Abstract
Aim:
Toxoplasma gondii has a clinical and veterinary importance as it is known to cause congenital disease and abortion both in humans and livestock. Since the contaminated lamb is one of the sources of human infection, this study was performed to determine the prevalence of T. gondii in sheep in south of Iran.
Materials and Methods:
Sera and tissue samples (diaphragm and heart) were collected from 370 sheep from slaughterhouse of Jahrom. The samples were taken from both sexes and from 6 to 60 months age. Specific immunoglobulin G antibodies to T. gondii were examined with enzyme-linked immunosorbent assay, and B1 gene nested-polymerase chain reaction detection was done to survey the tissue samples.
Results:
The total prevalence of Toxoplasma infection among sheep was found to be 35.94% and 34.32% based on serological and molecular method, respectively. According to serologic and molecular findings, the females were more positive than males for Toxoplasma; maximum frequency of positive samples was observed in 24-36 months and the positive samples had been collected more in spring than in summer, but no statistical correlation was observed between prevalence rate and the age and sex of animals or season of sampling.
Conclusion:
T. gondii is widely distributed in sheep in Jahrom with a rate comparable with other parts of Iran and the world. It suggested a widespread exposure of sheep in this region to T. gondii. Thus, consumption of undercooked or raw meat presents the transmission risk of the parasite and this might be considered as an important public health problem, mainly for high-risk groups such as the pregnant and the immunodeficient.
Keywords: B1 gene, enzyme-linked immunosorbent assay, meat consumers, nested-polymerase chain reaction, sheep, Toxoplasma gondii
Introduction
Zoonotic diseases are one of the major public health problems in many countries. One of these diseases is toxoplasmosis. Toxoplasmosis is a widespread zoonosis caused by Toxoplasma gondii, a ubiquitous coccidian parasite of felines, man and many wild or domestic warm-blooded animals [1]. Herbivores acquire infection generally by the ingestion of oocysts, shed by infected cats, in water or contaminated food, and humans become infected post-natally by ingesting tissue cysts from undercooked meat [2].
The parasite has clinical and veterinary importance as it is known to cause congenital disease and abortion both in humans and livestock [3]. Acquired toxoplasmosis is normally asymptomatic or has mild non-specific symptoms in immunocompetent persons, while in immunocompromised individuals might be life threatening [4].
It is well known that meat from persistently infected animals is one of the most important potential sources of human toxoplasmosis [5]. Hence, it is necessary to investigate the prevalence of T. gondii infection among meat producing animals. Sheep are important to the economy of many countries because they are a source of nutrition for humans. Epidemiological investigations have revealed a significant correlation between human toxoplasmosis and the consumption of raw or undercooked meat or its products [2].
T. gondii in sheep is a source of infection for humans and carnivorous animals [6]. Various serological and molecular tests have been widely used by researchers in epidemiological studies on animal and human toxoplasmosis worldwide [7-12]. The prevalence of toxoplasmosis was reported in different livestock such as sheep in different parts of Iran which is varied between 13.8% and 35% for sheep [13-15]. In addition, a seroprevalence rate of 51.8% has been reported for all parts of Iran [16]. The seroprevalence rate of Toxoplasma infection in Fars province, by focusing on Shiraz city, has been reported to be 26.5% in sheep [17]. Moreover, tissue cysts were observed in 38% of tissue samples of sheep by molecular methods in southwest of Iran [18].
There is little information concerning toxoplasmosis rate in sheep in southern parts of Iran. Furthermore, sheep breeding is significantly common in this area, and since the contaminated lamb is one of the sources of human infection, this study was performed to determine the prevalence of T. gondii in sheep using both serological and molecular methods in the south of Fars. This survey provides an accurate picture of the risk of exposure to T. gondii in a common source of meat products.
Materials and Methods
Ethical approval
The experiment on animals including all procedures of this study was approved by the local Ethical Committee in Jahrom University of Medical Sciences.
Study area and sampling
The animals were chosen from the main slaughterhouse of Jahrom district, south of Fars province, where animals gathered from different regions of the district, between Aprils and June 2013. Jahrom is situated in a zone with 1050 m height from sea level where the temperature can become high in summer and a mild winter. Within the study area, 370 sheep blood samples were randomly collected from slaughtered sheep. In addition, the tissue samples were taken from diaphragm and heart of all animals for molecular examination. The animals had been born and raised in the region and were intended for human consumption. Demographic information such as sex, age, and breeding area of samples was recorded. The age of animals was ranging from 6 to 60 months.
Serologic examination
Sera of samples were separated and stored at −20°C until assayed. Specific immunoglobulin G antibodies to T. gondii were examined with enzyme-linked immune assay using T. gondii Human Kit of DIA. PRO Italian Company and Abcam Company sheep serum conjugate. Negative control was obtained from a newborn sheep. After the study protocol had been approved by the Local Ethical Committee, the sheep was infected by two steps injection of live tachyzoites intramuscular and subdermal for obtaining positive control sample.
The optical density (OD) was read with a spectrophotometer (MULTISKAN MCC/340 P VERSION 2.33) at 492 nm. The absorbance average of each serum tested in duplicate was divided by the cut off (mean absorbance of negative serum samples plus three standard deviations) to determine the reactivity index (RI). Serum with RI 1 was considered positive.
Molecular examination
For extraction of DNA, the tissue samples were homogenized and DNA was extracted using phenol-chloroform and Proteinase K. The extracted DNA was stored at −20°C until use. Two polymerase chain reaction (PCR) primer pairs of the B1 gene were used has been showed on Table-1. These primers amplifying a 193 bp bond at the initial phase and a 96 bp fragment at the second round of nested-PCR.
Table-1.
Position and sequences of the primer pairs used in nestedPCR.
| Oligonucleotide primer | Sequence | Sequence position |
|---|---|---|
| Outer primer (sense strand) | 5’GGAACTGCATCCGTTCATGAG3’ | 694-714 |
| Outer primer (nonsense strand) | 5’TCTTTAAAGCGTTCGTGGTC3’ | 887-868 |
| Inner primer (sense strand) | 5’TGCATAGGTTGCAGTCACTG3’ | 757-776 |
| Inner primer (nonsense strand) | 5’GGCGACCAATCTGCGAATACACC3’ | 853-831 |
PCR: Polymerase chain reaction
The first amplification was performed in 20 µl of PCR-PreMix (Bioneer Company, South Korea) reaction mixture and 1 µl of each primer and 2.5 µl of extracted DNA (5-50 nanogram). The PCR condition was 93°C for 10 min, followed by 40 cycles of 93°C for 10 s of denaturation, 57°C for 10 s of annealing and 72°C for 30 s of extension, and the last extension step at 72°C for 5 min.
The second amplification was carried out in the same volumes as the first reaction with the 1 µl of the first round product as template and 1 µl of each inner primer. The PCR condition was 93°C for 10 min, followed by 40 cycles of 93°C for 10 s of denaturation, 62.5°C for 10 s of annealing and 72°C for 15 s of extension, and the last extension step at 72°C for 5 min.
Each amplification run contained two negative controls (doubly distilled water and negative control of DNA extraction) and one positive control (DNA extracted from RH T. gondii tachyzoite). The PCR products were analyzed by electrophoresis in a 2% agarose gel stained with ethidium bromide (0.5 mg/ml). The DNA fragments were visualized under ultraviolet illumination.
Statistical analysis
The Chi-square test was used to clarify whether sex, age, or season of sampling was associated with the prevalence rate of T. gondii in sheep. The results were analyzed by SPSS software (version 13) and a p<0.05 was considered as significant positive correlation.
Results
The number of 370 sheep (279 female and 91 male) were examined using both serological and molecular methods. The sampling was done during two seasons of spring (193 sheep) and summer (177 sheep). The animals were categorized in five age groups including 0-12 months (122), 12-24 months (70), 24-36 months (125), 36-48 months (34), and 48-60 months (19).
Serological findings
In this study, the samples with higher than 170 OD were considered positive. Anti-Toxoplasma antibodies were detected in sera of 133 out of 370 (35.94%) animals. Considering the sex of animals, however the females (100 cases) were more seropositive than males (33 cases) for Toxoplasma, but the differences were not statistically significant (p=0.942). Moreover, the maximum and minimum frequency of positive samples were observed in 24-36 months (42.40%) and 48-60 months (31.58%) age groups, respectively, however no significant correlation was found between age and seropositivity to toxoplasmosis (p=0.470). In addition, 76 positive samples had been taken in spring and 57 in summer, but statistical analysis was indicated no correlation between season and the seropositivity (p=0.151). Serological findings are shown in Table-2 in detail.
Table-2.
Distribution of T. gondii infection in sheep based on serological and molecular results in correlation to sex, age group and season of sampling.
| Category | Number of animal examined | Number of ELISA positive (%) | Number of nested-PCR positive (%) |
|---|---|---|---|
| Season | |||
| Spring | 193 | 76 (39.37) | 82 (42.48) |
| Summer | 177 | 57 (32.20) | 45 (25.42) |
| Sex | |||
| Male | 91 | 33 (36.26) | 31 (34.06) |
| Female | 279 | 100 (35.84) | 96 (34.40) |
| Age group | |||
| 0-12 | 122 | 39 (31.96) | 25 (20.49) |
| 12-24 | 70 | 23 (32.85) | 25 (35.71) |
| 24-36 | 125 | 53 (42.40) | 57 (45.60) |
| 36-48 | 34 | 12 (35.29) | 13 (38.23) |
| 48-60 | 19 | 6 (31.58) | 7 (36.84) |
| Total | 370 | 133 (35.94) | 127 (34.32) |
T. gondii: Toxoplasma gondii, ELISA: Enzyme-linked immunosorbent assay, PCR: Polymerase chain reaction
Molecular findings
A nested-PCR assay was done for tissue (heart and diaphragm) samples taken from 370 sheep to amplify the B1 gene. In general, 127 cases (34.32%) showed a 193 bp bond at initial step and a 96 bp bond at the second round of nested-PCR assay (Figures-1 and 2). All the positive samples had been identified positive based on serological results. The statistical difference between the prevalence rates of Toxoplasma infection among females (96 cases) and males (31 cases) was not significant (p=0.886). Furthermore, no positive correlation was found between the age of animals and the rate of infection, in which the highest and lowest prevalence rate were observed in 3 years old age group and 1 year old age group, respectively (p=0.055). Although 82 cases of positive samples had been collected in spring and 45 of them had been taken in summer, there was no positive relation observed between the rate of prevalence and season of sampling (p=0.138). Molecular findings are shown in Table-2 in detail.
Figure-1.
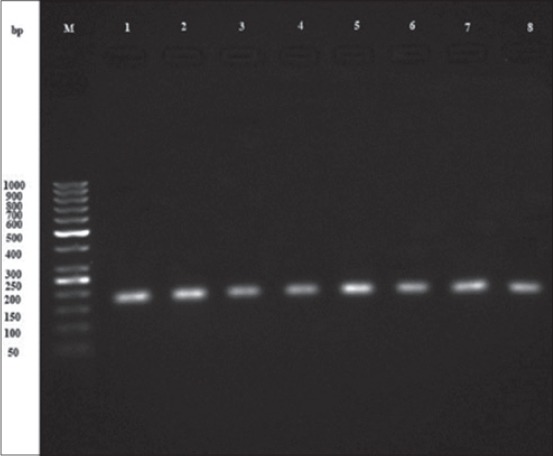
Electrophoresis result from primary step product of B1 gene nested-polymerase chain reaction products: M-100 bp marker, 1 - Positive control, 2-9: 193 bp bond of positive samples.
Figure-2.
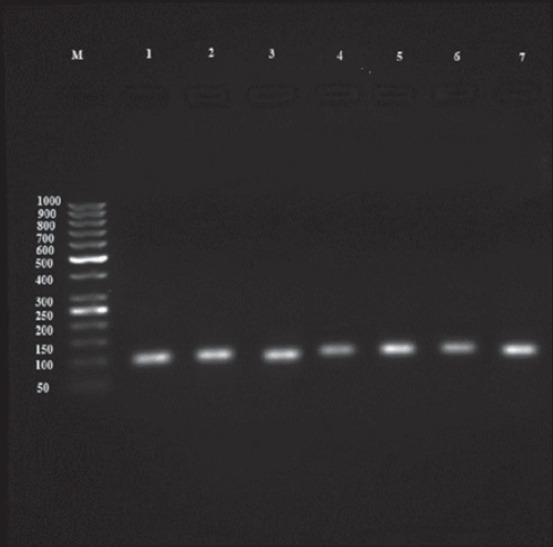
Electrophoresis result from secondary step product of B1 gene nested- Polymerase chain reaction: M-50 bp marker, 1- Positive control, 2-9: 96 bp bond of positive samples.
Discussion
Nowadays, toxoplasmosis is considered as one of the most important food-borne diseases. Humans are getting the disease by consuming infected meat of livestock including sheep [19]. The results of this study showed that 35.94% of sheep have anti T. gondii antibody in their sera. In addition, 34.32% of animals’ tissue contains T. gondii tissue cyst. Toxoplasmosis prevalence rate in the various zones of the world is variable, with ranging from 0% to 100% in different countries [20,21]. The differences observed could be due to diverse husbandry practices, lifestyles of the residents, traditions and the climatic variations from one region to another, which are known as essential elements in epidemiological investigations [22].
Our result is one of the highest rates which have been reported so far in different parts of Iran. Our findings were significantly more than those who reported in Kerman 3.3% seropositivity in sheep [23]. This could be due to the differences of weather in the two areas. Kerman is a desert zone, while Jahrom with the vast citrus gardens looks more like a forest zone rather than a poor average annual rainfall area. In other aspect, cats and other felids play an important role in preserving and spreading of T. gondii in hosts such as livestock (e.g., sheep) because they are considered as the major source of the oocysts that contaminate the environment and after sporulation become infectious to man and animals [22]. Humidity and temperate condition favor the oocyst survival. To that effect, Fayer has indicated that the toxoplasmosis prevalence rate is elevated in humid and hot zones compared to dry regions and this gap is more probably due to the high viability of the oocysts in these environments [24].
In addition, it seems that the prevalence of T. gondii in sheep in the Jahrom district was slightly more than other parts of Iran such as Uroomye [25], Shahre-Kord [26], and Kurdistan [27] provinces, in the west and the mountainous part of Iran. In the mentioned regions, there were reports of the seropositivity of 21.1%, 29.1%, and 21.74%, respectively. Moreover, the average seroprevalence in Iran which was reported by Hashemi [28] about 24.5% is distinctly lower than our finding in this area. In comparison with other parts of Fars province, again our results showed a little difference, in which Asgari observed that 29.5% of sheep in northern part of Fars province hold anti-Toxoplasma antibody in the sera [29].
More interestingly, Sharifi reported 35% sheep toxoplasmosis in three different regions, north of Iran, which is the most humid and forest part of the country. That was clearly similar to the findings of the present study. Sharifi, also, observed that seropositivity was higher in the western parts of the province where animals had been exposed to an environment contaminated with greater numbers of T. gondii oocysts, as a result of differences in the levels of humidity in these three areas [14]. Other studies across the world have confirmed this fact. In this regard, van der Puije in his study has indicated that the sheep toxoplasmosis varies from 20% in a dry region to 39% in forest areas [30]. It is worth mentioning that our findings were distinctly less than Greece, a Mediterranean country, where 48.6% of sheep were categorized seropositive [31]. Other studies in Ghana [30] and Portugal [32] which showed the same seroprevalence range as our study, around 33.2% and 33.6% infection, respectively.
To the best of this study, all the samples were subjected to a molecular survey to clarify the existence of parasite in the animals’ tissue. The major purpose of this step was to determine whether T. gondii was present as a contaminant of human foodstuffs, and if so, the level of contamination present. Our findings show a high rate of toxoplasmosis infection in sheep tissues. Detecting this high level of contaminant in a small part of animal tissue (5 g) poses an alarming public health risk because it means that considerable numbers of farm animals currently contributing to the food chain of people carry T. gondii.
Our finding demonstrates the presence of T. gondii DNA within the samples, regardless of the viability of parasites which might initiate a human infection. However, it seems highly probable that a significant portion of the parasites observed in the present study would have been viable. Because, it is well proved that T. gondii tissue cysts are considerably strong and remaining viable for weeks at temperatures of 1-4°C, and temperatures above 67°C or below −12°C are needed to see a significant loss of viability [33,34].
In other aspects, consuming the barbequed meat (kebab) and the processed meat products such as sausages have been dramatically increased in the recent years; which it clearly represents a significant risk of infection.
The results of this study showed that 33 out of 91 samples of male and 100 out of 279 samples of female animals were seropositive, but there was no statistical correlation between gender of sheep and the rate of seropositivity. This was in contrary to those who found a significant correlation between ewes and lambs [35]; whereas it was similar to the studies done in Iran and other parts of the world [26,29,31]. Some studies indicated a connection between the age of animals and the rate of Toxoplasma infection [11,14]; however, such connection was not observed in the current study like Asgari who did not find a statistical correlation in this kind [29]. In general, the prevalence of T. gondii varies with the methods of testing and cut-off values. It seems that the sensitivity and specificity of different tests are different that they might have some effects on results [36].
Conclusion
Regarding the foregone discussion, T. gondii is widely distributed in sheep in this region with a rate comparable with other parts of Iran and world. Obviously, the consumption of their undercooked or raw meat presents a risk of transmission of the parasite. This might be considered as an important public health problem, mainly for high-risk groups such as the pregnant and the immunodeficient. Likewise, other meats from other kinds of animals are currently used in Jahrom. It seems highly important to identify the other probable sources of infection to have a better vision to the role of meat diet in human infection with T. gondii and also to define the zoonotic aspect of this parasite.
Authors’ Contributions
BA and KS have designed the concept and supervised the plan of work and also have prepared the manuscript. MS and MSK have contributed in sample collection, administrative, technical, and material support. KS and MHD have analyzed and interpreted the data. All authors have read and approved the final manuscript.
Acknowledgment
The authors would like to thank the personnel of Jahrom main slaughterhouse for their kind assistance. This study was financially (grant number: 1389/08/19-3) supported by Zoonosis Research Center of Jahrom University of Medical Sciences, Iran.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Bowman D.D, Hendrix C.M, Lindsay D.S, Barr S.C. Feline Clinical Parasitology. Chichester, UK: John Wiley & Sons; 2008. [Google Scholar]
- 2.Webster J.P. Dubey J.P, editor. Review of Toxoplasmosis of Animals and Humans 2010. Parasit. Vectors. (Second Edition) 2010;3:112. [Google Scholar]
- 3.Bossi P, Bricaire F. Severe acute disseminated toxoplasmosis. Lancet. 2004;364(9434):579. doi: 10.1016/S0140-6736(04)16841-4. [DOI] [PubMed] [Google Scholar]
- 4.Pereira-Chioccola V.L, Vidal J.E, Su C. Toxoplasma gondii infection and cerebral toxoplasmosis in HIV-infected patients. Future Microbiol. 2009;4(10):1363–1379. doi: 10.2217/fmb.09.89. [DOI] [PubMed] [Google Scholar]
- 5.Lundén A, Uggla A. Infectivity of Toxoplasma gondii in mutton following curing, smoking, freezing or microwave cooking. Int. J. Food Microbiol. 1992;15(3):357–363. doi: 10.1016/0168-1605(92)90069-f. [DOI] [PubMed] [Google Scholar]
- 6.Dubey J.P. Toxoplasmosis in sheep-the last 20 years. Vet. Parasitol. 2009;163(1):1–14. doi: 10.1016/j.vetpar.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo K, Kamai R, Uetsu H, Goto H, Takashima Y, Nagamune K. Seroprevalence of Toxoplasma gondii infection in cattle, horses, pigs and chickens in Japan. Parasitol. Int. 2014;63(4):638–639. doi: 10.1016/j.parint.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Hill D.E, Dubey J.P. Toxoplasma gondii prevalence in farm animals in the United States. Int. J. Parasitol. 2013;43(2):107–113. doi: 10.1016/j.ijpara.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Dehkordi F.S, Haghighi Borujeni M.R, Rahimi E, Abdizadeh R. Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran. Pathog. Dis. 2013;10(2):120–125. doi: 10.1089/fpd.2012.1311. [DOI] [PubMed] [Google Scholar]
- 10.Asgari Q, Sarnevesht J, Kalantari M, Sadat S.J.A, Motazedian M.H, Sarkari B. Molecular survey of toxoplasma infection in sheep and goat from Fars province, Southern Iran. Trop. Anim. Health Prod. 2011;43(2):389–392. doi: 10.1007/s11250-010-9704-1. [DOI] [PubMed] [Google Scholar]
- 11.Habibi G.R, Imani A.R, Gholami M.R, Hablolvarid M.H, Behroozikhah A.M, Lotfi M, Bozorgi S. Detection and identification of Toxoplasma gondii type one infection in sheep aborted fetuses in Qazvin Province of Iran. J. Parasitol. 2012;7(3):64–72. [PMC free article] [PubMed] [Google Scholar]
- 12.Sharif M, Daryani A, Ebrahimnejad Z, Gholami S, Ahmadpour E, Borhani S, Lamsechi N. Seroprevalence of anti-toxoplasma IgG and IgM among individuals who were referred to medical laboratories in Mazandaran Province, Northern Iran. J. Infect. Public Health. 2016;9(1):75–80. doi: 10.1016/j.jiph.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Navidpour S, Hoghooghi-Rad N. Seroprevalence of anti-Toxoplasma gondii antibodies in buffaloes in Khoozestan Province, Iran. Vet. Parasitol. 1998;77(2):191–194. doi: 10.1016/s0304-4017(97)00148-9. [DOI] [PubMed] [Google Scholar]
- 14.Sharif M, Gholami S.H, Ziaei H, Daryani A, Laktarashi B, Ziapour S.P, Vahedi M. Seroprevalence of Toxoplasma gondii in cattle, sheep and goats slaughtered for food in Mazandaran Province, Iran, during 2005. Vet. J. 2007;174(2):422–424. doi: 10.1016/j.tvjl.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Ghazaei C. Serological survey of antibodies to Toxoplasma gondii. Afr. J. Health Sci. 2006;12(3):114–117. [PubMed] [Google Scholar]
- 16.Assmar M, Amirkhani A, Piazak N, Hovanesian A, Kooloobandi A, Etessami R. Toxoplasmosis in Iran. Results of a seroepidemiological study. Bull. Soc. Pathol. Exot. 1996;90(1):19–21. [PubMed] [Google Scholar]
- 17.Asgari Q, Moazzeni M, Mohajeri F.A, Kalantari M, Zarifi M, Ghalebi S.R, Mehrabani D. Seroprevalence of Toxoplasma gondii among caprines in Fars province, Southern Iran. J Vet Parasitol. 2007;21(2):153–155. [Google Scholar]
- 18.Azizi H, Shiran B, Boroujeni A.B, Jafari M. Molecular survey of Toxoplasma gondii in sheep, cattle and meat products in Chaharmahal va Bakhtiari Province, Southwest of Iran. Iran. J. Parasitol. 2014;9(3):429–434. [PMC free article] [PubMed] [Google Scholar]
- 19.Santos S.L, de Souza Costa K, Gondim L.Q, da Silva M.S.A, Uzêda R.S, Abe-Sandes K, Gondim L.F.P. Investigation of Neospora caninum Hammondia sp. and Toxoplasma gondii in tissues from slaughtered beef cattle in Bahia, Brazil. Parasitol. Res. 2010;106(2):457–461. doi: 10.1007/s00436-009-1686-4. [DOI] [PubMed] [Google Scholar]
- 20.Olivier A, Herbert B, Sava B, Pierre C, John D.C, Aline D.K. Surveillance and monitoring of toxoplasma in humans, food and animals: A scientific opinion of the panel on biological hazards. EFSA. J. 2007;583:1–64. [Google Scholar]
- 21.Tenter A.M, Heckeroth A.R, Weiss L.M. Toxoplasma gondii: From animals to humans. Int J. Parasitol. 2000;30(12):1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmore S.A, Jones J.L, Conrad P.A, Patton S, Lindsay D.S, Dubey J.P. Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends. Parasitol. 2010;26(4):190–196. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Derakhshan M, Mousavi M. Serological survey of antibodies to Toxoplasma gondii in cats, goats, and sheep in Kerman, Iran. Comp. Clin. Path. 2014;23(2):267–268. [Google Scholar]
- 24.Fayer R. Toxoplasmosis update and public health implications. Can Vet. J. 1981;22(11):344–352. [PMC free article] [PubMed] [Google Scholar]
- 25.Raeghi S, Akaberi A, Sedeghi S. Seroprevalence of Toxoplasma gondii in sheep, cattle and horses in Urmia North-West of Iran. Iran J Parasitol. 2011;6(4):90–94. [PMC free article] [PubMed] [Google Scholar]
- 26.Bonyadian M, Hematzade F, Manuchehri K. Seroprevalence of antibodies to Toxoplasma gondii in sheep in center of Iran. Pak. J. Biol. Sci. 2007;10(18):3228–3230. doi: 10.3923/pjbs.2007.3228.3230. [DOI] [PubMed] [Google Scholar]
- 27.Khezri M, Mohammadian B, Esmailnia K, Khezri O. Toxoplasmosis in sheep from Kurdistan Province, Iran. Afr. J. Microbiol. Res. 2012;6(18):3989–3992. [Google Scholar]
- 28.Hashemi-Fesharki R. Seroprevalence of Toxoplasma gondii in cattle, sheep and goats in Iran. Vet. Parasitol. 1996;61(1):1–3. doi: 10.1016/0304-4017(95)00818-7. [DOI] [PubMed] [Google Scholar]
- 29.Asgari Q, Sarkari B, Amerinia M, Panahi S, Mohammadpour I, Sarvestani A.S. Toxoplasma infection in farm animals: A seroepidemiological survey in Fars province, South of Iran. Jundishapur. J. Microbiol. 2013;6(3):269–272. [Google Scholar]
- 30.Van der Puije W.N.A, Bosompem K.M, Canacoo E.A, Wastling J.M, Akanmori B.D. The prevalence of anti -Toxoplasma gondii antibodies in Ghanaian sheep and goats. Acta Trop. 2000;76(1):21–26. doi: 10.1016/s0001-706x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 31.Tzanidakis N, Maksimov P, Conraths F.J, Kiossis E, Brozos C, Sotiraki S, Schares G. Toxoplasma gondii in sheep and goats: Seroprevalence and potential risk factors under dairy husbandry practices. Vet. Parasitol. 2012;190(3):340–348. doi: 10.1016/j.vetpar.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Lopes A.P, Dubey J.P, Neto F, Rodrigues A, Martins T, Rodrigues M, Cardoso L. Seroprevalence of Toxoplasma gondii infection in cattle, sheep, goats and pigs from the North of Portugal for human consumption. Vet. Parasitol. 2013;193(1):266–269. doi: 10.1016/j.vetpar.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Kotula A.W, Dubey J.P, Sharar A.K, Andrews C.D, Shen S.K, Lindsay D.S. Effect of freezing on infectivity of Toxoplasma gondii tissue cysts in pork. J Food Prot. 1991;54(9):687–690. doi: 10.4315/0362-028X-54.9.687. [DOI] [PubMed] [Google Scholar]
- 34.Dubey J.P, Kotula A.W, Sharar A, Andrews C.D, Lindsay D.S. Effect of high temperature on infectivity of Toxoplasma gondii tissue cysts in pork. The J. Parasitol. 1990;76(2):201–204. [PubMed] [Google Scholar]
- 35.Dumètre A, Ajzenberg D, Rozette L, Mercier A, Dardé M.L. Toxoplasma gondii infection in sheep from Haute-Vienne, France: Seroprevalence and isolate genotyping by microsatellite analysis. Vet. Parasitol. 2006;142(3):376–379. doi: 10.1016/j.vetpar.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Shaapan R.M, El-Nawawi F.A, Tawfik M.A.A. Sensitivity and specificity of various serological tests for the detection of Toxoplasma gondii infection in naturally infected sheep. Vet. Parasitol. 2008;153(3):359–362. doi: 10.1016/j.vetpar.2008.02.016. [DOI] [PubMed] [Google Scholar]


