Abstract
We reviewed the indexed literature regarding the efficacy of laser therapy in the treatment of peri-implantitis (PI). Databases were searched using combinations of the following keywords: peri-implantitis, bone loss, photodynamic therapy, laser, and light-activated disinfection. Titles and abstracts of publications from these search results were screened to determine which studies fulfilled the eligibility criteria. Full texts of relevant studies were read and independently assessed against the eligibility criteria. The resulting 28 studies described the role of lasers in the treatment of PI. The erbium:yttrium–aluminum-garnet laser can be used to sterilize implant surfaces without damaging them. Likewise, the carbon dioxide laser can disinfect implant surfaces and enhance the bone-to-implant contact around previously infected sites. Photodynamic therapy exhibits high target specificity and can destroy pathogens associated with the etiology of PI. Laser therapy can significantly reduce levels of clinical markers of peri-implant tissue inflammation (i.e., bleeding upon probing and clinical attachment loss) without jeopardizing the integrity of the implant or alveolar bone. In conclusion, laser therapy as an adjunct to conventional mechanical debridement therapy can be used effectively for the treatment of PI.
Keywords: Peri-implant bone loss, Dental lasers, Peri-implantitis, Photodynamic therapy, Decontamination, Review
1. Introduction
Peri-implantitis (PI) is a disease of the tissues surrounding dental implants. Roos-Jansåker et al., 2006a, Roos-Jansåker et al., 2006b defined PI as an inflammatory condition in which implants with varying degrees of bone loss are accompanied by a probing pocket depth (PPD) of at least 4 mm, bleeding on probing (BOP), and purulent discharge upon probing. PI occurs in 10% of implants and in 20% of patients within 5–10 years after implantation (Mombelli et al., 2012). However, the reported prevalence of PI is variable (Mombelli et al., 2012, Schuldt Filho et al., 2014, Costa et al., 2012). Risk factors associated with the etiology of PI include poor oral hygiene or plaque control (Javed et al., 2009), previous history of periodontal disease (Javed et al., 2009), stagnation of residual cement in or around the gingivae after implant prosthesis cementation (Pette et al., 2013), occlusal overloading (Naert et al., 2012, Tawil, 2008), smoking (Galindo-Moreno et al., 2014), and systemic diseases, such as poorly controlled diabetes (Javed and Romanos, 2009), osteoporosis (Chen et al., 2013), and human immunodeficiency virus infection (Hwang and Wang, 2007).
Ideally, treatment of PI should focus on infection control, detoxification of implant surfaces, regeneration of lost tissues, and plaque control regimes via mechanical debridement with or without raising a surgical flap (Bautista and Huynh-Ba, 2013, Schou et al., 2004). New innovative therapeutic regimes, such as laser-supported and photodynamic therapy (PDT), have emerged as useful treatments for periodontitis and PI (Qadri et al., 2011, Qadri et al., 2010, Vohra et al., 2014, Javed et al., 2013, Javed and Romanos, 2013, Leja et al., 2013, Romanos et al., 2009, Romanos et al., 2013, Romanos and Weitz, 2012). Romanos and Nentwig (2008) investigated the efficacy of a carbon dioxide (CO2) laser in the decontamination of failing implants. After a mean follow-up of 27 months, virtually complete bone regeneration occurred in the peri-implant defects. In a preclinical study in dogs, Nevins et al. (2014) assessed the efficacy of an erbium:yttrium–aluminum-garnet (Er:YAG) laser in reestablishing bone-to-implant contact around sites with PI. After 3 months of treatment, the animals were killed, and the jaw segments were resected and prepared for histologic assessment. Only animals treated with the Er:YAG laser demonstrated elimination of the inflammatory tissue and complete osseointegration with the implant surface (Nevins et al., 2014).
Laser dentistry has revolutionized modern clinical dental practice and research (Javed and Romanos, 2013). The aim of the present study was to provide a comprehensive review of literature concerning the efficacy of laser therapy in the treatment of PI.
2. Materials and methods
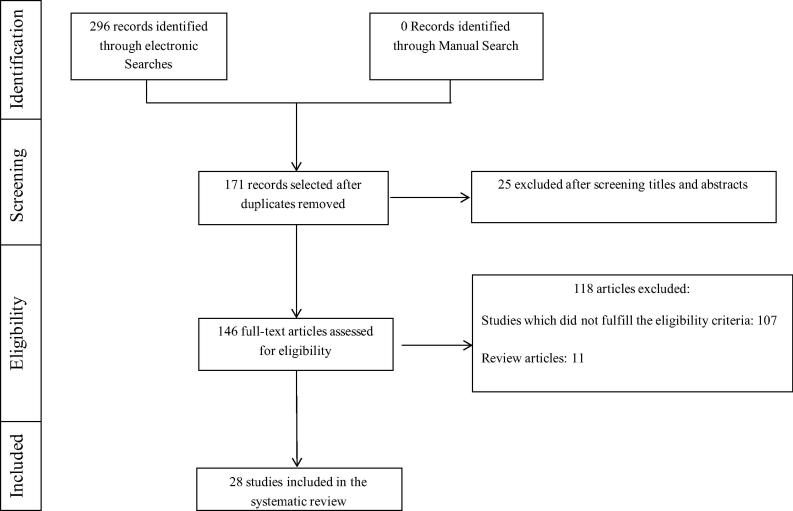
To determine which published studies were pertinent to laser therapy of PI, we established a set of eligibility criteria for inclusion in our study. The following eligibility criteria were imposed: (1) original articles; (2) experimental human studies; (3) experimental animal studies; (4) articles published only in English-language; and (6) full-text articles (Randomized and Controlled Clinical Trials). PubMed/Medline (National Library of Medicine, Bethesda, Maryland), EMBASE, Scopus, ISI Web of Knowledge and Google Scholar databases were searched for publications published between 1991 through August 2015, using different combinations of the following keywords: peri-implantitis, bone loss, photodynamic therapy, laser, and light-activated disinfection. Titles and abstracts of these studies were screened against the eligibility criteria (Fig. 1). Full texts of the remaining relevant studies were read and assessed against the eligibility criteria. During this search, potentially relevant articles that were cited within our primary library were also evaluated.
Figure 1.
Article selection flow chart for the screening process.
3. Results and discussion
Our initial search of the indexed literature yielded 171 unique publications (Fig. 1). After scanning the titles and abstracts, we excluded 25 publications that did not meet our eligibility criteria. We read the remaining 146 articles in full and eliminated 118 articles (including 11 reviews) that did not meet our criteria. The remaining 28 publications were included in our systematic review.
3.1. Disinfection of dental implant surfaces with the Er:YAG laser
Implant surface characteristics (e.g., surface roughness) play an important role in the osseointegration and long-term survival of dental implants (Javed et al., 2011). The Er:YAG laser has a high absorbability in water. This laser is capable of removing the microbe-infiltrated oxide layer from the surface of dental implants without compromising the implant surface characteristics or surrounding alveolar bone (Yamamoto and Tanabe, 2013, Takasaki et al., 2007).
In their study on dogs, Nevins et al. (2014) investigated the ability of the Er:YAG laser to treat PI by removing the contaminated titanium oxide layer from implant surfaces. After 3 months of follow-up, animals were examined clinically to assess the severity of peri-implant soft tissue inflammation. Afterward, the animals were killed, and their jaw segments (containing the implants and surrounding tissues) were assessed histologically. Minimal gingival inflammation was observed in the clinical examination. The histologic results showed bone formation with sufficiently enhanced bone-to-implant contact (Nevins et al., 2014). Yamamoto and Tanabe (2013) also reported that the Er:YAG laser is effective in stripping the titanium oxide layer from implant surfaces without damaging the implant surface or bone.
However, negative outcomes have also been attributed to Er:YAG laser use. In a 48-month follow-up clinical study, Schwarz et al. (2013) assessed the effect of two surface decontamination methods on the long-term outcomes after combined surgical resection and regenerative treatment of PI. Implant surfaces were treated with an Er:YAG laser (experimental group) or with plastic curettes, cotton pellets, and sterile saline (control group). Four-year follow-up results showed significantly reduced BOP, plaque index, and attachment loss among implants in the control group compared to the Er:YAG laser-treated group (Schwarz et al., 2013). One explanation for this discrepancy may be that the use of laser equipment is technique-sensitive and influenced by the operator’s level of experience (Lustosa-Pereira et al., 2011).
Alterations in implant surface characteristics have been reported when using lasers with energies exceeding 140–180 mJ/pulse (Kim et al., 2011). However, the Er:YAG laser has been shown to be safe for use on implant surfaces when used at 100 mJ/pulse and 10 pulses/s for 60 s (Monzavi et al., 2014). In this context, disinfection of implant surfaces using an Er:YAG laser system seems to be a promising therapeutic protocol for PI.
3.2. Increased osseointegration of dental implants with CO2 laser treatment
The CO2 laser is increasingly being used in implant dentistry because it is minimally absorbed at the implant surface and has a reduced risk of causing temperature-induced tissue damage (Romanos et al., 2009, Stubinger et al., 2005, Deppe et al., 2002, Deppe et al., 2001, Nammour and Majerus, 1991). Irradiation of titanium surfaces using a CO2 laser led to increased osteoblast attachment to implant surfaces, thereby augmenting bone formation (Romanos et al., 2006). Similarly, Stubinger et al. (2005) found that application of CO2 laser as an adjunct to mechanical debridement augmented new bone formation in peri-implant defect sites. In a clinical study of 32 patients, Deppe et al. (2007) assessed the efficacy of soft-tissue resection with and without adjunct CO2 laser therapy in the treatment of PI. The 5-year follow-up results showed that resolution of PI was significantly accelerated using a CO2 laser concomitant with soft-tissue resection (Deppe et al., 2007). However, heat production as a result of excessive CO2 laser application may jeopardize osseointegration to some extent (Romanos et al., 2000, Geminiani et al., 2011). Therefore, an understanding of the relationship between applied laser energy and therapeutic effect is crucial for optimal treatment.
3.3. Laser-assisted PI protocol
An emerging experimental technique for treating PI is the laser-assisted PI protocol (LAPIP; Aoki et al., 2015). The LAPIP technique is an implant-specific modification of the laser-assisted new attachment protocol (LANAP; Nevins et al., 2014). Both protocols use a neodymium-doped YAG (Nd:YAG) laser-ablation step to remove inflamed sulcular tissues and decontaminate the implant surface, followed by nonsurgical periodontal therapy. The LAPIP technique is designed to create a blood clot that allows the defect area to heal apico-coronally by preventing down-growth of the gingival epithelium. However, to our knowledge, no randomized controlled trials to date have assessed the efficacy of LAPIP for management of PI.
3.4. Role of PDT in treatment of PI
PDT is a modern therapeutic strategy that uses a monochromatic light source (typical λ: 630–700 nm) and a photosensitizer (e.g., toluidine or methylene blue) in the presence of oxygen to generate reactive oxygen species (Eichler et al., 2005, Vohra et al., 2014, Javed et al., 2014). Reactive oxygen species can cause oxidative damage to target cells, including microbial and tumor cells (Sperandio et al., 2013, Hamblin and Hasan, 2004). Some advantages of PDT include its high target specificity (Soukos and Goodson, 2011), biocompatibility with healthy human cells (Tremblay et al., 2002), low risk of chemical and/or thermal side effects (Nagayoshi et al., 2011), and low probability of microbial resistance (Donnelly et al., 2008).
In a study by Dortbudak et al. (2001), the pathogen load in peri-implant sulci of patients with PI was significantly reduced after PDT. Other researchers (Eick et al., 2013, Hayek et al., 2005, Marotti et al., 2013) have also reported reduced bacterial counts after PDT. These results suggest that PDT may be a useful treatment strategy in the management of PI.
Multiple research groups have speculated that PDT used as an adjunct therapy to mechanical debridement for PI may be more effective than conventional treatment alone (Eick et al., 2013, Hayek et al., 2005, Marotti et al., 2013). However, clinical studies (De Angelis et al., 2012, Esposito et al., 2013, Bassetti et al., 2014, Schar et al., 2013, Deppe et al., 2013) have reported contradictory results. For example, Esposito et al. (2013) compared the effects of mechanical and surgical debridement techniques with and without PDT in patients with PI. In this study, peri-implant inflammatory parameters (i.e., PPD, plaque, and bleeding scores) were investigated at baseline and 52 weeks after treatment. The results showed a comparable reduction in peri-implant inflammatory parameters when conventional treatments were performed either with or without PDT (Esposito et al., 2013). Similar results were reported by De Angelis et al. (2012).
One explanation for the discrepant results may be the wide variability in PI severity among clinical studies (De Angelis et al., 2012, Bassetti et al., 2014, Deppe et al., 2013, Dortbudak et al., 2001, Esposito et al., 2013, Schar et al., 2013). For example, in studies by Schar et al., 2013, De Angelis et al., 2012, the mean PPD was 4.29 and 6.34 mm, respectively. A study by Deppe et al. (2013) found no significant effect of PDT in patients with severe PI (PPD 5–8 mm) compared to patients with moderate PI (PPD 3–5 mm). Therefore, it may be that the efficacy of conventional debridement either with or without PDT depends on the severity of PI. Nevertheless, the frequency (1–4 times) and duration (10–80 s) of PDT varied considerably among the aforementioned clinical studies (De Angelis et al., 2012, Bassetti et al., 2014, Deppe et al., 2013, Dortbudak et al., 2001, Esposito et al., 2013, Schar et al., 2013). In addition, many of the clinical studies were missing either a standardized test group (mechanical debridement with adjunct PDT) or control group (mechanical debridement alone) (Bassetti et al., 2014, Deppe et al., 2013, Dortbudak et al., 2001, Schar et al., 2013). Thus, the clinical efficacy of PDT as an adjunct treatment to conventional debridement techniques in the treatment of PI remains debatable.
4. Conclusion
Laser therapy is a modern therapeutic technique that can be used effectively as an adjunct to conventional mechanical debridement therapy for PI. However, there is a need to reach a consensus regarding the standardization of laser-related parameters that could yield the most favorable outcomes in terms of peri-implant infection therapy.
Conflict of interest and financial disclosure
The author declares that he has no conflict of interest and there was no external source of funding for the present study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aoki A., Mizutani K., Schwarz F., Sculean A., Yukna R.A., Takasaki A.A., Romanos G.E., Taniguchi Y., Sasaki K.M., Zeredo J.L., Koshy G., Coluzzi D.J., White J.M., Abiko Y., Ishikawa I., Izumi Y. Periodontal and peri-implant wound healing following laser therapy 2000. Periodontology. 2015;68:217–269. doi: 10.1111/prd.12080. [DOI] [PubMed] [Google Scholar]
- Bassetti M., Schar D., Wicki B., Eick S., Ramseier C.A., Arweiler N.B., Sculean A., Salvi G.E. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin. Oral Implants Res. 2014;25:279–287. doi: 10.1111/clr.12155. [DOI] [PubMed] [Google Scholar]
- Bautista L., Huynh-Ba G. In patients with peri-implantitis, access flap surgery may be more effective than mechanical debridement in terms of clinical attachment gain although both treatments lead to improved clinical parameters (UT CAT #2432) Texas Dent. J. 2013;130:1112. [PubMed] [Google Scholar]
- Chen H., Liu N., Xu X., Qu X., Lu E. Smoking, radiotherapy, diabetes and osteoporosis as risk factors for dental implant failure: a meta-analysis. PLoS One. 2013;8:e71955. doi: 10.1371/journal.pone.0071955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F.O., Takenaka-Martinez S., Cota L.O., Ferreira S.D., Silva G.L., Costa J.E. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J. Clin. Periodontol. 2012;39:173–181. doi: 10.1111/j.1600-051X.2011.01819.x. [DOI] [PubMed] [Google Scholar]
- De Angelis N., Felice P., Grusovin M.G., Camurati A., Esposito M. The effectiveness of adjunctive light-activated disinfection (LAD) in the treatment of peri-implantitis: 4-month results from a multicentre pragmatic randomised controlled trial. Eur. J. Oral Implantol. 2012;5:321–331. [PubMed] [Google Scholar]
- Deppe H., Horch H.H., Henke J., Donath K. Per-implant care of ailing implants with the carbon dioxide laser. Int. J. Oral Maxillofac. Implants. 2001;16:659–667. [PubMed] [Google Scholar]
- Deppe H., Greim H., Brill T., Wagenpfeil S. Titanium deposition after peri-implant care with the carbon dioxide laser. Int. J. Oral Maxillofac. Implants. 2002;17:707–714. [PubMed] [Google Scholar]
- Deppe H., Horch H.H., Neff A. Conventional versus CO2 laser-assisted treatment of peri-implant defects with the concomitant use of pure-phase beta-tricalcium phosphate: a 5-year clinical report. Int. J. Oral Maxillofac. Implants. 2007;22:79–86. [PubMed] [Google Scholar]
- Deppe H., Mucke T., Wagenpfeil S., Kesting M., Sculean A. Nonsurgical antimicrobial photodynamic therapy in moderate vs severe peri-implant defects: a clinical pilot study. Quintessence Int. 2013;44:609–618. doi: 10.3290/j.qi.a29505. [DOI] [PubMed] [Google Scholar]
- Donnelly R.F., McCarron P.A., Tunney M.M. Antifungal photodynamic therapy. Microbiol. Res. 2008;163:1–12. doi: 10.1016/j.micres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dortbudak O., Haas R., Bernhart T., Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin. Oral Implants Res. 2001;12:104–108. doi: 10.1034/j.1600-0501.2001.012002104.x. [DOI] [PubMed] [Google Scholar]
- Eichler M., Lavi R., Shainberg A., Lubart R. Flavins are source of visible-light-induced free radical formation in cells. Lasers Surg. Med. 2005;37:314–319. doi: 10.1002/lsm.20239. [DOI] [PubMed] [Google Scholar]
- Eick S., Markauskaite G., Nietzsche S., Laugisch O., Salvi G.E., Sculean A. Effect of photoactivated disinfection with a light-emitting diode on bacterial species and biofilms associated with periodontitis and peri-implantitis. Photodiagn. Photodyn. Ther. 2013;10:156–167. doi: 10.1016/j.pdpdt.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Esposito M., Grusovin M.G., De Angelis N., Camurati A., Campailla M., Felice P. The adjunctive use of light-activated disinfection (LAD) with FotoSan is ineffective in the treatment of peri-implantitis: 1-year results from a multicentre pragmatic randomised controlled trial. Eur. J. Oral Implantol. 2013;6:109–119. [PubMed] [Google Scholar]
- Galindo-Moreno P., Leon-Cano A., Ortega-Oller I., Monje A.F.O.V., Catena A. Marginal bone loss as success criterion in implant dentistry: beyond 2 mm. Clin. Oral Implants Res. 2014 doi: 10.1111/clr.12324. [DOI] [PubMed] [Google Scholar]
- Geminiani A., Caton J.G., Romanos G.E. Temperature increase during CO(2) and Er:YAG irradiation on implant surfaces. Implant Dent. 2011;20:379–382. doi: 10.1097/ID.0b013e3182310d57. [DOI] [PubMed] [Google Scholar]
- Hamblin M.R., Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek R.R., Araujo N.S., Gioso M.A., Ferreira J., Baptista-Sobrinho C.A., Yamada A.M., Ribeiro M.S. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J. Periodontol. 2005;76:1275–1281. doi: 10.1902/jop.2005.76.8.1275. [DOI] [PubMed] [Google Scholar]
- Hwang D., Wang H.L. Medical contraindications to implant therapy: Part II: Relative contraindications. Implant Dent. 2007;16:13–23. doi: 10.1097/ID.0b013e31803276c8. [DOI] [PubMed] [Google Scholar]
- Javed F., Romanos G.E. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: a systematic literature review. J. Periodontol. 2009;80:1719–1730. doi: 10.1902/jop.2009.090283. [DOI] [PubMed] [Google Scholar]
- Javed F., Romanos G.E. Does photodynamic therapy enhance standard antibacterial therapy in dentistry? Photomed. Laser Surg. 2013;31:512–518. doi: 10.1089/pho.2012.3329. [DOI] [PubMed] [Google Scholar]
- Javed F., Sundin U., Altamash M., Klinge B., Engstrom P.E. Self-perceived oral health and salivary proteins in children with type 1 diabetes. J. Oral. Rehabil. 2009;36:39–44. doi: 10.1111/j.1365-2842.2008.01895.x. [DOI] [PubMed] [Google Scholar]
- Javed F., Almas K., Crespi R., Romanos G.E. Implant surface morphology and primary stability: is there a connection? Implant Dent. 2011;20:40–46. doi: 10.1097/ID.0b013e31820867da. [DOI] [PubMed] [Google Scholar]
- Javed F., Hussain H.A., Romanos G.E. Re-stability of dental implants following treatment of peri-implantitis. Interv. Med. Appl. Sci. 2013;5:116–121. doi: 10.1556/IMAS.5.2013.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed F., Samaranayake L.P., Romanos G.E. Treatment of oral fungal infections using antimicrobial photodynamic therapy: a systematic review of currently available evidence. Photochem. Photobiol. Sci. 2014;13:726–734. doi: 10.1039/c3pp50426c. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Herr Y., Chung J.H., Shin S.I., Kwon Y.H. The effect of erbium-doped: yttrium, aluminium and garnet laser irradiation on the surface microstructure and roughness of double acid-etched implants. J. Periodontal Implant Sci. 2011;41:234–241. doi: 10.5051/jpis.2011.41.5.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leja C., Geminiani A., Caton J., Romanos G.E. Thermodynamic effects of laser irradiation of implants placed in bone: an in vitro study. Lasers Med. Sci. 2013;28:1435–1440. doi: 10.1007/s10103-012-1215-z. [DOI] [PubMed] [Google Scholar]
- Lustosa-Pereira A.C., Pozza D.H., Cunha A., Dedavid B.A., Duarte-de Moraes J.F., Gerhardt-de Oliveira M. Analysis of the morphology and composition of tooth apices apicectomized using three different ablation techniques. Med. Oral Patol. Oral Cir. Bucal. 2011;16:e225–e230. doi: 10.4317/medoral.16.e225. [DOI] [PubMed] [Google Scholar]
- Marotti J., Tortamano P., Cai S., Ribeiro M.S., Franco J.E., de Campos T.T. Decontamination of dental implant surfaces by means of photodynamic therapy. Lasers Med. Sci. 2013;28:303–309. doi: 10.1007/s10103-012-1148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombelli A., Muller N., Cionca N. The epidemiology of peri-implantitis. Clin. Oral Implants Res. 2012;23(Suppl. 6):67–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- Monzavi A., Shahabi S., Fekrazad R., Behruzi R., Chiniforush N. Implant surface temperature changes during Er:YAG laser irradiation with different cooling systems. J. Dent. (Tehran) 2014;11:210–215. [PMC free article] [PubMed] [Google Scholar]
- Naert I., Duyck J., Vandamme K. Occlusal overload and bone/implant loss. Clin. Oral Implants Res. 2012;23(Suppl. 6):95–107. doi: 10.1111/j.1600-0501.2012.02550.x. [DOI] [PubMed] [Google Scholar]
- Nagayoshi M., Nishihara T., Nakashima K., Iwaki S., Chen K.K., Terashita M., Kitamura C. Bactericidal effects of diode laser irradiation on Enterococcus faecalis using periapical lesion defect model. ISRN Dent. 2011;2011:870364. doi: 10.5402/2011/870364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nammour S., Majerus P. Sterilization potential of the CO2 laser. Acta Stomatol. Belg. 1991;88:183–186. [PubMed] [Google Scholar]
- Nevins M., Nevins M.L., Yamamoto A., Yoshino T., Ono Y., Wang C.W., Kim D.M. Use of Er:YAG laser to decontaminate infected dental implant surface in preparation for reestablishment of bone-to-implant contact. Int. J. Periodontics Restorative Dent. 2014;34:461–466. doi: 10.11607/prd.2192. [DOI] [PubMed] [Google Scholar]
- Pette G.A., Ganeles J., Norkin F.J. Radiographic appearance of commonly used cements in implant dentistry. Int. J. Periodontics Restorative Dent. 2013;33:61–68. doi: 10.11607/prd.1466. [DOI] [PubMed] [Google Scholar]
- Qadri T., Poddani P., Javed F., Tuner J., Gustafsson A. A short-term evaluation of Nd:YAG laser as an adjunct to scaling and root planing in the treatment of periodontal inflammation. J. Periodontol. 2010;81:1161–1166. doi: 10.1902/jop.2010.090700. [DOI] [PubMed] [Google Scholar]
- Qadri T., Javed F., Poddani P., Tuner J., Gustafsson A. Long-term effects of a single application of a water-cooled pulsed Nd:YAG laser in supplement to scaling and root planing in patients with periodontal inflammation. Lasers Med. Sci. 2011;26:763–766. doi: 10.1007/s10103-010-0807-8. [DOI] [PubMed] [Google Scholar]
- Romanos G.E., Nentwig G.H. Regenerative therapy of deep peri-implant infrabony defects after CO2 laser implant surface decontamination. Int. J. Periodontics Restorative Dent. 2008;28:245–255. [PubMed] [Google Scholar]
- Romanos G.E., Weitz D. Therapy of peri-implant diseases. Where is the evidence? J. Evid. Based Dent. Pract. 2012;12:204–208. doi: 10.1016/S1532-3382(12)70038-6. [DOI] [PubMed] [Google Scholar]
- Romanos G.E., Everts H., Nentwig G.H. Effects of diode and Nd:YAG laser irradiation on titanium discs: a scanning electron microscope examination. J. Periodontol. 2000;71:810–815. doi: 10.1902/jop.2000.71.5.810. [DOI] [PubMed] [Google Scholar]
- Romanos G., Crespi R., Barone A., Covani U. Osteoblast attachment on titanium disks after laser irradiation. Int. J. Oral Maxillofac. Implants. 2006;21:232–236. [PubMed] [Google Scholar]
- Romanos G., Ko H.H., Froum S., Tarnow D. The use of CO(2) laser in the treatment of peri-implantitis. Photomed. Laser Surg. 2009;27:381–386. doi: 10.1089/pho.2008.2280. [DOI] [PubMed] [Google Scholar]
- Romanos G.E., Gupta B., Yunker M., Romanos E.B., Malmstrom H. Lasers use in dental implantology. Implant Dent. 2013;22:282–288. doi: 10.1097/ID.0b013e3182885fcc. [DOI] [PubMed] [Google Scholar]
- Roos-Jansaker A.M., Lindahl C., Renvert H., Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part I: Implant loss and associations to various factors. J. Clin. Periodontol. 2006;33:283–289. doi: 10.1111/j.1600-051X.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- Roos-Jansaker A.M., Lindahl C., Renvert H., Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part II: Presence of peri-implant lesions. J. Clin. Periodontol. 2006;33:290–295. doi: 10.1111/j.1600-051X.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- Schar D., Ramseier C.A., Eick S., Arweiler N.B., Sculean A., Salvi G.E. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin. Oral Implants Res. 2013;24:104–110. doi: 10.1111/j.1600-0501.2012.02494.x. [DOI] [PubMed] [Google Scholar]
- Schou S., Berglundh T., Lang N.P. Surgical treatment of peri-implantitis. Int. J. Oral Maxillofac. Implants. 2004;(19 Suppl.):140–149. [PubMed] [Google Scholar]
- Schuldt Filho G., Dalago H.R., Souza J.G., Stanley K., Jovanovic S., Bianchini M.A. Prevalence of peri-implantitis in patients with implant-supported fixed prostheses. Quintessence Int. 2014 doi: 10.3290/j.qi.a32566. [DOI] [PubMed] [Google Scholar]
- Schwarz F., Hegewald A., John G., Sahm N., Becker J. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J. Clin. Periodontol. 2013;40:962–967. doi: 10.1111/jcpe.12143. [DOI] [PubMed] [Google Scholar]
- Soukos N.S., Goodson J.M. Photodynamic therapy in the control of oral biofilms. Periodontology 2000. 2011;55:143–166. doi: 10.1111/j.1600-0757.2010.00346.x. [DOI] [PubMed] [Google Scholar]
- Sperandio F.F., Huang Y.Y., Hamblin M.R. Antimicrobial photodynamic therapy to kill gram-negative bacteria. Recent Pat. Antiinfect Drug Discovery. 2013 doi: 10.2174/1574891x113089990012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubinger S., Henke J., Donath K., Deppe H. Bone regeneration after peri-implant care with the CO2 laser: a fluorescence microscopy study. Int. J. Oral Maxillofac. Implants. 2005;20:203–210. [PubMed] [Google Scholar]
- Takasaki A.A., Aoki A., Mizutani K., Kikuchi S., Oda S., Ishikawa I. Er:YAG laser therapy for peri-implant infection: a histological study. Lasers Med. Sci. 2007;22:143–157. doi: 10.1007/s10103-006-0430-x. [DOI] [PubMed] [Google Scholar]
- Tawil G. Peri-implant bone loss caused by occlusal overload: repair of the peri-implant defect following correction of the traumatic occlusion. A case report. Int. J. Oral Maxillofac. Implants. 2008;23:153–157. [PubMed] [Google Scholar]
- Tremblay J.F., Dussault S., Viau G., Gad F., Boushira M., Bissonnette R. Photodynamic therapy with toluidine blue in Jurkat cells: cytotoxicity, subcellular localization and apoptosis induction. Photochem. Photobiol. Sci. 2002;1:852–856. doi: 10.1039/b204385h. [DOI] [PubMed] [Google Scholar]
- Vohra F., Al-Rifaiy M.Q., Lillywhite G., Abu Hassan M.I., Javed F. Efficacy of mechanical debridement with adjunct antimicrobial photodynamic therapy for the management of peri-implant diseases: a systematic review. Photochem. Photobiol. Sci. 2014;13:1160–1168. doi: 10.1039/c4pp00083h. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Tanabe T. Treatment of peri-implantitis around TiUnite-surface implants using Er:YAG laser microexplosions. Int. J. Periodontics Restor. Dent. 2013;33:21–30. doi: 10.11607/prd.1593. [DOI] [PubMed] [Google Scholar]