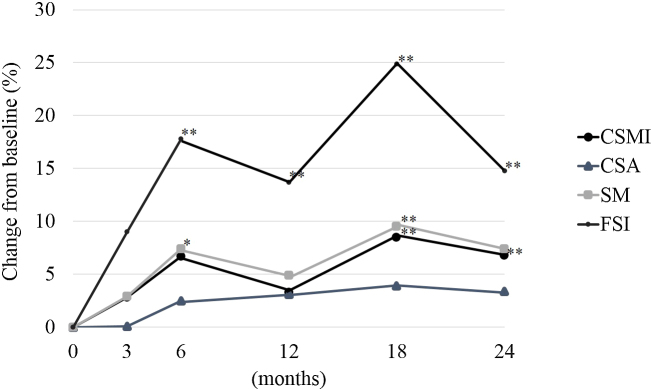
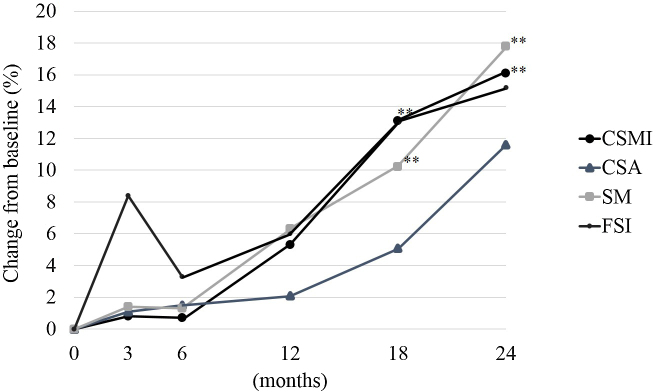
Fig. 1, Fig. 2, Fig. 3 shows the percent change from baseline at 3, 6, 12, 18, and 24 months for all patients, the group without RA, and the RA group for CSMI, CSA, SM, and FSI.
Fig. 1.
The percent change from baseline for CSMI, CSA, SM, and FSI for all patients by intention-to-treat. CSMI: cross sectional moment of inertia; CSA: cross-sectional area; SM: section modulus; FSI: femoral strength index. Paired t-test: *p < 0.05, **p < 0.01.
Fig. 2.
The percent change from baseline for CSMI, CSA, SM, and FSI for postmenopausal women with osteoporosis without RA (group without RA) by intention-to-treat. CSMI: cross sectional moment of inertia; CSA: cross-sectional area; SM: section modulus; FSI: femoral strength index; RA: rheumatoid arthritis. Paired t-test: *p < 0.05, **p < 0.01.
Fig. 3.
The percent change from baseline for CSMI, CSA, SM, and FSI for postmenopausal women with osteoporosis with RA (RA group) by intention-to-treat. CSMI: cross sectional moment of inertia; CSA: cross-sectional area; SM: section modulus; FSI: femoral strength index; RA: rheumatoid arthritis. Paired t-test: *p < 0.05, **p < 0.01.
In all patients, the percent changes from baseline at 3, 6, 12, 18, and 24 months for BR were 6.1%, 6.2%, 2.5%, 4.1%, and 3.3%; for neck CW were −0.1%, 3.7%, 11.5%, 9.9%, and 12.4%; and for shaft CW were −1.8%, 3.8%, 4.2%, 5.4%, and 6.2%, respectively.
In the group without RA, the percent changes from baseline at 3, 6, 12, 18, and 24 months for BR were 3.7%, 7.0%, 1.2%, 1.6%, and 2.1%; for neck CW were 0.4%, 2.7%, 15.0%, 15.1%, and 15.6%; and for shaft CW were 4.0% (p < 0.05), 3.8%, 3.8%, 3.9%, and 3.0%, respectively.
In the RA group, the percent changes from baseline at 3, 6, 12, 18, and 24 months for BR were 11.2%, 4.4%, 5.1%, 9.2%, and 5.6%; for neck CW were −5.9%, 4.7%, 4.1%, −0.9%, and 5.7%; and for shaft CW were −4.3%, 3.8%, 5.2%, 6.3%, and 12.9%, respectively.
3.3. Efficacy on BMD based on ITT
The increases in lumbar spine BMD from baseline for all patients, patients without RA, and the RA group, respectively, were 4.4%, 4.8%, and 3.4% at 3 months; 8.4%, 8.6%, and 8.0% at 6 months; 9.0%; 11.3%, 11.5%, and 10.8% at 12 months; 12.9%, 13.3%, and 12.1% at 18 months; and 14.1%, 14.7%, and 12.8% at 24 months. At all time points, lumbar spine BMD was significantly increased in all groups (p < 0.01).
The increases in total hip BMD from baseline for all patients, patients without RA, and the RA group, respectively, were 1.0%, 1.0%, and 0.9% at 3 months; 1.4% (p < 0.05), 1.9% (p < 0.05), and 0.6% at 6 months; 3.2% (p < 0.01), 3.5% (p < 0.01), and 2.4% at 12 months; 3.8% (p < 0.01), 4.0% (p < 0.01), and 3.5% (p < 0.05) at 18 months; and 4.7% (p < 0.01), 5.2% (p < 0.01), and 3.7% (p < 0.01) at 24 months.
The changes in femoral neck BMD from baseline for all patients, the group without RA, and the RA group, respectively, were −0.1%, −0.2%, and −0.1% at 3 months; 0.1%, 0.6%, and −1.0% at 6 months; 0.1%, 0.2%, and −0.1% at 12 months; 1.9% (p < 0.05), 2.0%, and 1.8% at 18 months; and 2.6%, 2.6%, and 2.6% at 24 months.
The changes in femoral shaft BMD from baseline for all patients, the group without RA, and the RA group, respectively, were 0.0%, 0.7%, and −1.6% at 3 months; 0.7%, 2.0%, and −2.0% at 6 months; 3.4%, 4.7% (p < 0.01), and 0.7% at 12 months; 3.8%, 5.2% (p < 0.01), and 0.9% at 18 months; and 4.9%, 6.4% (p < 0.01), and 2.5% at 24 months.
The changes in hand BMD from baseline for all patients, the group without RA, and the RA group, respectively, were 1.1%, 1.0% and 1.4% at 3 months; 0.0%, 0.5%, and −0.9% at 6 months; 0.9%, 0.5%, and 1.8% at 12 months; 0.1%, 0.3%, and −0.4% at 18 months; and 0.4%, 0.1%, and 1.1% at 24 months.
3.4. Efficacy of HSA and BMD by PP
For all patients, the group without RA, and RA group, the percent changes from baseline at 3, 6, 12, 18, and 24 months for CSMI, SM, neck CW, shaft CW, FSI, lumbar spine BMD, total hip BMD, femoral neck BMD, femoral shaft BMD, and hand BMD are shown in Table 2.
Table 2.
The percent change from baseline in variables of interest for all patients (a), postmenopausal women with osteoporosis without RA (group without RA) (b), and postmenopausal women with osteoporosis with RA (RA group) (c) results by per protocol.
| At 3 months | At 6 months | At 12 months | At 18 months | At 24 months | |
|---|---|---|---|---|---|
| (a) All patients | |||||
| CSMI (mm3), mean (SD) | 2.2 (15.8) | 4.6 (27.4) | 3.1 (16.1) | 10.4 (19.5)** | 10.7 (22.6)** |
| CSA (mm3), mean (SD) | 0.5 (12.8) | 2.2 (9.0) | 2.7 (13.0) | 4.6 (17.9)** | 8.6 (19.4)** |
| SM (cm3), mean (SD) | 2.4 (24.2) | 5.3 (21.6)* | 4.4 (15.6)* | 8.8 (19.5)** | 9.6 (22.4)** |
| BR, mean (SD) | 6.1 (57.5) | 4.0 (38.7) | −0.5 (35.6) | 1.9 (39.6) | 2.8 (41.9) |
| Neck CW (mm), mean (SD) | 0.0 (0.4) | 4.7 (31.1) | 14.2 (50.0) | 11.5 (52.0) | 14.1 (45.1) |
| Shaft CW (mm), mean (SD) | −1.8 (32.6) | 3.0 (35.9) | 4.7 (38.4)* | 5.6 (42.6)* | 5.7 (41.0)* |
| FSI, mean (SD) | 8.8 (29.6)* | 13.6 (38.1)** | 12.6 (35.0) | 24.2 (50.7)** | 17.9 (38.2)** |
| Lumbar spine BMD (g/cm2), mean (SD) | 4.4 (8.0)** | 8.7 (8.8)** | 11.6 (10.5)** | 13.6 (11.8)** | 16.0 (14.4)** |
| Total hip BMD (g/cm2), mean (SD) | 1.0 (8.0) | 1.4 (5.6)* | 2.9 (10.3)* | 3.8 (6.1)** | 5.0 (8.0)** |
| Femoral neck BMD (g/cm2), mean (SD) | −0.1 (7.8) | 0.0 (7.6) | −0.2 (6.6) | 1.9 (7.8) | 3.0 (11.2) |
| Femoral shaft BMD (g/cm2), mean (SD) | 1.0 (6.7) | 1.7 (7.3) | 4.2 (10.5)* | 4.8 (10.8)* | 6.4 (11.2)** |
| Hand BMD (g/cm2), mean (SD) | 1.1 (9.8) | 0.0 (10.1) | 0.3 (9.6) | −0.7 (12.5) | −1.3 (10.6) |
| (b) Group without RA | |||||
| CSMI (mm3), mean (SD) | 2.8 (16.6) | 6.6 (31.5) | 1.9 (14.0) | 8.4 (20.8)** | 7.3 (17.0)* |
| CSA (mm3), mean (SD) | 0.1 (14.7) | 2.7 (9.5) | 3.3 (14.2) | 4.3 (20.4)** | 6.4 (13.8)* |
| SM (cm3), mean (SD) | 2.9 (16.6)* | 7.4 (24.5)* | 3.4 (14.7) | 7.7 (15.6)** | 7.2 (16.0) |
| BR, mean (SD) | 3.7 (65.9) | 4.2 (41.2) | −2.3 (36.5) | −1.8 (39.7) | 1.2 (45.4) |
| Neck CW (mm), mean (SD) | 6.5 (43.3) | 7.0 (34.5) | 19.2 (56.7) | 20.0 (59.8) | 18.8 (52.8) |
| Shaft CW (mm), mean (SD) | −0.6 (38.0) | 2.0 (41.6) | 3.7 (44.6) | 5.3 (51.0) | 1.0 (44.5) |
| FSI, mean (SD) | 9.0 (31.0) | 19.1 (42.8)* | 14.3 (35.7) | 29.3 (56.0)** | 19.5 (39.7)* |
| Lumbar spine BMD (g/cm2), mean (SD) | 4.8 (8.8)* | 8.9 (8.7)** | 12.1 (10.5)** | 14.5 (11.7)** | 17.4 (13.7)** |
| Total hip BMD (g/cm2), mean (SD) | 1.0 (9.1) | 1.9 (6.2)* | 3.2 (11.9)* | 4.0 (5.7)** | 5.7 (8.7)** |
| Femoral neck BMD (g/cm2), mean (SD) | −0.2 (7.4) | 0.8 (8.1) | 0.0 (6.4) | 0.6 (8.5) | 3.5 (12.7) |
| Femoral shaft BMD (g/cm2), mean (SD) | 0.7 (7.1) | 2.0 (7.6) | 4.4 (11.8)* | 5.2 (1.3)* | 6.6 (12.2)** |
| Hand BMD (g/cm2), mean (SD) | 1.0 (8.7) | 0.3 (9.1) | −0.7 (8.5) | −0.9 (13.8) | −3.0 (10.4)* |
| (c) RA group | |||||
| CSMI (mm3), mean (SD) | 0.8 (14.1) | 0.5 (15.8) | 5.2 (19.4) | 13.9 (30.1)** | 16.4 (29.1)** |
| CSA (mm3), mean (SD) | 1.1 (7.9) | 1.1 (8.1) | 1.7 (10.4) | 5.0 (12.7) | 12.4 (26.0) |
| SM (cm3), mean (SD) | 1.4 (37.7) | 1.0 (13.1) | 6.1 (17.2) | 10.8 (21.0)* | 13.6 (30.0) |
| BR, mean (SD) | 11.2 (34.2) | 3.5 (33.5) | 2.5 (34.3) | 8.4 (39.3) | 5.3 (36.1) |
| Neck CW (mm), mean (SD) | −5.9 (37.5) | 5.8 (32.8) | 5.2 (33.8) | 0.1 (24.9) | 6.3 (27.5) |
| Shaft CW (mm), mean (SD) | −4.3 (15.4) | 5.1 (20.2) | 6.6 (24.4) | 6.2 (21.2) | 13.4 (34.0) |
| FSI, mean (SD) | 8.4 (26.7) | 2.4 (22.4) | 9.5 (34.1) | 14.9 (33.6)* | 15.3 (36.2)* |
| Lumbar spine BMD (g/cm2), mean (SD) | 3.4 (6.1)* | 8.1 (9.3)** | 10.8 (10.7)** | 12.2 (12.0)** | 13.8 (15.3)** |
| Total hip BMD (g/cm2), mean (SD) | 0.9 (5.3) | 0.4 (4.2) | 2.3 (6.4) | 3.5 (7.0)** | 4.0 (6.7)* |
| Femoral neck BMD (g/cm2), mean (SD) | −0.1 (8.7) | −1.4 (6.2) | −0.5 (6.9) | 1.3 (6.5) | 2.3 (8.3) |
| Femoral shaft BMD (g/cm2), mean (SD) | 1.7 (5.7) | 1.0 (6.7) | 3.9 (7.6)* | 4.2 (9.8)* | 6.0 (9.6)** |
| Hand BMD (g/cm2), mean (SD) | 1.4 (12.0) | −0.7 (12.0) | 2.1 (11.2) | −0.3 (9.8) | 1.3 (10.6) |
RA: rheumatoid arthritis; CSMI: cross-sectional moment of inertia; SD: standard deviation; CSA: cross-sectional area; SM: section modulus; BR: buckling ratio; CW: cortical width; FSI: femoral strength index; BMD: bone mineral density.
p < 0.05 (paired t-test).
p < 0.01 (paired t-test).
4. Discussion
Our study demonstrated the effects of daily TPTD treatment for 24 months on HSA and BMD in postmenopausal women with and without RA in clinical practice. The main HSA finding in this study was that FSI was significantly increased at 12 months in all patients, including postmenopausal women without RA. This result suggests that TPTD treatment for preventing hip fracture should continue for more than 12 months in postmenopausal women. On the other hand, the TPTD treatment was not as effective on hip strength in RA patients. Uusi-Rasi et al. compared the effects of TPTD and placebo on hip strength efficacy and reported that CSA and SM were significantly increased and BR was significantly decreased at 1 year and 1.8 years based on DXA. Moreover, the neck cortical thickness was increased.16 Our study showed similar results for CSA and neck CW. In this study, BR negatively changed but not significantly from baseline. Thus, these studies suggest that TPTD treatment does not affect cortical bone stability. Even though the cortical bone is wide, cortical stability was not impaired. This result suggests that the improvement in bone structure was simultaneously performed. Actually, paired iliac crest biopsy images from 2 postmenopausal women with osteoporosis treated with TPTD showing pre-treatment and post-treatment findings showed improvement of bone structure. The 24 months of teriparatide treatment increased cortical bone formation and cortical turnover in patients.17 We think that in the hip bone structures, TPTD promoted bone formation at the site where osteoclasts were undergoing bone resorption. Hence, whereas FSI was significantly increased at 6 months, neck BMD was significantly increased at 18 months. In Japanese women, hip fracture has been increasing every year and is affecting younger women.18 Our results suggest that researchers should investigate methods of strengthening bone structures in the hip during TPTD treatment lasting more than 18 months.
In previous reports, the efficacy of TPTD was indicated, particularly for increasing BMD. In a domestic, third-phase double-blind comparative study, TPTD increased lumbar spine BMD by 4.46% after 3 months of administration, by 7.35% after 6 months, by 10.04% after 12 months, by 11.93% after 18 months, and by 13.42% after 24 months, whereas neck BMD increased by 2.72% at 12 months, by 3.02% at 18 months, and by 3.67% after 24 months.19 In the FACT (Forteo Alendronate Comparison Trial) study, in which TPTD was compared to alendronate (ALN), TPTD increased lumbar spine BMD by 10.92% after 18 months, compared to 5.51% with ALN, which was a significant increase from the 6-month follow-up.20 Similarly, in our study, BMD changed from baseline by 14.1% in the lumbar spine, by 4.7% in the total hip, and by 0.4% in the hand at 24 months. Furthermore, lumbar spine BMD had significantly increased by 3 months in both the group without RA and the RA group, and the effect was observed early in both disease types. However, the significant increase in total hip BMD was slower compared to that in the group without RA, and FSI was not significantly increased from baseline in the RA group. In the efficacy of TPTD treatment for the hip, the group without RA and the RA group were different. In RA, tumor necrosis factor-α is produced in large amounts from synovial cells, as are interleukin (IL)-1 and IL-6 and the expression of the receptor activator NF-κB ligand, in order to promote osteoclast differentiation and activation.21, 22 Therefore, bone turnover markers may be affected by disease activity and anti-rheumatic drugs, such as biological products, and do not necessarily reflect the therapeutic effect of TPTD. A correlation between markers of inflammation and bone resorption markers has been observed.23 In the RA group, osteoporosis treatment and RA treatment are both important for bone structure.
In previous reports, including our study, TPTD was highly effective for the lumbar spine BMD; however, it and was less effective for hip, radius, and hand BMD. In the radius, based on high-resolution peripheral QCT, TPTD increased CSA at 18 months, along with cortical thickness and cortical porosity at 6 and 18 months. Cortical density was decreased at month 18. Trabecular number was increased at month 6, but was unchanged from baseline at month 18. In the tibia, TPTD increased cortical thickness and cortical porosity at months 6 and 18, whereas CSA was unchanged. Total density increased at month 18, despite a decrease in cortical density.24 In the hip, based on DXA, the mean changes were 3.5% for CSA, 3.6% for SM, and −5.5% for BR at 1.8 years with TPTD treatment.16
In Japan, where the society is aging, the need for preventing fractures due to osteoporosis is urgent. TPTD, with its ability to increase lumbar vertebral body BMD rapidly and significantly, should be considered for use as the primary drug of choice in severe osteoporotic cases with high risk of new vertebral body fractures. Moreover, we must prevent hip fractures. In daily practice, physicians should consider whether it is necessary to strengthen any part of the hip joint.
In conclusion, the results of our study revealed that TPTD was effective for the hip structure in HSA by DXA, and suggested that for the significant increase of various indicators of HAS, TPTD is better to administered over 18 months.
Conflicts of interest
T. Mochizuki has received speaking fees from Asahi Kasei Pharma, Astellas, Eisai, Briatol Myers Squibb, Chugai Pharma, Daiichi Sankyo, Eli Lilly, Janssen Pharma, Mitsubishi Tanabe Pharma, and Takeda Pharma. K. Ikari has received speaking fees from Astellas, Briatol Myers Squibb, Chugai Pharma, Eisai, Janssen Pharma, Mitsubishi Tanabe Pharma and Takeda Pharma. All other authors have no conflicts of interest. The sponsors were not involved in the study design: collection, analysis, and interpretation of data; writing of the paper; and/or decision to submit for publication.
References
- 1.Cauley J.A., Thompson D.E., Ensrud K.C., Scott J.C., Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 2.Kanis J.A., Borgstrom F., De Laet C. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 3.Bone H.G., Hosking D., Devogelaer J.P. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 4.Black D.M., Schwartz A.V., Ensrud K.E. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 5.Harris S.T., Watts N.B., Genant H.K. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 6.Kushida K., Shiraki M., Nakamura T. Alendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: a 3-year follow-up study. J Bone Miner Metab. 2004;22:462–468. doi: 10.1007/s00774-004-0508-0. [DOI] [PubMed] [Google Scholar]
- 7.Kushida K., Fukunaga M., Kishimoto H. A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidoronate: a randomized, double-masked trial. J Bone Miner Metab. 2004;22:469–478. doi: 10.1007/s00774-004-0509-z. [DOI] [PubMed] [Google Scholar]
- 8.Hagino H., Nishizawa Y., Sone T. A double-blinded head-to-head trial of minodronate and alendronate in women with postmenopausal osteoporosis. Bone. 2009;44:1078–1084. doi: 10.1016/j.bone.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Shane E., Burr D., Ebeling P.R. Atypical subtrochanteric and diaphyseal femoral fracture: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 10.Park-Wyllie L.Y., Mamdani M.M., Juurlink D.N. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305:783–789. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 11.Reeve J., Meunier P.J., Parsons J.A. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Br Med J. 1980;280:1340–1344. doi: 10.1136/bmj.280.6228.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesp R., Hulme P., Williams D., Reeve J. The relationship between changes in femoral bone density and calcium balance in patients with involutional osteoporosis treated with human parathyroid hormone fragment (hPTH 1–34) Metab Bone Dis Relat Res. 1981;2:331–334. [Google Scholar]
- 13.Dobnig H., Turner R.T. The effect of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology. 1997;138:4607–4612. doi: 10.1210/endo.138.11.5505. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa T., Turner C.H., Peacock M. Geometric structure of the femoral neck measured using dual-energy X-ray absorptiometry. J Bone Miner Res. 1994;9:1053–1064. doi: 10.1002/jbmr.5650090713. [DOI] [PubMed] [Google Scholar]
- 15.Ramamurthi K., Ahmad O., Engelke K. An in vivo comparison of hip structure analysis (HSA) with measurements obtained by QCT. Osteoporos Int. 2012;23:543–551. doi: 10.1007/s00198-011-1578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uusi-Rasi K., Semanick L.M., Zanchetta J.R. Effects of teriparatide [rhPTH (1–34)] treatment on structural geometry of the proximal femur in elderly osteoporotic women. Bone. 2005;36:948–958. doi: 10.1016/j.bone.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y.L., Zeng Q.Q., Chiang A.Y. Effects of teriparatide on cortical histomorphometric variables in postmenopausal women with or without prior alendronate treatment. Bone. 2014;59:139–147. doi: 10.1016/j.bone.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura N., Suzuki T., Hosoi T., Orimo H. Epidemiology of hip fracture in Japan: incidence and risk factors. J Bone Miner Metab. 2005;23:78–80. doi: 10.1007/BF03026328. [DOI] [PubMed] [Google Scholar]
- 19.Miyauchi A., Matsumoto T., Sugimoto T., Tsujimoto M., Warner M.R., Nakamura T. Effects of teriparatide on bone mineral density and bone turnover makers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone. 2010;47:493–502. doi: 10.1016/j.bone.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 20.McClung M.R., San Martin J., Miller P.D. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–1768. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 21.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclaest differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 22.Udagawa N., Kotake S., Kamatani N., Takahashi N., Suda T. The molecular mechanism of osteoclastogenesis in rheumatoid arthritis. Arthritis Res. 2002;4:281–289. doi: 10.1186/ar431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momohara S., Okamoto H., Yago T. The study of bone mineral density and bone turnover markers in postmenopausal women with active rheumatoid arthritis. Mod Rheumatol. 2005;15:410–414. doi: 10.1007/s10165-005-0435-5. [DOI] [PubMed] [Google Scholar]
- 24.Hansen S., Hauge E.M., Beck Jensen J.E., Brixen K. Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28:736–745. doi: 10.1002/jbmr.1784. [DOI] [PubMed] [Google Scholar]