Abstract
Objective. Systematic review and meta-analysis to observe the efficacy and safety of stem cell transplantation therapy in patients with brain ischemia. Methods. We searched Cochrane Library, PubMed, Ovid, CBM, CNKI, WanFang, and VIP Data from its inception to December 2015, to collect randomized controlled trials (RCT) of stem cell transplantation for the ischemic stroke. Two authors independently screened the literature according to the inclusion and exclusion criteria, extracted data, and assessed the risk of bias. Thereafter, meta-analysis was performed. Results. Sixteen studies and eighteen independent treatments were included in the current meta-analysis. The results based upon the pooled mean difference from baseline to follow-up points showed that the stem cell transplantation group was superior to the control group with statistical significance in the neurologic deficits score (NIHSS, MD = 1.57; 95% CI, 0.64–2.51; I 2 = 57%; p = 0.001), motor function (FMA, MD = 4.23; 95% CI, 3.08–5.38; I 2 = 0%; p < 0.00001), daily life ability (Barthel, MD = 8.37; 95% CI, 4.83–11.91; I 2 = 63%; p < 0.00001), and functional independence (FIM, MD = 8.89; 95% CI, 4.70–13.08; I 2 = 79%; p < 0.0001). Conclusions. It is suggested that the stem cell transplantation therapy for patients with brain ischemic stroke can significantly improve the neurological deficits and daily life quality, with no serious adverse events. However, higher quality and larger data studies are required for further investigation to support clinical application of stem cell transplantation.
1. Introduction
Ischemic stroke, also known as cerebrovascular accident, is caused by the decreased or interrupted blood supply in the part of the brain. It is of the dominant diseases affecting human health and causing the most mortalities, together with coronary heart disease and cancer [1]. There are more than fifty million people suffering with varying degrees of ischemic stroke in the world; the rate of deaths was close to 10% each year; meanwhile most survivors were disabled, and not only did it affect patients physically, but also there are huge impacts economically and to spirit of patients and their families [1, 2]. The main pathological manifestation of ischemic stroke is the ischemic brain tissue (hypoxia/necrosis) for a short time, and it results in reduced number of neurons, interrupted neural axon network, and formation of local free oxygen radical species, leading to harsh environments in the peripheries of ischemic region, damaging brain self-healing, and eventually resulting in the permanent loss of nerve tissue or disabling of brain function. Currently there is no efficient therapy after stroke, except tPA (tissue plasminogen activator) which is the only efficient treatment drug in clinical settings [3]. However, it plays a major role in the early stage of ischemia, which has a very short time window (4.5 hours, and less than 6 hours) and can increase the risk of cerebral hemorrhage. In addition, only approximately 5% of stroke patients can receive this treatment in the United States [4].
In recent decades, with the rapid development of stem cell research, many types of stem cells (including adult stem cells) give rise to more and more attentions in clinic and showed that transplanted stem cells could promote therapies for ischemic stroke [5, 6]. Stem cells have high self-replication and self-differentiation potentials and can differentiate into many types of cells, such as neural stem cells (NSC) which may further differentiate into neurons, astrocytes, and oligodendrocytes, and so forth [7], with lower immunogenicity and better histocompatibility. It has been considered as good tools and the most promising natural resources for the treatment of brain stroke [8]. Although many studies regarding stem cells transplantation therapies have been carried out in experimental models or preclinically, because of safety, ethical, and therapeutic effect issues, there is still a long distance for clinical application of stem cells.
Therefore, the purpose of the current study is to systematically review and evaluate the potential efficacy and safety of stem cell transplantation therapy for patients with ischemic brain stroke. We collected the randomized controlled trials (RCT) of stem cell transplantation for ischemic stroke in recent 20 years and used systematical review and meta-analysis approaches to identify the possible publication bias and to investigate the impact of various aspects of reporting outcomes in clinical ischemic stroke studies.
2. Methods
2.1. Search Strategy
We searched the literatures in Cochrane Library, PubMed, Ovid, CBM, CNKI, WanFang, and VIP Data from its inception to December 2015, to collect randomized controlled trials (RCT) of stem cell transplantation for ischemic stroke. Published languages contain English and Chinese. The search words contain stem cell, transplantation, brain ischemia, and cerebrovascular stroke, and the details of search strategy are described in the following list (take PubMed, for instance):
-
#1 brain ischemia OR cerebrovascular stroke OR ischemic stroke.
-
#2 stem cell OR stem cell transplantation.
-
#3 transplantation.
-
#4 #1 AND #2 AND #3 (filters: randomized controlled trial; limited: humans).
2.2. Inclusion and Exclusion Criteria
Intervention measurements contain all types of stem cells, such as neural stem cell (NSC), bone marrow mesenchymal stem cell (MSC), and umbilical cord mesenchymal stem cell (UC-MSC). Research subjects (ischemic stroke patients) must meet WHO diagnostic criteria of brain stroke and exclude cerebral hemorrhage by brain CT or MRI. Each group must have more than 5 patients. The outcomes of treatment from baseline to follow-up points (or before/after treatment) should contain National Institutes of Health Stroke Scale (NIHSS), Fugl-Meyer Assessment (FMA), Barthel index, or Functional Independence Measure (FIM). We also excluded literature reviews, meta-analysis, meeting abstracts, case reports, repeated studies, experimental model researches, and other diseases researches.
2.3. Data Extraction
Two authors independently screened the literatures according to the inclusion and exclusion criteria, extracted data, and assessed the risk of bias. We extracted these data including authors, year of publication, patients' age, gender, time/type of cerebral ischemia, number of patients (treatment group/control group), type of SCs (stem cells) intervention, intervention dose, time of administration, route of delivery, and outcome assessments, mean, SD (standard deviation) or SE (standard error), and adverse effects. We defined the treatment comparison as the outcomes in the control group compared to the treated group. If more than one intervention was given in one study, we regarded these to be another independent intervention. Furthermore, if the treatment was administered in multiple doses, we considered the sum of all doses administered. Finally, if the functional outcomes were reported for >1 time points, we only included the last time of assessment. We used the manual of Cochrane systematic review (5.1.0 RCT bias risk assessment tools) to evaluate the bias risk of all included studies.
2.4. Data Analysis/Statistical Analysis
The pooled outcome difference of stem cell transplantation in the treatment of ischemic stroke between each treatment group and the control group used the quantitative data of mean difference (MD) or standardized mean difference (SMD) and 95% confidence interval (CI) to meta-analysis (RevMan 5.3 software). Heterogeneity between the results of included studies was analyzed using χ 2 test (significance level α = 0.05) and quantitatively determined the size of heterogeneity by combining with I 2. If no significant heterogeneity or little heterogeneity between studies was found, the fixed effects model was then used. If the large statistical heterogeneity between studies was observed, the random effects model was then used after excluding the significant clinical heterogeneity. When the heterogeneity was obvious, the subgroup analysis or sensitivity analysis was used, or just descriptive analysis. Finally, we used the funnel plot analysis to evaluate the publication bias.
3. Results
3.1. Study Characteristics
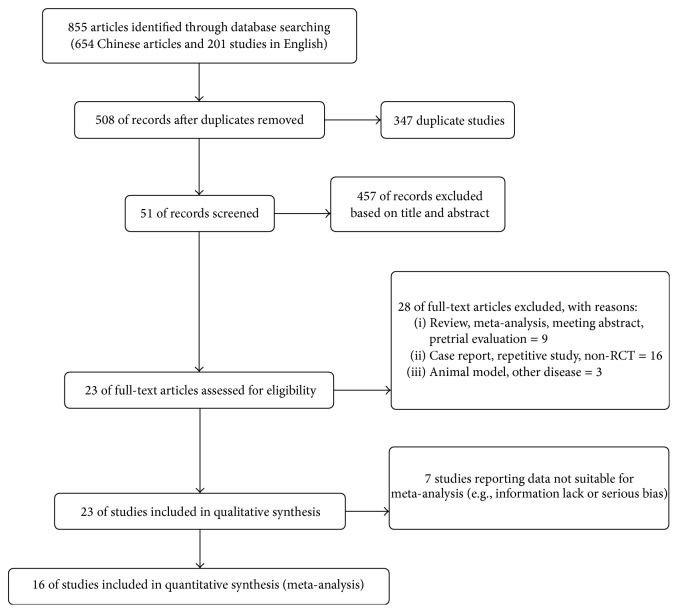
We identified a total of 23 studies from 855 publications (654 Chinese articles and 201 studies in English) for this systematic review. The number of patients is 680 in the stem cell treatment group and 694 in the control group, respectively. However, there are seven researches which were excluded due to lack of information or serious bias, and finally 16 studies (18 independent interventions) were conducted in the meta-analysis (Figure 1).
Figure 1.
Flow diagram showing summary of study selection procedure.
There were four interventions with NSC transplantation, one with UC-MSC, one with PBSC, and twelve combined with MSC transplantation. There were 6 out of 18 interventions which were performed in acute cerebral ischemia, 7 were done in chronic cerebral ischemic states, and time of treatment was not mentioned in remaining 5 interventions. Furthermore, there were 5 of 18 treatments which were injected in subarachnoid space, 1 through carotid artery, 1 by intracerebral transplantation, and 11 interventions administrated intravenously. The duration of follow-up varied from 1 month to 12 months (Table 1).
Table 1.
Study characteristic report.
| Author | Year of publish | Numbers (SCs/Con) |
Age | Type of stroke Acute/chronic |
Gender Male/female |
Type of interventions | Cells dose ×106 |
Route of injection | Follow-up (month) |
Outcome effect indicators | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stem cell | Control | ||||||||||
| Bang et al. [9] | 2005 | 5/25 | 63.0 ± 7.5 | Acute | 18/12 | MSC | Routine | 100 | Intravenous | 12 | Barthel |
| Sun et al. [10] | 2008 | 20/22 | 57.8 ± 8.9 | X | 32/10 | MSC | Routine | X | Intravenous | 3 | NIHSS |
| Niu et al. [11] | 2010 | 16/20 | 56 ± 7 | Chronic | 22/14 | NSC | Routine | X | Subarachnoid | 6 | FIM |
| Bhasin et al. [12] | 2011 | 6/6 | 42 | Chronic | 8/4 | MSC | Routine | 50–60 | Intravenous | 6 | FMA, Barthel |
| Deng et al. [13] | 2012 | 15/15 | X | Acute | X | MSC | Routine | 10–50 | Intravenous | 1 | NIHSS |
| He [14] | 2012 | 20/18 | 56.4 ± 7.9 | X | 23/15 | MSC | Routine | 100 | Intravenous | 3 | NIHSS |
| Bhasin et al. [15] | 2012 | 12/12 | 46.5 | Chronic | X | MNC | Routine | 50–60 | Intravenous | 6 | FMA, Barthel |
| Xiao [16] | 2013 | 78/82 | 56.9 ± 9.2 | X | 103/57 | NSC | Routine | 8000 | Subarachnoid | X | FIM |
| Bhasin et al. [17] | 2013 | 14/20 | 45.1 ± 12.1 | Chronic | 32/8 | MNC | Routine | 50–60 | Intravenous | 6 | FMA, Barthel |
| 6/20 | 45.1 ± 12.1 | Chronic | 32/8 | MSC | Routine | 50–60 | Intravenous | 6 | FMA, Barthel | ||
| Liu et al. [18] | 2014 | 29/29 | 55.3 ± 3.6 | Acute | 38/20 | MSC | Routine | 100 | Subarachnoid | 3 | NIHSS, Barthel, FMA |
| Xie et al. [19] | 2014 | 30/30 | 51.4 ± 7.2 | Acute | 37/23 | MSC | Routine | X | Subarachnoid | 6 | NIHSS, Barthel |
| Chen et al. [20] | 2014 | 15/15 | 50.1 ± 7.7 | Chronic | 20/10 | PBSC | Routine | 3–8 | Stereotactic | 12 | NIHSS |
| Prasad et al. [21] | 2014 | 60/60 | 50.7 ± 11.6 | Acute | 77/43 | MSC | Routine | 280.75 | Intravenous | 12 | NIHSS, Barthel |
| Zhang et al. [22] | 2015 | 14/28 | 57.2 ± 7.3 | X | 34/26 | NSC | Routine | 40 | Intravenous | 6 | FIM |
| 18/28 | 56.9 ± 7.5 | X | 34/26 | NSC | Routine | 40 | Subarachnoid | 6 | FIM | ||
| Shen [23] | 2015 | 16/16 | 52 ± 10.4 | Acute | X | UC-MSC | Routine | 1000 | Intra-carotid | 3 | FIM |
| Cai et al. [24] | 2015 | 21/21 | 61.4 ± 6.7 | Chronic | 27/15 | MSC | Routine | 150–600 | Intravenous | 6 | FIM, FMA, Barthel |
MSC: mesenchymal stem cell; NSC: neural stem cell; MNC: bone marrow mononuclear stem cells; PBSC: peripheral blood stem cell; UC-MSC: umbilical cord blood mesenchymal stem cell; X: unknown.
3.2. Quality Assessment and Bias Risk
Most studies had a certain risk of bias, especially the Chinese articles in which the trial design was not rigorous and had more defects in the blinding and data's integrity. The English studies have less bias risk than Chinese articles due to the trial design which followed the RCT policy (Table 2).
Table 2.
Study quality or risk of bias report.
| Author | Year | Random allocation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Complete outcome data | Nonselective reporting | No other bias risk |
|---|---|---|---|---|---|---|---|---|
| Bang et al. [9] | 2005 | √ | √ | √ | √ | √ | √ | √ |
| Sun et al. [10] | 2008 | √ | Χ | Χ | Χ | Χ | Χ | Χ |
| Niu et al. [11] | 2010 | √ | Χ | Χ | Χ | Χ | Χ | Χ |
| Bhasin et al. [12] | 2011 | Χ | Χ | Χ | √ | √ | √ | √ |
| Deng et al. [13] | 2012 | Χ | Χ | Χ | Χ | Χ | Χ | Χ |
| He [14] | 2012 | √ | Χ | Χ | Χ | Χ | Χ | Χ |
| Bhasin et al. [15] | 2012 | Χ | Χ | Χ | √ | √ | √ | √ |
| Xiao [16] | 2013 | √ | Χ | Χ | Χ | Χ | Χ | Χ |
| Bhasin et al. [17] | 2013 | Χ | Χ | Χ | √ | √ | √ | √ |
| Liu et al. [18] | 2014 | √ | Χ | Χ | Χ | Χ | Χ | Χ |
| Xie et al. [19] | 2014 | √ | Χ | Χ | Χ | Χ | Χ | Χ |
| Chen et al. [20] | 2014 | √ | √ | √ | √ | √ | √ | √ |
| Prasad et al. [21] | 2014 | √ | √ | √ | √ | √ | √ | Χ |
| Zhang et al. [22] | 2015 | √ | Χ | Χ | Χ | Χ | Χ | Χ |
| Shen [23] | 2015 | √ | Χ | Χ | Χ | Χ | Χ | Χ |
| Cai et al. [24] | 2015 | √ | √ | Χ | Χ | Χ | Χ | Χ |
3.3. Meta-Analysis and Effect Evaluation
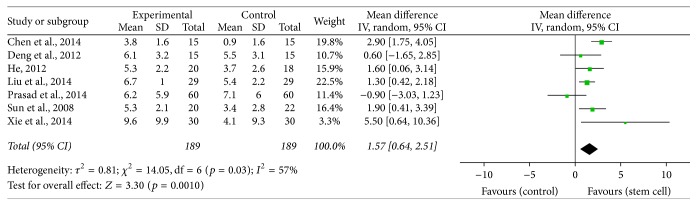
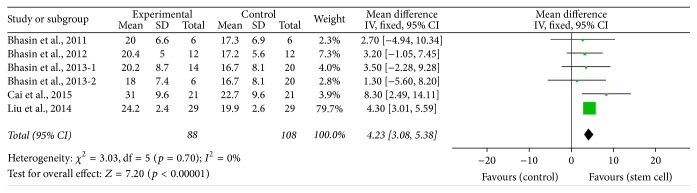
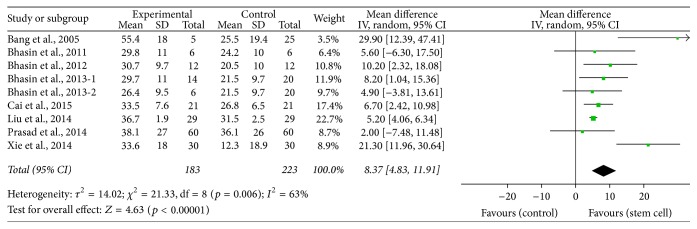
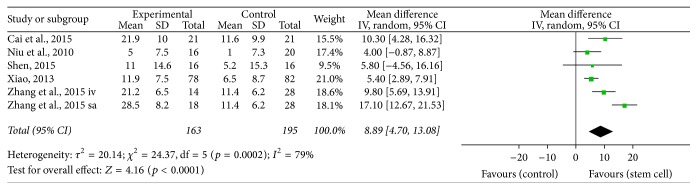
We performed the pooled data for meta-analysis and found that 7 interventions reported NIHSS improvement [10, 13, 14, 18–21], 6 interventions determined FMA functional optimization [12, 15, 17, 18, 24], 9 treatments pointed out Barthel index enhancement [9, 12, 15, 17–19, 21, 24], and 6 treatment groups showed FIM functional improvement [11, 16, 22–24]. The results of meta-analysis were as follows: NIHSS (MD = 1.57, 95% CI 0.64–2.51, I 2 = 57%, p = 0.001, Figure 2), FMA (MD = 4.23, 95% CI 3.08–5.38, I 2 = 0%, p < 0.00001, Figure 3), Barthel (MD = 8.37, 95% CI 4.83–11.91, I 2 = 63%, p < 0.00001, Figure 4), and FIM (MD = 8.89, 95% CI 4.70–13.08, I 2 = 79%, p < 0.0001, Figure 5). All the outcomes after the merger were in favor of stem cell transplantation group, and each of the differences was statistically significant. Only four studies reported adverse effects, which included mild fever or headache and these were self-relieved within a short period.
Figure 2.
The effect size of NIHSS improvement across studies by meta-analysis.
Figure 3.
The effect size of improved FMA across studies by meta-analysis.
Figure 4.
The effect size of Barthel index improvement across studies by meta-analysis.
Figure 5.
The effect size of improved FIM across studies by meta-analysis.
Then we performed subgroup analysis for further evaluation of the clinical efficacy based on several clinical variables, and we focused on four clinical variables: time/type of cerebral ischemia, type of SCs interventions, route of delivery, and follow-up period. Each clinical variable was analyzed by interacting with NIHSS or Barthel index which were reported consistently, including evaluation of subgroup effects and heterogeneity analysis (Tables 3 and 4), and the results were in favor of the nonvein injected group (e.g., subarachnoid space and carotid artery administration) and long-time follow-up period (more than 6 months). However, when the time and type of cerebral ischemia were analyzed, NIHSS and Barthel index were improved in chronic cerebral ischemia and acute cerebral ischemic stroke patients, respectively. Due to the reason that most studies used MSC transplantation, the effect of SCs type interventions could not be obtained.
Table 3.
The correlations of NIHSS and clinical variables across studies by subgroup and heterogeneity analysis.
| Subgroup | Mean difference (95% CI) | I 2 (%) |
|---|---|---|
| Patients' characteristics | ||
| Acute stoke | 0.93 (−0.65, 2.51) | 57 |
| Chronic stroke | 2.28 (1.47, 3.09) | 6 |
| Cell type | ||
| MSC | 1.25 (0.35, 2.14) | 38 |
| Non-MSC | 2.9 (1.75, 4.05) | — |
| Route of delivery | ||
| Intravenous injection | 1.0 (−0.18, 2.17) | 41 |
| Non-IV | 2.38 (0.77, 3.99) | 71 |
| Follow-up period | ||
| <6 months | 1.41 (0.76, 2.06) | 0 |
| ≥6 months | 2.07 (−1.10, 5.24) | 82 |
Table 4.
The correlations of Barthel and clinical variables across studies by subgroup and heterogeneity analysis.
| Subgroup | Mean difference (95% CI) | I 2 (%) |
|---|---|---|
| Patients' characteristics | ||
| Acute stoke | 12.61 (2.39, 22.84) | 84 |
| Chronic stroke | 7.19 (4.18, 10.20) | 0 |
| Cell type | ||
| MSC | 8.37 (4.83, 11.91) | 63 |
| Non-MSC | — | — |
| Route of delivery | ||
| Intravenous injection | 7.58 (3.85, 11.30) | 30 |
| Non-IV | 12.56 (−3.16, 28.27) | 91 |
| Follow-up period | ||
| <6 months | 5.2 (4.06, 6.34) | — |
| ≥6 months | 9.52 (4.92, 14.13) | 58 |
4. Discussion
Stem cells have several special biological characteristics, such as proliferation ability, multidirectional differentiation, and good histocompatibility, and numerous studies have focused on the therapeutic potential of stem cell therapy for various refractory diseases. With the rise of stem cell therapy in recent years, different types of stem cells have been widely investigated and applied for ischemic stroke therapy, especially with a sharp increase in NSC and MSC transplantation. Lees et al. evaluated 117 studies on preclinical experimental model of stem cell therapy by meta-analysis and showed that stem cell transplantation had improved the animals' neural function and reduced the area of cerebral infarct [25]. Vu et al. studied the efficacy of preclinical mesenchymal stromal cells transplantation therapy for ischemic stroke models and demonstrated the favorable (MSC) outcomes, and the outcomes were related with the type of MSC source, route of delivery, time of injection, and intervention dose [26]. Because of safety, efficacy, and ethical reasons, there still remains a long way before the stem cell therapy can be actually used in clinical settings. Liu et al. searched the MSC transplantation therapy for cerebral ischemic patients and found 6 studies (332 patients); they confirmed that MSC transplantation could significantly improve patients' neural functional defects, motor functions, and daily life abilities [27]. Jeong et al. analyzed the safety and efficacy of stem cell transplantation therapy for brain stroke; they included 14 researches which independently use stem cell therapy (not RCT studies) and determined that stem cell therapy improved the patients' grades of NIHSS, Barthel index, and Rankin functions [28]. All those studies confirmed the therapeutic potential of stem cells clinically.
Here we have searched the RCT studies of stem cell transplantation for ischemic stroke and used meta-analysis approach to identify the possible publication bias and to investigate the clinical efficacy of reported outcomes (NIHSS, FMA, Barthel and FIM) in ischemic stroke studies. A total of 23 studies recruited 1374 patients, and the patients of ischemic stroke must have met WHO diagnostic criteria of brain stroke. Cerebral hemorrhage was ruled out in these patients by brain CT or MRI. Each group must have more than 5 patients. The type of interventions contained NSC, MSC, UC-MSC, and PBSC. Finally, 16 articles and 18 treatment interventions have been analyzed by meta-analysis; the outcomes of treatment from baseline to follow-up points included NIHSS, FMA, Barthel index, or FIM which all improved to varying degrees, with fewer adverse effects. Then we used 4 clinical variables (time/type of cerebral ischemia, type of SCs interventions, route of delivery, and follow-up period) to interact with 2 outcome indicators (NIHSS and Barthel) and found that patients who received SCs transplantation via subarachnoid space, carotid artery, and intracerebral were superior to those in which SCs were administered intravenously, and long-term follow-up was associated with better outcome than short-term follow-up. But there was no relation with time and type of cerebral infarct or type of SC interventions with functional improvement. Due to the fact that most studies had defects in blinding, data's integrity, and trial designs, our meta-analysis might have some risks of bias.
In conclusion, the current meta-analysis of clinical studies of SCs therapy for ischemic stroke demonstrated significant and favorable effects on behavioral, motor, and life quality outcomes. However, because of few clinical trials for stem cell transplantation therapy, lower frequency of patient participation, and possible bias issues in trial designs, there is possibility of bias occurrence. Therefore, further high quality and bigger data studies are needed in the future to investigate the possibilities of stem cells transplantation in clinical settings.
Acknowledgments
This study was supported by National Natural Science Foundation of China (no. 81671819).
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Lukui Chen and Guilong Zhang contributed equally to this study.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;385(9963):117–171. doi: 10.1016/s0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Li J., Zhao X., Wang Y., Wu D., Wang Y. Stroke care development in Mainland China: past, present and future. International Journal of Stroke. 2008;3(4):288–289. doi: 10.1111/j.1747-4949.2008.00218.x. [DOI] [PubMed] [Google Scholar]
- 3.Ning M., Sarracino D. A., Buonanno F. S., et al. Proteomic protease substrate profiling of tPA treatment in acute ischemic stroke patients: a step toward individualizing thrombolytic therapy at the bedside. Translational Stroke Research. 2010;1(4):268–275. doi: 10.1007/s12975-010-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer S. C., Stradling D., Brown D. M., et al. Organization of a United States county system for comprehensive acute stroke care. Stroke. 2012;43(4):1089–1093. doi: 10.1161/strokeaha.111.635334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer S. C. Repairing the human brain after stroke. II. Restorative therapies. Annals of Neurology. 2008;63(5):549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- 6.Lee M.-C., Jin C.-Y., Kim H.-S., et al. Stem cell dynamics in an experimental model of stroke. Chonnam Medical Journal. 2011;47(2):90–98. doi: 10.4068/cmj.2011.47.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daadi M. M. In vitro assays for neural stem cell differentiation. Methods in Molecular Biology. 2002;198:149–155. doi: 10.1385/1-59259-186-8:149. [DOI] [PubMed] [Google Scholar]
- 8.Martino G., Pluchino S. The therapeutic potential of neural stem cells. Nature Reviews Neuroscience. 2006;7(5):395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 9.Bang O. Y., Lee J. S., Lee P. H., Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Annals of Neurology. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 10.Sun H. B., Xia S. M., Yang S. S., et al. The dinical effect of rhG-CSF combined transplantation of autologous bone marrow mesenchymal stem cells on acute cerebral infarction. Journal of Chinese Physician. 2008;10(4):441–443. [Google Scholar]
- 11.Niu G. Q., Guo B. R., Chang X. Y., An Y. H. Sequelae analysis of neural stem cell transplantation for treating with cerebral infarction. China Medicine. 2010;5(7):615–616. [Google Scholar]
- 12.Bhasin A., Srivastava M. P., Kumaran S. S., et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovascular Diseases Extra. 2011;1(1):93–104. doi: 10.1159/000333381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Y. G., Zhang Z. P., Yu W. Clinical efficacy of salvia miltiorrhiza injection combined with marrow stem cells in treatment of cerebral infarction. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease. 2012;20(3):424–425. [Google Scholar]
- 14.He Z. D. The functional mechanism of mesenchymal stem cell transplantation therapy for patients with cerebral infarction. Asia-Pacific Traditional Medicine. 2012;8(12):126–127. [Google Scholar]
- 15.Bhasin A., Srivastava M. V., Bhatia R., Mohanty S., Kumaran S. S., Bose S. Autologous intravenous mononuclear stem cell therapy in chronic ischemic stroke. Journal of Stem Cells & Regenerative Medicine. 2012;8(3):181–189. doi: 10.46582/jsrm.0803011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao X. H. 78 Cases Clinical Observation of Stem cell transplantation in the treatment of cerebral infarction. Shandong Medical Journal. 2013;53(9):52–53. [Google Scholar]
- 17.Bhasin A., Padma Srivastava M. V., Mohanty S., Bhatia R., Kumaran S. S., Bose S. Stem cell therapy: a clinical trial of stroke. Clinical Neurology and Neurosurgery. 2013;115(7):1003–1008. doi: 10.1016/j.clineuro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Liu D. H., Han B. J., Hong S. S., et al. Transplanting autologous mesenchymal nerve stem cells in the treatment of cerebral infarction. Chinese Journal of Physical Medicine and Rehabilitation. 2014;36(6):425–428. [Google Scholar]
- 19.Xie X. F., Liu S. Y., Jin G. H., Qu X. H., Zhang K. N., Wu X. M. Clinical analysis of autologous bone marrow mesenchymal stem cell transplantation for treating cerebral infarction. Laboratory Medicine and Clinic. 2014;(21):2955–2957. [Google Scholar]
- 20.Chen D.-C., Lin S.-Z., Fan J.-R., et al. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: a randomized phase II study. Cell Transplantation. 2014;23(12):1599–1612. doi: 10.3727/096368914x678562. [DOI] [PubMed] [Google Scholar]
- 21.Prasad K., Sharma A., Garg A., et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45(12):3618–3624. doi: 10.1161/strokeaha.114.007028. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M., Wu S. T., Liu L., et al. Clinical observation of neural stem cells transplantation in the treatment of cerebral infarction. Chinese Journal of Practical Nervous Diseases. 2015;(22):8–10. [Google Scholar]
- 23.Shen D. P. Umbilical cord mesenchymal stem cell early single transplantation in treatment of neurological recovery in acute cerebral infarction. Chinese Journal of Trauma and Disability Medicine. 2015;(2):26–28. [Google Scholar]
- 24.Cai M. S., Shen C. L., Zeng L. H., Huang X. Q., Song C. W. Effect of stem cell transplantation on serum homocysteine, CRP and BDNF in patients with ischemic stroke. Chinese Journal of Biochemical Pharmaceutics. 2015;(9):91–93. [Google Scholar]
- 25.Lees J. S., Sena E. S., Egan K. J., et al. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. International Journal of Stroke. 2012;7(7):582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- 26.Vu Q., Xie K., Eckert M., Zhao W., Cramer S. C. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82(14):1277–1286. doi: 10.1212/wnl.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Wu H.-Q., Yan P., et al. Efficacy and safety of bone mesenchymal stem cells transplantation for ischemic stroke: a systematic review. Chinese Journal of Evidence-Based Medicine. 2015;15(6):659–663. doi: 10.7507/1672-2531.20150110. [DOI] [Google Scholar]
- 28.Jeong H., Yim H. W., Cho Y.-S., et al. Efficacy and safety of stem cell therapies for patients with stroke: a systematic review and single arm meta-analysis. International Journal of Stem Cells. 2014;7(2):63–69. doi: 10.15283/ijsc.2014.7.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]