Abstract
Amyloid-β (Aβ) peptides are the main components of the plaques found in the brains of patients with Alzheimer’s disease. However, Aβ peptides are also detectable in secretory compartments and peripheral blood contains a complex mixture of more than 40 different modified and/or N- and C-terminally truncated Aβ peptides. Recently, anti-infective properties of Aβ peptides have been reported. Here, we investigated the interaction of Aβ peptides of different lengths with various bacterial strains and the yeast Candida albicans. The amyloidogenic peptides Aβ1-42, Aβ2-42, and Aβ3p-42 but not the non-amyloidogenic peptides Aβ1-40 and Aβ2-40 bound to microbial surfaces. As observed by immunocytochemistry, scanning electron microscopy and Gram staining, treatment of several bacterial strains and Candida albicans with Aβ peptide variants ending at position 42 (Aβx-42) caused the formation of large agglutinates. These aggregates were not detected after incubation with Aβx-40. Furthermore, Aβx-42 exerted an antimicrobial activity on all tested pathogens, killing up to 80% of microorganisms within 6 h. Aβ1-40 only had a moderate antimicrobial activity against C. albicans. Agglutination of Aβ1-42 was accelerated in the presence of microorganisms. These data demonstrate that the amyloidogenic Aβx-42 variants have antimicrobial activity and may therefore act as antimicrobial peptides in the immune system.
Amyloid-β (Aβ) peptides are the main component of the plaques in the brains of patients with Alzheimer’s disease but are also found in healthy individuals1,2. Amyloid deposits are also observed after intranasal infection of mice with bacteria3. In soluble form, Aβ peptides are predominantly located in the cerebrospinal fluid, but these peptides are also generated in most other tissues and are detectable in several bodily fluids, including saliva and urine4,5. Aβ is a phylogenetically ancient peptide that is highly conserved across species, but its physiological function remains to be elucidated6. Aβ peptides are generated via sequential proteolytic cleavage from the membrane-anchored amyloid precursor protein. In addition to the long-known β- and γ-secretases, several other enzymes, such as meprin-β, caspase and aminopeptidase A, are potentially involved in this process7,8,9,10. To date, more than 40 different N- and C-terminal truncated Aβ peptide variants consisting of 37 to 43 amino acids have been identified11,12,13.
Due to their structural homology, it has been suggested that Aβ peptides are antimicrobial peptides involved in the innate immune defense system14,15,16. Antimicrobial peptides are peptide antibiotics that act on a variety of Gram-positive and Gram-negative bacteria, fungi and viruses17,18. Similar to Aβ peptides, antimicrobial peptides are amphiphilic peptides of up to 40 amino acids. They are soluble in aqueous solutions but can also interact with lipid-rich membranes17.
In specific conditions, both antimicrobial peptides and Aβ peptides build α-helical structures within pathogen cell membranes, forming ion channels that disturb cell homeostasis and ultimately induce cell death15,19,20,21,22.
Another antimicrobial function of antimicrobial peptides is pathogen agglutination, which prevents the spread of infection and facilitates phagocytosis23. Aggregates of the eosinophil cationic protein—a model for other amyloid-like peptides—induce bacterial agglutination and cell death24. Due to their hydrophobic nature, Aβ peptides are prone to aggregation, so their antimicrobial activity may also involve the agglutination of pathogens. Here, we evaluated the antimicrobial and agglutinating activity of several Aβ peptide variants which differ in their C- and N-terminal lengths.
Materials and Methods
Cultures of bacteria and fungi
Enterococcus faecalis (ATCC 29212), Listeria monocytogenes (VA 15110/93), Escherichia coli (DH5α) and Staphylococcus aureus (ATCC 25923) were grown aerobically on blood agar plates. Candida albicans (ATCC 10231) was plated on Sabouraud dextrose agar plates. E. coli, S. aureus and C. albicans were cultured at 37 °C; E. faecalis and L. monocytogenes were cultured at 37 °C with an additional 5% CO2. Before the experiments, the organisms were subcultured to generate mid-logarithmic growth cultures for use as inoculates. Colonies from the agar were transferred using a sterile loop to Mueller-Hinton broth (Roth, Karlsruhe, Germany) and incubated for 100 min at 39 °C to achieve a McFarland density of 0.5. The C. albicans and bacterial inocula were normalized in Mueller-Hinton broth to 5 × 105 cells/ml immediately before use. For the experiments, an inoculum of 5 × 105 cells/ml was dispensed into 96-well plates containing Mueller-Hinton broth growth medium with Aβ peptides, as indicated below.
Peptide pretreatment
Aβ1-40, Aβ1-42, Aβ2-40, Aβ2-42 and Aβ3p-42 (Anaspec, Fremont, CA, USA) were reconstituted in 1% NH4OH (Anaspec, Fremont, CA, USA), diluted with H2Odd to reach a final concentration of 1 mg/ml in H2Odd/0.8% NH4OH and stored at −20 °C. Immediately before the experiments, all peptides were diluted in Mueller-Hinton broth to reach a final concentration of 100 μg/ml.
Flow cytometry
After 6 h of incubation for bacteria and 20 h for C. albicans, 50 μl of the cell suspension was diluted in 1% paraformaldehyde (Sigma-Aldrich, Munich, Germany) and stained with DAPI (Sigma-Aldrich, Munich, Germany). Flow cytometry analysis was performed using a Gallios Cytometer (Beckman Coulter, Krefeld, Germany), and the results were evaluated using Kaluza® software (Beckman & Coulter, Krefeld, Germany). Experiments were repeated at least three times for each organism, and duplicates were included for each assay condition. Cultures treated with antibiotics, Mueller-Hinton broth without cells and untreated cells served as controls. Viability was assessed based on the increase in autofluorescence and reduction of forward scatter as signs of microbial damage25,26.
Gram staining
The same cultures analysed by flow cytometry were used for the gram staining. After incubating the bacteria and C. albicans with the peptides, 10 μl of the cell suspensions were air-dried on microscope slides and flame-fixated. The smears were flooded with crystal violet solution for 2 min. The slides were rinsed and then incubated for 2 min with iodine solution. The slides were then flooded with 96% ethanol for approximately 10 seconds and washed with distilled water. The slides were flooded with safranin for 1 min, washed with distilled water and examined on an Olympus IX 70 microscope (Olympus, Hamburg, Germany) with NIS Elements BR software (Nikon, Duesseldorf, Germany) using a 100× oil immersion objective (1000× magnification).
Immunocytochemistry
The immunocytochemistry of E. faecalis and S. aureus was examined using the Gram preparations specified above. To achieve the robust adherence of pathogens on the glass slides during the staining procedure, cells were incubated with 2% paraformaldehyde (Sigma-Aldrich, Munich, Germany) on the slides and air dried for 60 min at room temperature, followed by washing with PBS (Biochrom, Berlin, Germany). Slides were blocked with 1% bovine serum albumin/5% goat serum (Sigma-Aldrich, Munich, Germany) in PBS for 1 h at room temperature before incubation with the Aβ peptide-specific monoclonal antibody 6E10 (Covance, Princeton, New Jersey, USA) at a concentration of 4 μg/ml for 60 min at room temperature. Labeling with the secondary AF488-conjugated goat anti-mouse antibody (Life Technologies, Darmstadt, Germany) at a concentration of 2 μg/ml was performed for 1 h at room temperature. Slides were mounted with Roti®-Mount FluorCare DAPI (Roth, Kralsruhe, Germany), and photographs were taken on an automated screening epifluorescence microscope (Eclipse Ti-E, Nikon, Duesseldorf, Germany) equipped with a 100× objective (Plan Apo VC 100× oil, N.A.: 1.4, W.D.: 0.13 mm, Nikon, Duesseldorf, Germany), a motorized stage (TI-S-ER Motorized Stage, Nikon, Duesseldorf, Germany), an sCMOS camera (Neo, Andor, Belfast, UK), a 100-W lamp for illumination (Lambda LS Xenon Arc, Sutter Instrument, Novato, CA, USA) and a filter set (Nikon, Duesseldorf, Germany). Gain and scaling were kept constant throughout all measurements.
Quantification of Aβ peptides and cell aggregation on the immunocytochemistry slides
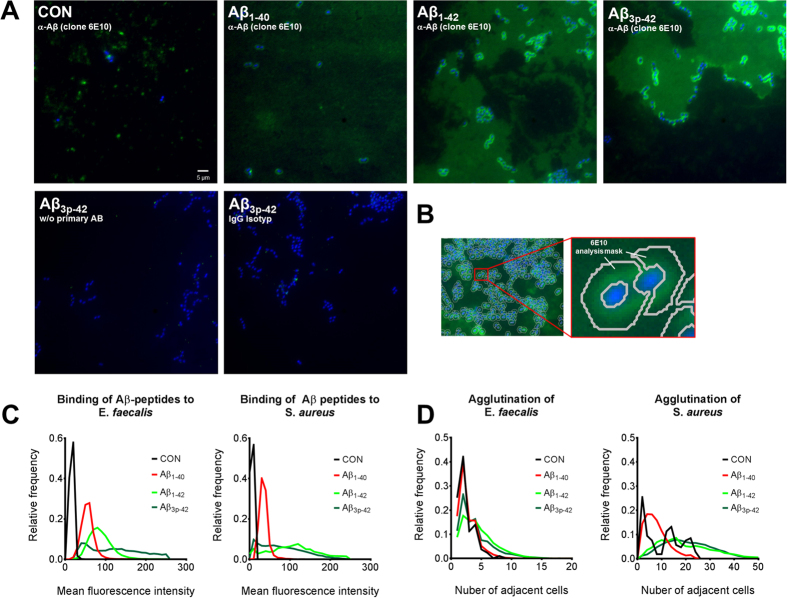
All images were visually quality controlled. Images that did not fulfill pre-defined criteria based on image acquisition in the adequate focal plane and adequate cell density were discarded. Of the 2,304 images acquired, 2,072 (~90%) were used for further analysis. An automated quantitative phenotypic image analysis was performed using a custom-adapted version of the image analysis software DetecTiff 27. Pericellular Aβ peptide levels were quantified from the AF488 channel within donut-shaped masks (see Fig. 1B), which were generated from the DAPI channel in a three-step procedure. First, the images of DAPI-stained bacteria were automatically segmented by combined dynamic intensity thresholding and size-dependent particle filtering. In the second step, the binary masks of bacteria were iteratively dilated using a morphological operator to cover the area of the pericellular AF488 signal. Finally, the donut-shaped masks were computed by subtracting the binary masks generated in step one from the masks generated in step two. A total of 57,249 individual donut-shaped analysis masks were constructed from all images. The Aβ peptide level in an individual cell was calculated as the arithmetic mean of all pixel values within the area of a donut-shaped mask and reported as the mean pixel intensity. Cell aggregation was quantified in an automated manner from segmented images of DAPI-stained nuclei by counting the number of bacteria adjacent to a selected individual bacterium within a rectangular 50-pixel2 region.
Figure 1. Aβ peptides bind and agglutinate E. faecalis and S. aureus.
Cultures of E. faecalis and S. aureus were supplemented with 50 μg/ml Aβ1-40, Aβ1-42, Aβ1-42 or Aβ3p-42. After 6 h, a sample was transferred to glass slides, flame-fixed and stained with DAPI (blue) and the anti-Aβ monoclonal antibody 6E10 (green) or an IgG-control (A). The amount of Aβ peptide bound to E. faecalis and S. aureus was quantified by calculating donut-shaped masks (B) around each bacterium and measuring the mean pixel intensity in this area (C). Aggregation was measured by counting the number of cells around each bacterium (D). The results are presented as frequency distributions of one representative experiment with E. faecalis and one with S. aureus; each assay included at least 1,000 bacteria counted in more than 160 images per condition.
Scanning electron microscopy of Enterococcus faecalis
The specimens were attached to aluminum holders and sputtered with platinum in 15-nm layers using a LEICA EM SCD-500 (Leica, Wetzlar, Germany). The surface morphology of the platinum sputter-coated samples was investigated using a JSM 6 610 scanning electron microscope (JEOL, Peabody, MA, USA) at a working distance of 10 mm and an acceleration voltage of 10 kV at room temperature.
Assessment of proliferation and viability
An inoculum of 5 × 105 cells/ml was dispensed into 96-well plates containing Mueller-Hinton broth growth medium and 3.13 μg/ml or 6.25 μg/ml Aβ-peptides or LL-37 (Anaspec, Fremont, CA, USA). Bacterial plates were incubated aerobically at 37 °C for 6 h, and C. albicans was cultured for 20 h at 37 °C. To assess proliferation, 10 μl Alamar Blue® reagent (Life Technologies, Darmstadt, Germany) was added to each well containing a 100-μl sample, followed by incubation for 60 min at 37 °C in the dark. The resulting absorbance was measured with a SpectraMax 340 PC 384 microplate reader (Molecular Devices, Biberach, Germany). The proliferation of C. albicans was assessed by measuring turbidity at 570 nm.
The viability of E. faecalis, E. coli and C. albicans was further examined by plating Aβ-treated cultures on agar plates. Cultures of E. coli, E. faecalis and C. albicans were prepared as described above. After incubation with 50 μg/ml Aβ1-40, Aβ1-42 or Aβ3p-42 for 6 h for bacteria and 20 h for C. albicans, the cell suspensions were diluted in Mueller-Hinton broth, and the microbial load was determined using an Eddy Jet Spiral Plater (IUL Instruments, Germany) by depositing 50 μl of sample on a rotating agar plate. Bacteria were grown on Mueller-Hinton agar plates, and C. albicans was grown on Sabouraud dextrose agar plates. After 24 h of incubation, colonies were counted and translated to colony-forming units (CFU)/ml.
Assessment of Aβ peptide aggregation
Thioflavin T was used to monitor the aggregation of Aβ1-42 in the presence of microorganisms. In a black 96-well plate, 160 μg/ml of Aβ1-42 in PBS/0.4% NH4OH were mixed with heat inactivated E. coli, S. aureus or C. albicans at a final density of McFarland 0.5. Leaving away the microorganisms or adding 10 μg/ml carboxylated microbeads (Micromod, Rostock, Germany) with a diameter of 0.5 μm served as control. After adding Thioflavin T (Sigma-Aldrich, Munich, Germany) in final concentration of 0.2 mM, kinetic measurement was performed at 37 °C with excitation at 450 nm and emission at 484 nm in a CLARIOstar® microplate reader (BMG labtech, Ortenberg, Germany). Measurements containing the respective microorganisms but no Aβ1-42 served as blank.
Statistical analysis
The statistical analysis was performed using GraphPad Prism® 6.0 software (GraphPad, La Jolla, CA, USA). Although normality tests could not be calculated, due to the small sample sizes, a Gaussian distribution of the data can be assumed. No pairing of the experiments was assumed. Therefore, one-way ANOVA, followed by the Dunnett’s post-test for multiple comparisons against the control were calculated.
Results
Aβ peptides bind to bacterial surfaces
E. faecalis and S. aureus were cultivated in the presence of the Aβ peptide variants Aβ1-40, Aβ2-40, Aβ1-42 and Aβ3p-42 at a concentration of 50 μg/ml. After 6 h, the bacteria were smeared on glass slides, labeled with the Aβ peptide-specific mouse monoclonal antibody 6E10 and counterstained with DAPI. Immunofluorescence microscopy revealed Aβ peptide-specific staining on the surfaces of both, E. faecalis and S. aureus (Fig. 1A). Automated quantification of experiments with E. faecalis and with S. aureus including more than 1,000 bacteria per condition revealed that Aβx-42 bound more strongly to the surface of the microorganisms than did Aβ1-40 (Fig. 1A,C). Aβ-reactive material was also observed in between the bacteria after incubation with Aβ1-42 and Aβ3p-42, but the intensity was strongest directly around the bacteria.
Aβ peptides agglutinate microorganisms
Automated analysis of the immunocytochemistry slides further revealed that treatment with Aβ1-42 and Aβ3p-42 led to agglutination of E. faecalis and S. aureus. By contrast, incubation with Aβ1-40 resulted in almost no agglutination (Fig. 1A,D).
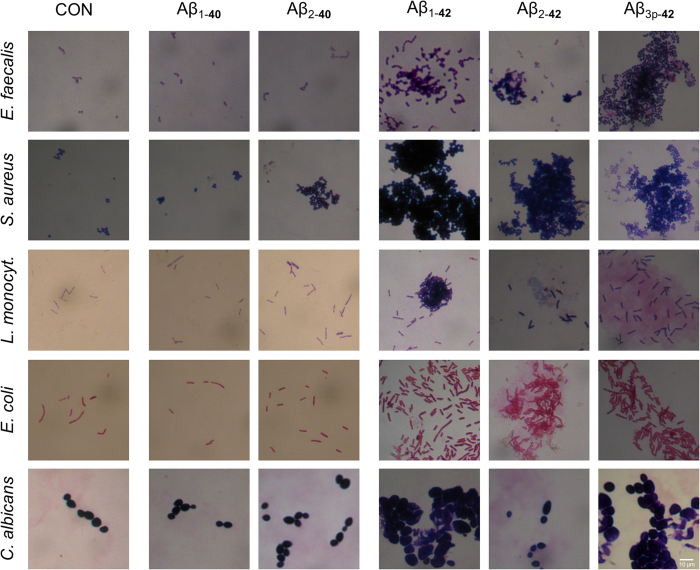
To extend these findings to other pathogens and Aβ peptide fragments, four different bacteria (E. faecalis, E. coli, S. aureus and L. monocytogenes) and one fungus (C. albicans) were cultured in the presence of the Aβ peptide variants Aβ1-40, Aβ1-42, Aβ2-40, Aβ2-42 and Aβ3p-42. Gram staining revealed Aβ peptide variant-dependent agglutination of all microorganisms. Neither Aβ1-40 nor Aβ2-40 induced microbial agglutination, whereas Aβx-42 agglutinated the microorganisms into large clusters (Figs 1 and 2).
Figure 2. Aβx-42 but not Aβx-40 agglutinates several microorganisms.
Gram stains of E. faecalis, S. aureus, L. monocytogenes, E. coli and C. albicans were prepared after 6 h of incubation (20 h for C. albicans) with the indicated Aβ peptide variants at a concentration of 50 μg/ml. Large clusters of agglutinated microorganisms were observed only in cultures treated with Aβx-42. Within the clusters of agglutinated microorganisms, large amounts of amorphic material are evident.
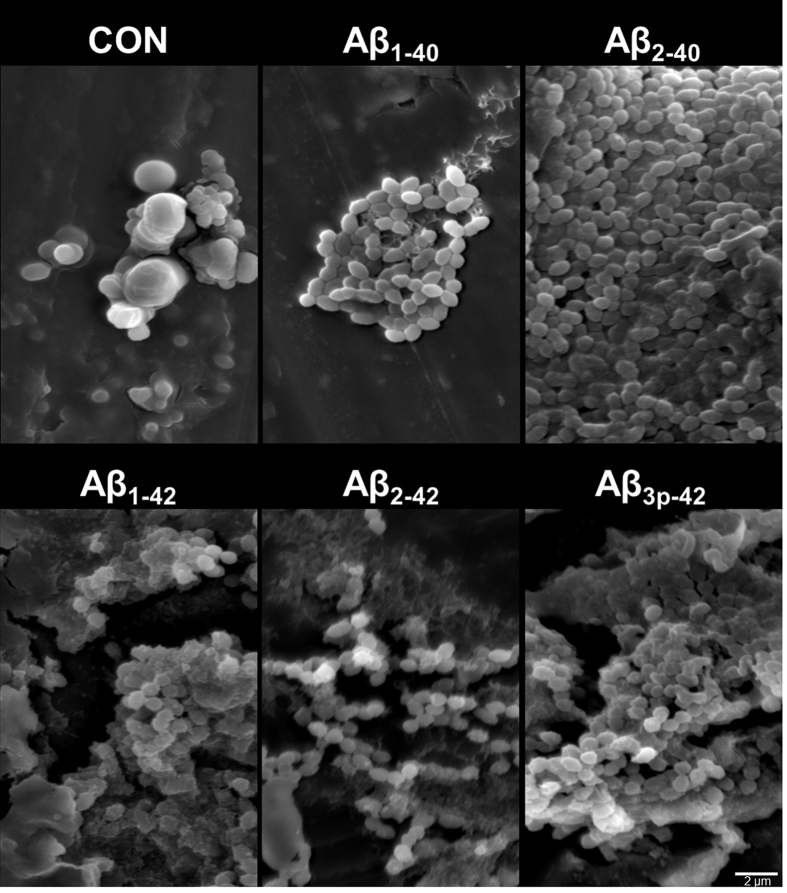
Scanning electron microscopy of E. faecalis incubated with the different Aβ peptide variants confirmed the agglutinating activity of Aβx-42. Additionally, bacteria treated with Aβx-42 were dysmorphic, and large amounts of amorphous material were present within the aggregates. In cultures of E. faecalis incubated with Aβ2-40, there were larger aggregates of bacteria that retained their vital morphology. Only minor aggregates were observed in the absence of Aβ peptides and in cultures incubated with Aβ1-40. Taken together, these results suggest that Aβx-42 peptides induce agglutination of microorganisms (Fig. 3).
Figure 3. E. faecalis agglutinated by Aβx-42 exhibits an irregular, dysmorphic shape and the accumulation of large amounts of amorphic material between cells.
Scanning electron microscopy of E. faecalis after incubation for 6 h with the indicated Aβ peptide variants at a concentration of 50 μg/ml. The scale bar represents 2 μm.
Aβ peptides exert antimicrobial activity
Flow cytometry analysis of the microorganisms treated with Aβx-42 peptides revealed a population characterized by increased autofluorescence at 525 nm and/or reduced forward scatter (AF+/FSC−). Increased autofluorescence and reduced forward scatter are common features of damaged cells25,26. This population was therefore categorized as damaged microorganisms. DAPI staining was performed to differentiate the microorganisms from the background, and only DAPI-positive events were gated for further analysis. The microbial aggregates observed via immunocytochemistry, Gram staining and scanning electron microscopy were only partially detected by flow cytometry; Compared with the size of untreated and consequently unaggregated microorganisms Aβ peptide treated microorganisms did not show increased forward or side-scatter characteristics.
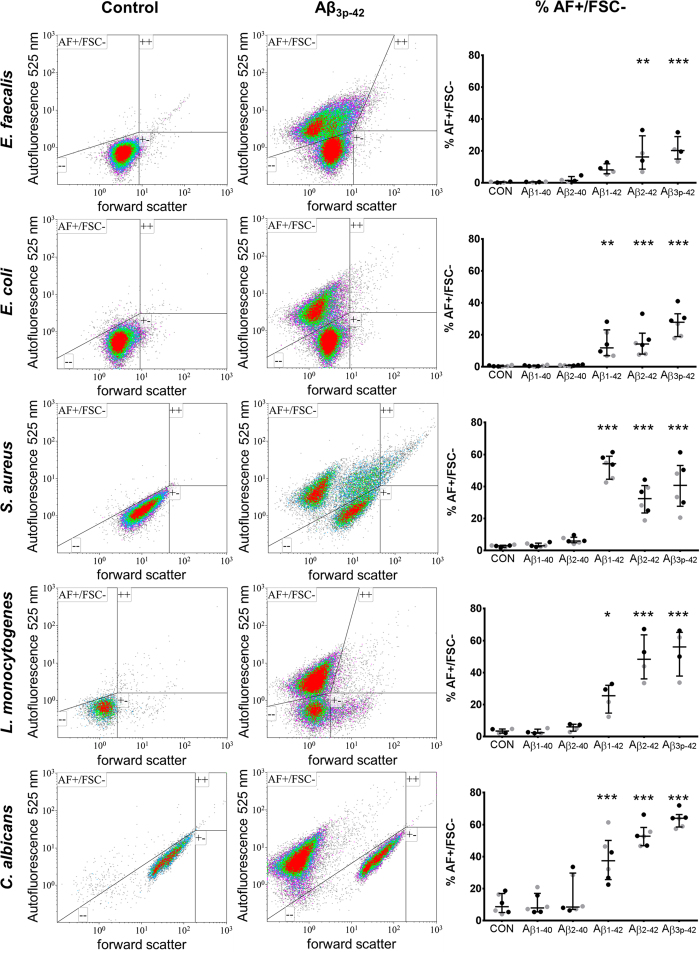
Aβ1-42 exerted antimicrobial activity on all tested microorganisms. As characterized by reduced forward scatter and increased autofluorescence, damaged microorganisms represented up to 70% of cells in the analyzed samples. N-terminal truncation and pyroglutaminylation of Aβ1-42 further increased microbicidal activity against all microorganisms except S. aureus (Fig. 4). No microbicidal activity was observed for Aβ1-40 or Aβ2-40. Identical concentrations of Aβ peptides did not agglutinate human THP-1 cells and were not toxic to those cells within 24 h.
Figure 4. Antimicrobial activity of Aβx-42.
Flow cytometry analysis of E. faecalis, E. coli, S. aureus, L. monocytogenes and C. albicans after 6 h of incubation with the indicated Aβ peptide variants at a concentration of 25 μg/ml (gray) or 50 μg/ml (black). Column one (control) depicts representative density plots of forward scatter vs. autofluorescence at 525 nm in untreated microorganisms gated for DAPI positivity. Column two (Aβ3p-42) shows the same cultures after incubation with Aβ3p-42. Column three (% AF+/FSC−) shows the percentage of microorganisms with increased autofluorescence at 525 nm or reduced forward scatter (upper left quadrant) which are supposed to be damaged. The results are depicted as scatter plots with the median ± interquartile range. Cultures treated with 25 μg/ml and 50 μg/ml Aβ-peptides were grouped for the statistical analysis. Asterisks indicate significant differences calculated by the Friedman test followed by Dunn’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001.
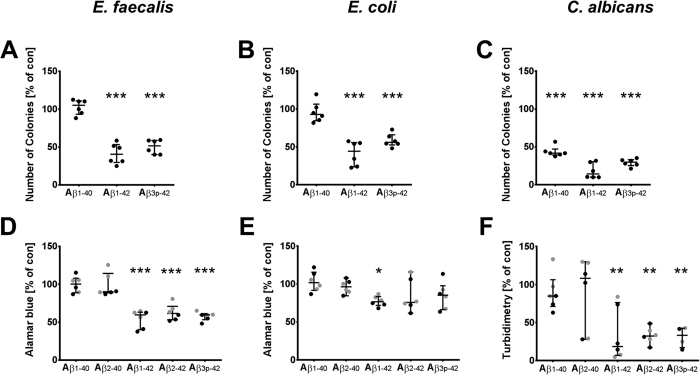
The antimicrobial activities of Aβ1-42 and Aβ3p-42 were further confirmed by the Alamar blue test for bacteria, by turbidimetry for Candida and by seeding the bacterial and fungal cultures on agar plates (Fig. 5). Incubating E. coli and E. faecalis with 50 μg/ml Aβ1-42 or Aβ3p-42 reduced the number of colony forming units by 50% compared to untreated cultures (Fig. 5A,B). The number of colony forming units was not reduced by Aβ1-40. The toxic effects of the Aβ peptides were even stronger against C. albicans. Incubation with Aβ1-42 or Aβ3p-42 reduced the number of colony forming units by 85% (Fig. 5C). Aβ1-40 also had a moderate antimicrobial effect against C. albicans in culture.
Figure 5. Aβx-42 reduces the number of colony-forming units and proliferation of microorganisms.
Colony forming units (CFU) of E. faecalis, E. coli and C. albicans were determined after treatment with 50 μg/ml Aβ1-40, Aβ1-42 and Aβ3p-42 for 6 h (24 h for Candida) by plating the cultures on agar plates and incubating for 24 h (A–C). The proliferation of the bacteria incubated with the indicated Aβ peptides at a concentration of 6.25 μg/ml (black) or 3.13 μg/ml (grey) was assessed by the Alamar Blue assay after 6 h (D,E). The proliferation of C. albicans was assessed by turbidimetry after incubation for 24 h (E). The results are expressed relative to untreated cultures and are depicted in a scatter plot together with the median and interquartile range. Asterisks indicate significant differences calculated by the Friedman test followed by Dunn’s post-hoc test. *p < 0.05, **p < 0.01, ***p < 0.001.
Agglutination of Aβ1-42 is accelerated by microorgansims
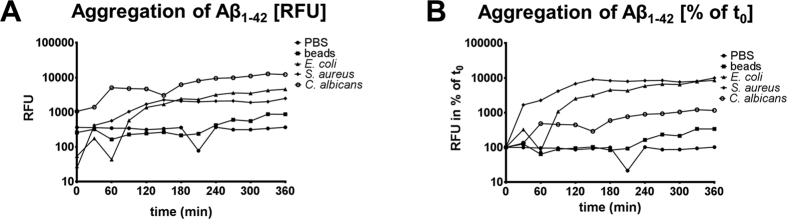
The agglutination of Aβ1-42 in PBS was monitored by the Thioflavin assay over 6 h at 37 °C. Adding heat inactivated E. coli, S. aureus or C. albicans resulted in a more rapid increase in Thioflavin fluorescence than agglutination of Aβ1-42 in PBS alone or in the presence of carboxylated polystyrene microbeads with a diameter of 0.5 μm. This difference was obvious in terms of relative fluorescence units (RFU, Fig. 6A) or when expressed in relation to fluorescence at the beginning of the measurement (% of t0, Fig. 6B).
Figure 6. Microorganisms accelerate the agglutination of Aβ1-42.
Thioflavin fluorescence (Ex 450 nm, Em 484 nm) was monitored for 6 h at 37 °C in solutions of 160 μg/ml Aβ1-42 in PBS. Each measurement was corrected for the fluorescence in absence of Aβ1-42. Blank corrected Relative fluorescence units (RFU) are depicted in (A) whereas the change of RFU in relation to the first measurement at t0 is graphed in (B).
Discussion
We observed that the more amyloidogenic Aβx-42 peptides led to the agglutination and death of microorganisms. For Aβ1-40 only an antifungal activity was seen.
Microbial agglutination induced by Aβx-42 was observed by Gram staining, immunocytochemistry and scanning electron microscopy. Microbial agglutination was accompanied by binding of the Aβ peptide to the microbial surface. Only in cultures of L. monocytogenes and C. albicans no agglutinated bacteria were observed after the treatment with Aβ2-42. As there can still be seen this red amorphic material, it is well possible that the bacteria already disintegrated and left only debris. This is supported by the fact that a very strong microbicidal effect for Aβ2-42 is also observed by flow cytometry. Also, when looking at the gram stains of Aβ3p-42 treated L. monocytogenes it seems that there are already several bacteria “missing” within the agglutinate. The antimicrobial peptides eosinophil cationic protein and salivary agglutinin23,24 also induce agglutination. The agglutination of pathogens by Aβ-peptides may contribute to antimicrobial control via several mechanisms. First, agglutination may prevent the distribution of microorganisms by causing physical immobilization. Second, agglutination facilitates phagocytosis23,24. Third, Aβ peptides act as opsonins for phagocytosis28.Thereby, the Aβ peptide variants with the highest microbicidal activity are also the most effective in inducing phagocytosis28. Finally, in addition to bacterial agglutination, Aβx-42-peptides have direct antimicrobial activity. Microorganisms exposed to Aβx-42 exhibited reduced forward scatter and increased autofluorescence in flow cytometry analyses. A loss of forward scatter is a common feature of damaged cells and bacteria, but increased autofluorescence of bacteria exposed to bactericidal agents has only recently been observed25. Renggli et al. suggested that this autofluorescence is caused by a change of cell morphology25.
Autofluorescence after excitation with ultraviolet and blue light was also observed in amyloid plaques containing full length Aβ peptides and after aggregation of synthetic Aβ peptides29,30,31. As can be seen from the IgG staining control in Fig. 1, the detected signals do not result from autofluorescence. In the flow cytometry experiments, the reported autofluorescence after treatment with Aβ peptides also occurred after treatment with antibiotics (data not shown). Therefore we suppose, that autofluorescence in our experiments is due to cell death. However, we cannot fully exclude, that part of the observed fluorescence is due to autofluorescence of Aβ peptides.
The antimicrobial activity of amyloidogenic Aβ peptides was confirmed by reduced Alamar blue turnover and a reduction in colony forming units in our study. In case of E. faecalis, both assays showed a consistent reduction of bacterial growth by the Aβx-42 peptides. In contrast, the decrease in E. coli CFU by these peptides was paralleled by a weaker effect on Alamar blue turnover (Fig. 5B,E), indicating that agglutination of E. coli may lead to overestimation of the killing activity in the CFU assay. On the other hand, the Alamar blue test and turbidimetry might have underestimated the antimicrobial effect, as the microbial aggregates could disturb the optical path, leading to heterogeneous and false high readings. This could explain, why we miss the cytotoxic effect of Aβ1-40 and Aβ2-40 on C. albicans with the Alamar blue assay.
C- and N-terminal modifications of the Aβ peptides greatly affected their aggregation and antimicrobial activity, such that Aβx-42 was much more effective than Aβx-40. N-terminal truncation and pyroglutaminylation further enhanced the antimicrobial activity. The differences in the effects of the Aβ peptide variants are most likely due to their physicochemical characteristics. Aβx-42 is much more hydrophobic than Aβx-40. The truncated and pyroglutaminylated N-terminus further enhances this hydrophobicity32,33,34,35. The increased hydrophobicity of Aβx-42 peptides is also reflected by their increased binding to the surface of the microorganisms. Therefore, there is a good correlation between Aβ-peptide hydrophobicity and the binding/agglutination of microorganisms. While the exact mechanism by which Aβ-peptides kill microorganisms remains to be elucidated, it seems as if the heparin binding domain of the Aβ peptide sequence is involved. Mannan and glucan are suggested to bind to Aβ peptides via its heparin-binding site36,37,38. By competitive binding of the heparin binding site by mannan and glucan, the agglutination and binding of Aβ peptides to microbes was effectively inhibited36. Furthermore, binding of the heparin binding site of Aβ peptides promotes their aggregation and the formation of β-sheet structures39,40. This explains, why we found an accelerated aggregation of Aβ1-42 when incubated together with E. coli, S. aureus and C. albicans. Binding of proteoglycans to the heparin-binding site of Aβ peptides has been suggested as an early step in plaque formation39. Eosinophil cationic protein is also an amyloid-like protein. Its antimicrobial activity depends on its ability to agglutinate bacteria and form amyloid fibrils on the bacterial surface24. The eosinophil cationic protein mediated agglutination of bacteria is followed by membrane leakage and cell death24.
The antimicrobial activity of Aβx-42-peptides demonstrated in this study is consistent with previous reports16,36. However, Soscia et al. found, that Aβ1-40 is not as effective as Aβ1-42 but still possesses antimicrobial activity against bacteria and fungi. This effect strongly differed between the investigated microorganisms and was in several bacteria near the limit of detection. In respect to the colony forming units, we only observed an antimicrobial activity of Aβ1-40 against C. albicans. In accordance with the report of Kumar et al., this activity was weaker as for the Aβx-42 peptides. The discrepancy concerning the effect against bacteria might be due to the selection of different bacterial strains or differences in Aβ peptide pretreatment. Aβ peptides are prone to self-aggregation, and the method of synthesis, the solvents used and the time until application strongly affect their conformation and, consequently, their biological activity. Thus, while an antifungal effect of Aβ1-40 was consistently shown, its impact against bacteria needs to be further investigated. Having observed increased phagocytotic activity of monocytes after stimulation with Aβ2-40, we previously suggested, that the more soluble Aβx-40 peptides, may also act as auto- or paracrine factors regulating immune activity28.
The physiological relevance of Aβ peptide antimicrobial activity is supported by several lines of evidence.
In an experimental model of meningitis, survival was reduced in mice that were not able to produce Aβ peptides due to a knockout of its precursor. Reciprocally, mice overexpressing Aβ peptides showed reduced mortality in this model36.
Acute infections of the brain result in reduced Aβ42 levels (but not Aβ40) in cerebrospinal fluid but increased deposition in brain tissue3,41,42,43,44.
Aβ peptide deposition has also been reported in chronic infections of the central nervous system, such as neuroborreliosis, neurosyphilis, HIV or Herpes simplex encephalitis22,45,46,47. Recently, Diana Pisa and her colleagues observed Candida species in brain regions affected by Alzheimer’s disease pathology but not in normal controls48. In our study, Candida was particularly sensitive to Aβ-peptide-induced aggregation and cell death.
The γ-secretase blocker DAPT impairs recovery from lipopolysaccharide-induced inflammation in the rat brain49.
Monocytes, microglia and astrocytes increase the expression of amyloid precursor protein and release Aβ peptides upon activation by lipopolysaccharide50,51,52. In several mouse models, lipopolysaccharide induces amyloid precursor protein expression and subsequent Aβ plaque deposition22.
These findings suggest that the secretion of Aβ-peptides is part of the innate immune defense in the CNS. Aβ-lowering therapies might therefore hamper the resistance of the brain to infections and malignant disorders. The increased neonatal mortality of BACE1/BACE2 knock-out mice only in animals housed under non-sterile conditions supports this assumption53. Special attention should therefore be given to these events during clinical tests of Aβ-lowering therapies. Others have even suggested that an infectious agent may be involved in Alzheimer’s disease pathogenesis22,46,54. Our finding of accelerated Aβ1-42 agglutination in the presence of microorganisms may support this hypothesis. Further research is needed to determine whether Aβ plaque deposition is the consequence of a microbial infection or a mechanism of CNS immune defense that is misled in Alzheimer’s disease.
Additional Information
How to cite this article: Spitzer, P. et al. Amyloidogenic amyloid-β-peptide variants induce microbial agglutination and exert antimicrobial activity. Sci. Rep. 6, 32228; doi: 10.1038/srep32228 (2016).
Acknowledgments
This work was supported by grants from the Interdisciplinary Center for Clinical Research (IZKF), Erlangen. We acknowledge support by Deutsche Forschungsgemeinschaft (DFG) and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding program Open Access Publishing. The present work was performed in fulfillment of the requirements for obtaining the degree “Dr. med” of MC. Technical support from Katrin Jozefowski is gratefully acknowledged.
Footnotes
Author Contributions M.C., M.H., J.M.M., R.L., T.J.O., J.K. and P.S. designed the study. M.C., M.H., R.L. and P.S. performed most of the experiments. Immunocytochemistry was performed and quantified by D.F.G., O.F. and T.G. Electron microscopy was performed by M.-S.M., M.C. and P.S. drafted the manuscript. All authors reviewed the manuscript and provided constructive comments to improve the quality of the manuscript. All authors read and approved the final manuscript.
References
- Chow V. W., Mattson M. P., Wong P. C. & Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med 12, 1–12, doi: 10.1007/s12017-009-8104-z (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltfang J. et al. Highly conserved and disease-specific patterns of carboxyterminally truncated Abeta peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer’s disease and in patients with chronic neuroinflammation. J Neurochem 81, 481–496 (2002). [DOI] [PubMed] [Google Scholar]
- Little C. S. et al. Detection of bacterial antigens and Alzheimer’s disease-like pathology in the central nervous system of BALB/c mice following intranasal infection with a laboratory isolate of Chlamydia pneumoniae. Frontiers in aging neuroscience 6, 304, doi: 10.3389/fnagi.2014.00304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Pareja F., Antequera D., Vargas T., Molina J. A. & Carro E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer’s disease: a pilot study. BMC Neurol 10, 108, doi: 10.1186/1471-2377-10-108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M. et al. Detection of amyloid beta protein in the urine of Alzheimer’s disease patients and healthy individuals. Neurosci Lett 435, 126–130, doi: 10.1016/j.neulet.2008.02.019 (2008). [DOI] [PubMed] [Google Scholar]
- Tharp W. G. & Sarkar I. N. Origins of amyloid-beta. BMC Genomics 14, 290, doi: 10.1186/1471-2164-14-290 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevalle J. et al. Aminopeptidase A contributes to the N-terminal truncation of amyloid beta-peptide. J Neurochem 109, 248–256, doi: 10.1111/j.1471-4159.2009.05950.x (2009). [DOI] [PubMed] [Google Scholar]
- Takeda K., Araki W., Akiyama H. & Tabira T. Amino-truncated amyloid beta-peptide (Abeta5-40/42) produced from caspase-cleaved amyloid precursor protein is deposited in Alzheimer’s disease brain. FASEB J 18, 1755–1757, doi: 10.1096/fj.03-1070fje (2004). [DOI] [PubMed] [Google Scholar]
- Bien J. et al. The metalloprotease meprin beta generates amino terminal-truncated amyloid beta peptide species. J Biol Chem 287, 33304–33313, doi: 10.1074/jbc.M112.395608 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C. Take five–BACE and the gamma-secretase quartet conduct Alzheimer’s amyloid beta-peptide generation. Embo J 23, 483–488 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maler J. M. et al. Urea-based two-dimensional electrophoresis of beta-amyloid peptides in human plasma: evidence for novel Abeta species. Proteomics 7, 3815–3820, doi: 10.1002/pmic.200700311 (2007). [DOI] [PubMed] [Google Scholar]
- Guntert A., Dobeli H. & Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience 143, 461–475 (2006). [DOI] [PubMed] [Google Scholar]
- Portelius E. et al. Identification of novel APP/Abeta isoforms in human cerebrospinal fluid. Neurodegener Dis 6, 87–94, doi: 10.1159/000203774 (2009). [DOI] [PubMed] [Google Scholar]
- Schluesener H. J., Su Y., Ebrahimi A. & Pouladsaz D. Antimicrobial peptides in the brain: neuropeptides and amyloid. Front Biosci (Schol Ed) 4, 1375–1380 (2012). [DOI] [PubMed] [Google Scholar]
- Kagan B. L. et al. Antimicrobial properties of amyloid peptides. Mol Pharm 9, 708–717, doi: 10.1021/mp200419b (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soscia S. J. et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One 5, e9505, doi: 10.1371/journal.pone.0009505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadpanah A. & Gallo R. L. Antimicrobial peptides. J Am Acad Dermatol 52, 381–390; quiz 391-382, doi: 10.1016/j.jaad.2004.08.026 (2005). [DOI] [PubMed] [Google Scholar]
- Kagan B. L. Mode of action of yeast killer toxins: channel formation in lipid bilayer membranes. Nature 302, 709–711 (1983). [DOI] [PubMed] [Google Scholar]
- Zaiou M. Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. J Mol Med (Berl) 85, 317–329, doi: 10.1007/s00109-006-0143-4 (2007). [DOI] [PubMed] [Google Scholar]
- Kriz Z., Klusak J., Kristofikova Z. & Koca J. How ionic strength affects the conformational behavior of human and rat beta amyloids–a computational study. PLoS One 8, e62914, doi: 10.1371/journal.pone.0062914 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise-Scira O., Xu L., Kitahara T., Perry G. & Coskuner O. Amyloid-beta peptide structure in aqueous solution varies with fragment size. J Chem Phys 135, 205101, doi: 10.1063/1.3662490 (2011). [DOI] [PubMed] [Google Scholar]
- Miklossy J. Emerging roles of pathogens in Alzheimer disease. Expert Rev Mol Med 13, e30, doi: 10.1017/S1462399411002006 (2011). [DOI] [PubMed] [Google Scholar]
- Gorr S. U., Sotsky J. B., Shelar A. P. & Demuth D. R. Design of bacteria-agglutinating peptides derived from parotid secretory protein, a member of the bactericidal/permeability increasing-like protein family. Peptides 29, 2118–2127, doi: 10.1016/j.peptides.2008.09.019 (2008). [DOI] [PubMed] [Google Scholar]
- Torrent M., Pulido D., Nogues M. V. & Boix E. Exploring new biological functions of amyloids: bacteria cell agglutination mediated by host protein aggregation. PLoS Pathog 8, e1003005, doi: 10.1371/journal.ppat.1003005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renggli S., Keck W., Jenal U. & Ritz D. Role of autofluorescence in flow cytometric analysis of Escherichia coli treated with bactericidal antibiotics. J Bacteriol 195, 4067–4073, doi: 10.1128/JB.00393-13 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayles K. W. Bacterial programmed cell death: making sense of a paradox. Nature reviews. Microbiology 12, 63–69, doi: 10.1038/nrmicro3136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. F., Meinhof T., Pepperkok R. & Runz H. DetecTiff: a novel image analysis routine for high-content screening microscopy. J Biomol Screen 14, 944–955, doi: 10.1177/1087057109339523 (2009). [DOI] [PubMed] [Google Scholar]
- Condic M. et al. N-truncation and pyroglutaminylation enhances the opsonizing capacity of Abeta-peptides and facilitates phagocytosis by macrophages and microglia. Brain Behav Immun, doi: 10.1016/j.bbi.2014.05.003 (2014). [DOI] [PubMed] [Google Scholar]
- Thal D. R., Ghebremedhin E., Haass C. & Schultz C. UV light-induced autofluorescence of full-length Abeta-protein deposits in the human brain. Clin Neuropathol 21, 35–40 (2002). [PubMed] [Google Scholar]
- Chan F. T. et al. Protein amyloids develop an intrinsic fluorescence signature during aggregation. The Analyst 138, 2156–2162, doi: 10.1039/c3an36798c (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercatelli R. et al. Clasmatodendrosis and beta-amyloidosis in aging hippocampus. FASEB J 30, 1480–1491, doi: 10.1096/fj.15-275503 (2016). [DOI] [PubMed] [Google Scholar]
- Schlenzig D. et al. Pyroglutamate formation influences solubility and amyloidogenicity of amyloid peptides. Biochemistry 48, 7072–7078, doi: 10.1021/bi900818a (2009). [DOI] [PubMed] [Google Scholar]
- Pike C. J., Overman M. J. & Cotman C. W. Amino-terminal deletions enhance aggregation of beta-amyloid peptides in vitro. The Journal of biological chemistry 270, 23895–23898 (1995). [DOI] [PubMed] [Google Scholar]
- Meral D. & Urbanc B. Discrete molecular dynamics study of oligomer formation by N-terminally truncated amyloid beta-protein. J Mol Biol 425, 2260–2275, doi: 10.1016/j.jmb.2013.03.010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling S. et al. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro). Biochemistry 45, 12393–12399, doi: 10.1021/bi0612667 (2006). [DOI] [PubMed] [Google Scholar]
- Kumar D. K. et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 8, 340ra372, doi: 10.1126/scitranslmed.aaf1059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K. & Rabenstein D. L. Interaction of the Heparin-Binding Consensus Sequence of beta-Amyloid Peptides with Heparin and Heparin-Derived Oligosaccharides. The journal of physical chemistry. B 120, 2187–2197, doi: 10.1021/acs.jpcb.5b12235 (2016). [DOI] [PubMed] [Google Scholar]
- Cardin A. D. & Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9, 21–32 (1989). [DOI] [PubMed] [Google Scholar]
- Klajnert B., Cortijo-Arellano M., Bryszewska M. & Cladera J. Influence of heparin and dendrimers on the aggregation of two amyloid peptides related to Alzheimer’s and prion diseases. Biochem Biophys Res Commun 339, 577–582, doi: 10.1016/j.bbrc.2005.11.053 (2006). [DOI] [PubMed] [Google Scholar]
- McLaurin J., Franklin T., Zhang X., Deng J. & Fraser P. E. Interactions of Alzheimer amyloid-beta peptides with glycosaminoglycans effects on fibril nucleation and growth. European journal of biochemistry/FEBS 266, 1101–1110 (1999). [DOI] [PubMed] [Google Scholar]
- Krut J. J. et al. Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. J Neurol 260, 620–626, doi: 10.1007/s00415-012-6688-y (2013). [DOI] [PubMed] [Google Scholar]
- Mattsson N. et al. Neuroinflammation in Lyme neuroborreliosis affects amyloid metabolism. BMC Neurol 10, 51, doi: 10.1186/1471-2377-10-51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren M., Gisslen M., Vanmechelen E. & Blennow K. Low cerebrospinal fluid beta-amyloid 42 in patients with acute bacterial meningitis and normalization after treatment. Neurosci Lett 314, 33–36, doi: S0304394001022856 [pii] (2001). [DOI] [PubMed] [Google Scholar]
- McManus R. M., Higgins S. C., Mills K. H. & Lynch M. A. Respiratory infection promotes T cell infiltration and amyloid-beta deposition in APP/PS1 mice. Neurobiol Aging 35, 109–121, doi: 10.1016/j.neurobiolaging.2013.07.025 (2014). [DOI] [PubMed] [Google Scholar]
- Andras I. E. & Toborek M. Amyloid beta accumulation in HIV-1-infected brain: The role of the blood brain barrier. IUBMB Life 65, 43–49, doi: 10.1002/iub.1106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawanda F. & Wallace R. Can Infections Cause Alzheimer’s Disease? Epidemiol Rev, doi: 10.1093/epirev/mxs007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki R. F. Herpes simplex virus type 1 and Alzheimer’s disease: increasing evidence for a major role of the virus. Frontiers in aging neuroscience 6, 202, doi: 10.3389/fnagi.2014.00202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa D., Alonso R., Rabano A., Rodal I. & Carrasco L. Different Brain Regions are Infected with Fungi in Alzheimer’s Disease. Scientific reports 5, 15015, doi: 10.1038/srep15015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasoohi S., Hemmati A. A., Moradi F. & Ahmadiani A. The gamma-secretase blocker DAPT impairs recovery from lipopolysaccharide-induced inflammation in rat brain. Neuroscience 210, 99–109, doi: 10.1016/j.neuroscience.2012.02.051 (2012). [DOI] [PubMed] [Google Scholar]
- Spitzer P. et al. Phagocytosis and LPS alter the maturation state of b-amyloid precursor protein and induce different Ab peptide release signatures in human mononuclear phagocytes. Journal of neuroinflammation 7, 59 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitting L., Naidu A., Cordell B. & Murphy G. M. Beta-amyloid peptide secretion by a microglial cell line is induced by beta-amyloid-(25-35) and lipopolysaccharide. The Journal of biological chemistry 271, 16084–16089 (1996). [DOI] [PubMed] [Google Scholar]
- Monning U. et al. Synthesis and secretion of Alzheimer amyloid beta A4 precursor protein by stimulated human peripheral blood leucocytes. FEBS Lett 277, 261–266, doi: 0014-5793(90)80861-C [pii] (1990). [DOI] [PubMed] [Google Scholar]
- Dominguez D. et al. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem 280, 30797–30806, doi: 10.1074/jbc.M505249200 (2005). [DOI] [PubMed] [Google Scholar]
- Itzhaki R. F. et al. Microbes and Alzheimer’s Disease. J Alzheimers Dis 51, 979–984, doi: 10.3233/JAD-160152 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]