Abstract
Unlike other organs that operate continuously, such as the heart and kidneys, many of the operations of the nervous system shut down during sleep. The evolutionarily conserved unconscious state of sleep that puts animals at risk from predators indicates that it is an indispensable integral part of systems operation. A reasonable expectation is that any hypothesis for the mechanism of the nervous system functions should be able to provide an explanation for sleep. In this regard, the semblance hypothesis is examined. Postsynaptic membranes are continuously being depolarized by the quantally-released neurotransmitter molecules arriving from their presynaptic terminals. In this context, an incidental lateral activation of the postsynaptic membrane is expected to induce a semblance (cellular hallucination of arrival of activity from its presynaptic terminal, which forms a unit for internal sensation) of the arrival of activity from its presynaptic terminal as a systems property. This restricts induction of semblance to a context of a very high ratio of the duration of the default state of neurotransmitter-induced postsynaptic depolarization to the total duration of incidental lateral activations of the postsynaptic membrane. This requirement spans within a time-bin of a few sleep-wake cycles. Since the duration of quantal release remains maximized, the above requirement can be achieved only by ceiling the total duration of incidental lateral activations of the postsynaptic membrane, which necessitates a state of sleep.
Keywords: Quantal release, Duration of quantal release, Systems requirement for sleep, Semblance, Semblance hypothesis, Lateral postsynaptic activation
1. Introduction
Several functional roles are attributed to sleep. One view is that sleep is required for energy conservation and nervous system recuperation [1]. Even though glucose consumption is reduced during the slow wave activity (SWA) phase of sleep relative to the wake state [3], a certain amount of energy is consumed for synaptic activity during sleep [2]. Cellular energetics during sleep are found to be associated with metabolic function and gene transcription [4] and sleep promotes mRNA translation for protein synthesis [5]. However, there are no causal factors indicating the need for sleep either for gene expression or metabolic activity. Even though unwanted metabolic products from the adult brain are cleared during sleep [6], there are no indications why this function requires a reduction in the level of consciousness. Other systems in the body that operate throughout the life of animals, such as the heart and kidneys, do not shut down their major functions at any time. In contrast, the nervous system alters the level of consciousness during sleep, putting the animal at risk for attack by its predators. This leads to the question – What specific function of the nervous system necessitates altering its basic function of maintaining the state of consciousness, a necessary background state required for carrying out other higher brain functions?
Sleep is thought to facilitate learning and memory [7], memory consolidation [8], consolidation of cortical plasticity [9] and cortical response potentiation following visual experience [10]. However, these experimental findings are not sufficient to provide an adequate explanation for the state of unconsciousness associated with sleep [11]. The observation that mutation in a transcriptional repressor reduces sleep by two hours every day [12] has not yet provided an explanation for the necessity for sleep. In this context, different proposals for possible mechanisms for sleep were put forward [13], [14]. Even though energy conservation, rejuvenation and facilitation of learning and memory can be contributing factors, a mechanism that results in losing consciousness that puts the animal at severe risk for survival is still lacking. This naturally raises the question – Why did not evolution eliminate the need for sleep that would have enabled animals to remain conscious throughout the twenty-four-hour period? Conservation of sleep indicates that sleep has an indispensable role in nervous system operations. Sleep-like states observed in lower-level animal species such as flies and worms [15], [16], [17] indicate a universal functional role for sleep.
Since the normal mechanism of functioning of the system has not yet been discovered, any new theoretical framework of a mechanism is expected to provide an explanation for the functional role of sleep [18]. Difficulties in understanding the mechanisms of higher brain functions still continue, primarily because these functions are first-person internal sensations to which only the owner of the nervous system has access. In this context, the semblance hypothesis [19], [20] that was put forward to explain the observations made from both the first- and third-person frames of reference at various levels is examined. The hypothesis was developed from the premise that the basic units of internal sensations, namely semblances, are induced as a systems property at the level of the postsynaptic terminals and occur in synchrony with extracellularly-recorded oscillating potentials of specific frequencies. To obtain an explanation for the necessity for sleep, essential conditions for the systems property of induction of semblances are examined.
2. Background
The quantal release of neurotransmitter molecules from the presynaptic terminal occurs all the time (tq), including during rest and sleep (Fig. 1A). The binding of these molecules to the postsynaptic membrane receptors induces a very tiny voltage that is reflected in the measured miniature postsynaptic potentials (minis) from the latter's neuronal soma. These quantal release-mediated potentials are continuously being induced at the postsynaptic membrane (postsynapse or dendritic spine). In addition, various stimuli from both the environment and from within the body, which arrive at the sensory receptors activate the sensory neurons, which are then transmitted through different higher neuronal orders. When this activity arrives at the presynaptic terminal, it leads to the entry of calcium into the cytoplasm that, in turn, leads to the release of a volley of neurotransmitter molecules from the presynaptic terminal into the synaptic cleft. This induces a large postsynaptic potential (Fig. 1B). Let this duration of activity-induced postsynaptic activation be (ts). The unidirectional flow of activity from the presynaptic terminal to the postsynaptic terminal at the chemical synapses can contribute to the vertical component of the extracellularly-recorded oscillatory potentials. Since recurrent collaterals do not present in sufficient numbers at different neuronal orders and since the presence of gap junctions between excitatory neurons is sparse [21], mechanisms that can contribute to the horizontal component of the oscillating potentials are yet to be discovered.
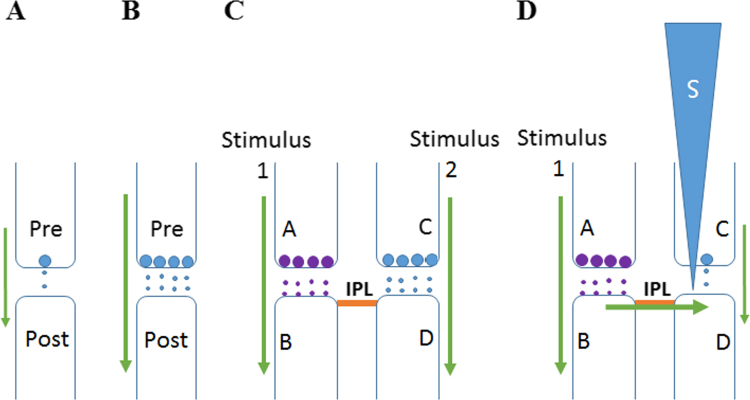
Fig. 1.
Illustration showing the structural mechanism for the formation of internal sensations of various higher brain functions. A: Continuous quantally released neurotransmitter molecules from the presynaptic terminal bind to the receptors on the postsynaptic membrane and induce a very tiny potential at the postsynaptic membrane. B: The intermittent arrival of a sensory stimulus induces an action potential, which when reaches the presynaptic terminal releases a volley of neurotransmitter molecules into the synaptic cleft. This induces a large postsynaptic potential at the postsynaptic membrane. C: When two abutted postsynaptic terminals are activated simultaneously, during events such as associative learning, it leads to the formation of an inter-postsynaptic functional LINK (IPL). D: At a later time, when the cue stimulus arrives at presynaptic terminal A and activates its postsynaptic terminal B, it leads to the reactivation of IPL and activates inter-LINKed postsynaptic terminal D. The lateral activation of postsynaptic terminal D in the absence of arrival of activity from its presynaptic terminal C induces a cellular hallucination or semblance of arrival of activity from presynaptic terminal C. The sensory identity of the semblance induced at postsynaptic terminal D can be determined by extrapolating towards the sensory receptor level to discover hypothetical packets of sensory stimuli capable of activating presynaptic terminal C (for details see [20]). The induction of semblances is viewed as a systems property of systems where the lateral entry of activity through the IPL contributes to the horizontal component of the extra-cellularly recorded oscillating potentials.
Each event or item in the environment is capable of stimulating more than one sensory system. When the event or item is close to the animal, the simultaneous arrival of these sensory inputs enables them to get associated within the animal's nervous system. Later, when the event or item is located away from the animal, the fastest arriving stimulus (usually visual) from the event or item induces a virtual internal sensation of the remaining stimuli from the latter. This provides the animal with a survival advantage in the environment where it has to obtain food and stay protected from its predators. These processes require a mechanism to both associatively store the information and then make internal sensations of the late-arrived or even non-arrived sensations, so that the animal can respond at appropriate times to the environmental stimuli. This is essential for both the prey and the predator to make quick internal decisions that enable them to survive in the environment. The process of associative learning during the exposure to a novel event or item was hypothesized to occur through the induction of an inter-postsynaptic functional LINK (IPL) between the simultaneously activated postsynaptic terminals at locations of convergence of different sensory stimuli [19], [20] (Fig. 1C).
At a later point of time, when the fastest arriving stimulus used in a previous associative learning event reaches the nervous system, it reactivates the IPL and activates the inter-LINKed postsynaptic terminal from a lateral direction (Fig. 1D). When the postsynaptic terminal is in a background state of continuous depolarization induced by the neurotransmitter molecules released from its presynaptic terminal (Fig. 1A), any “incidental” lateral activation of the postsynaptic terminal through the IPL is expected to induce a semblance of the arrival of activity from its presynaptic terminal as a systems property (see Fig. 1 for further description). Semblance is an expected event of cellular hallucination induced at the inter-LINKed postsynaptic terminal [19], [20]. Such an operation is essential for inducing the function of internal sensations; for example, memory [22]. Another integral part of the system property is that the lateral entry of activity through the IPL can contribute to the horizontal component of the oscillating potentials recorded extracellularly [23]. During the wake state, maintaining the extracellularly-recorded oscillating potentials at a certain frequency range is essential for inducing the internal sensations of various higher brain functions. For example, as the background frequency recorded by the electro-encephalogram (EEG) waves is reduced, there is a gradual reduction in the level of awareness that eventually leads to a state of unconsciousness [24].
3. Theoretical findings
3.1. Systems requirement for sleep
In the context of the operational mechanism of semblances, the question “Why would sleep be evolutionarily conserved across species although putting the animals at risk for survival?” is examined for possible explanations. The fact that sleep deprivation reduces cognitive abilities [25], [26], indicates that the ability to induce specific internal sensations of memory is reduced during the wake state after sleep deprivation. What systems requirement can be fulfilled by sleep in preparing the system for maintaining optimal cognitive abilities? How will the integral nature of sleep enable the induction of specific internal sensations during the wake state? Why is there a period of sleep during which no internal sensations are generated in response to ordinary environmental stimuli?
According to the semblance hypothesis, it is expected that an incidental lateral activation of the inter-LINKed postsynaptic terminal can induce semblances. There are two possible mechanisms by which the incidental nature of the lateral activation can be accomplished. One is maximizing the duration of the quantal release of neurotransmitter molecules from the presynaptic terminal towards the postsynaptic membrane, as reflected by the recorded miniature postsynaptic potentials (minis). Since quantal release takes place all the time in a twenty-four-hour period, it is already a saturated phase of the system. The fact that there are no natural or synthetic molecules that can completely block the miniature postsynaptic potentials is strong evidence for the latter's evolutionary conservation on Earth. The second possible mechanism is to reduce the total duration of the lateral activation of the inter-LINKed postsynaptic terminal by various cue stimuli. This can only be achieved by either reducing the arrival of the cue stimuli or by reducing the ability of the arriving stimuli to reach the IPLs. These methods can substantially increase the “relative” total duration of quantal release of neurotransmitter molecules in comparison to that of the total duration of events that activate the inter-LINKed postsynaptic terminal (tq+ts)≫tIPL (Fig. 2).
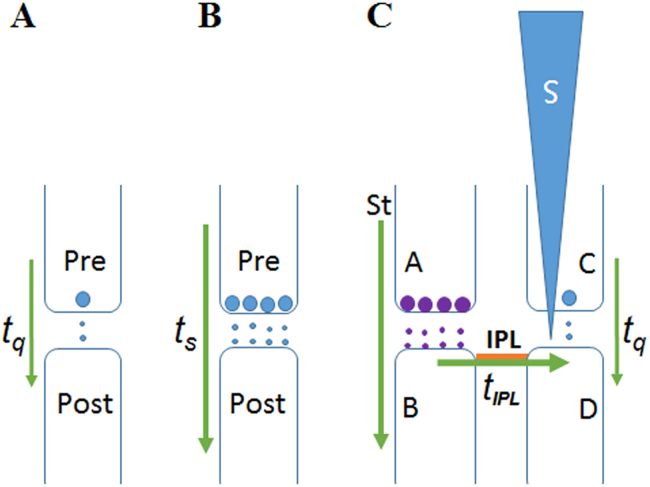
Fig. 2.
Diagram showing the durations of quantal release, activation of synapse and reactivation of inter-postsynaptic functional LINK (IPL). A: Quantal release of the neurotransmitter molecules takes place from the presynaptic terminal all the time. Therefore, this duration (tq) is a saturated phase for a given system. B: Intermittent release of a volley of transmitter molecules when activity arrives at the presynaptic terminal. Let the duration be (ts). C: This figure shows lateral activation of inter-LINKed postsynaptic terminal D by the arrival of activity through the IPL. Let this duration be tIPL. Semblance is expected to be induced only when the lateral activation of postsynaptic terminal D occurs infrequently when compared to the relatively highly frequent occurrence of the neurotransmitter-induced depolarization of postsynaptic terminal D (from presynaptic terminal C). In other words, it is necessary to maintain a high [(tq+ts)/tIPL] ratio. The only means by which this can be accomplished is by stopping the lateral activation of postsynaptic terminal D for a certain period of time. This is achieved by inducing a state of sleep. A and C: Presynaptic terminals. B and D: Postsynaptic terminals. tq: Duration of quantal release; ts: Duration of neurotransmission; tIPL: Duration of lateral activation of the inter-LINKed postsynaptic terminal D by activity arriving at synapse A-B. S: Semblance. IPL: Inter-postsynaptic functional LINK. St: Stimulus.
3.2. Can semblances stop forming beyond a breakpoint?
If sleep is an integral systems requirement to increase the relative total duration of quantal release of neurotransmitter molecules in comparison to that of the induction of internal sensations, then for how long can the semblances be induced after continuous sleep deprivation, if everything else remains optimal? Assuming that the relative total duration of quantal release of the neurotransmitter molecules has been higher than the total duration of the induction of internal sensations during the previous days, normal semblances can be induced during the initial days after stopping sleep. However, as sleep deprivation continues, the robustness of internal sensations of higher brain functions will be reduced. From the requirements, it can be seen that the system needs constant updating of the relative increase in [(tq+ts)/tIPL] ratio. This ratio from the previous few days likely gets carried forward to some extent. This may explain why a few nights of sleep deprivation may be followed by an increased requirement for sleep. Therefore, it is reasonable to arrive at the conclusion that sleep helps to increase the [(tq+ts)/tIPL] ratio within a time-bin of a few sleep-wake cycles. This ratio likely determines the robustness of the semblances for an optimal internal sensation of various higher brain functions during the wake period. Such a mechanism provides an explanation for why different components of memory are affected after continuous sleep deprivation [25], [26], [27]. Since the induction of semblances is essential for maintaining the net C-semblance for consciousness [28], eventually there will be a loss of consciousness, which will put the animal back into a sleep state. In some animals, sleep deprivation is reported to cause death [29]. If sleep deprivation does not produce damage to the IPL-operated neural circuit system [20] and the organs whose functions are dependent on them, the animal is expected to wake up from sleep.
3.3. Sequential implementation of the mechanism
The nervous system requires sleep for an average of eight hours in a twenty-four-hour period. During this period, the postsynaptic terminals are undergoing continuous depolarization from quantal release, whereas the activation of inter-LINKed postsynaptic terminals occurs much less frequently. During sleep, the nervous system has to be kept in a state from which it can be aroused. This requires the maintenance of C-semblance at certain threshold levels ready to be activated at the arrival of sensory stimuli above certain level of intensity. Depending on the alteration in the conformation of C-semblance during different stages of sleep, the nervous system remains at different arousable states. When the frequency of oscillating potentials keeps changing during different stages of sleep, the sets of reactivated inter-postsynaptic functional LINKs that contribute to the horizontal component of the oscillatory potentials also change. This provides rest for several other sets of inter-postsynaptic functional LINKs at different stages of sleep. In this regard, the observation that wakefulness, rapid eye movement (REM) and non-REM sleep are not mutually exclusive states is significant [30]. The absence of lateral activation of inter-LINKed postsynaptic terminals provides ample time for updating the required [(tq+ts)/tIPL] ratio well above the threshold level. The frequency of oscillating potentials during the REM sleep stage returns to a state close to that of the wake state during the last few cycles of sleep. When the C-semblance during the final REM sleep stage reaches a state almost identical to that of the C-semblance for the conscious state, the nervous system wakes up.
3.4. Mechanism to shut down the sensory inputs
Since light travels faster than all other sensory stimuli, visual cue stimuli from an item or event in the environment provide the animal with the internal sensation of the remaining associatively learned sensory qualities of that item or event. This is highly important for survival. The presence of light during the day is optimal for diurnal animals – it enables predators to capture prey and prey to escape from predators. The lack of light at night reduces visibility for both the prey and the predator, making them equally disadvantaged. This makes night-time an optimal period for sleep for diurnal animals. Reduced light at night reduces visual inputs arriving at the nervous system. However, night-time is not completely devoid of light; the presence of moonlight is an example. During sleep, eyelid closure may be sufficient to stop visual inputs arriving. However, other primary sensations such as hearing, smell, touch, and vibration have no mechanism to shut their receptors from getting activated when the corresponding stimuli arrive. These stimuli propagate to higher neuronal orders to activate the IPLs along their route. Some of these inputs are essential for the survival of the animal. For example, pain induced while sleeping in one position encourages change of position, thus avoiding tissue injury and the formation of decubitus ulcers. However, the sensation of pain is not felt consciously, because the conformation of C-semblance for normal consciousness is altered when the oscillating potentials are of different frequencies during sleep [28].
3.5. Range of total duration of lateral activation
A rough estimate of the [(tq+ts)/tIPL] ratio can be calculated for one normal sleep-wake cycle as follows. Since the quantal release of neurotransmitter molecules takes place continuously, tq is equal to 24 h. Since stimuli from the environment lead to activity-mediated synaptic transmission, the duration of ts is a fraction of the awake period of 16 h. ts can vary from synapse to synapse. Let ts1 be the total duration of arrival of activity at one synapse from its presynaptic terminal. Therefore, the total duration of depolarization of the postsynaptic membrane of that synaspe by the neurotransmitter molecules is equal to (24+ts1) hours. Semblance is expected to get induced for a wide range of duration of lateral activation of that postsynaptic terminal – ranging from a tiny fraction (few seconds) to the entire duration of the wake period (nearly 16 h). Even though it is very rare for a single stimulus (cue) to arrive during the entire wake period, shared physical properties of the items and events in the environment can lead to the continuous arrival of stimulus components that are shared among different environmental stimuli. This may activate certain specific IPLs for long periods of time during the wake period. On the other hand, as the animal explores new environments, the tIPL for a certain specific inter-LINKed postsynapse is going to be very small. Therefore, semblances are expected to be associated with a wide range of tIPL.
The duration of lateral activation of the inter-LINKed postsynaptic terminals inducing semblances is expected to vary among different higher brain functions. It is possible that the ratio [(tq+ts)/tIPL] varies depending on the nature of semblances, locations where they are induced and the mode of their integration. For example, consciousness and memory retrieval can be examined. The surgical removal of large portions of the cortex has no effect on consciousness [31]. This indicates that a large number of redundant units for inducing internal sensations are likely to contribute to the generation of C-semblance for consciousness [28]. Based on the present work, such redundancy may provide rest to different subsets of IPLs during the wake period. It is also possible that the quality of semblances that contribute to consciousness are different from other higher brain functions. In contrast to consciousness, highly robust semblances are expected to induce internal sensations of memory of specific events or items in response to a cue stimulus. The number of specific IPLs that can be formed by a specific novel associative learning event can be finite. Changes in the environment may not always bring very high cue stimulus specificity for the lateral activation of a specific set of postsynapases to induce internal sensations for specific memories. Due to this limitation, each structural unit capable of inducing robust semblance is expected to maintain an optimal [(tq+ ts)/tIPL] ratio for inducing semblances. A high quality of semblances is required for their integrated product to form the internal sensation of an expected memory matching that of a specific event or item.
3.6. Supporting evidence
Is the theoretical observation explained here testable? One method is to examine whether an alteration in the duration and/or number of external environmental stimuli has any relationship with the duration of sleep. This can be examined in conditions where the subjects are exposed to different amounts of environmental stimuli. Staying awake for up to twenty-four hours results in progressively higher slow-wave activity (SWA) levels (power density in the 0.75 Hz to 4.5 Hz range) levels at sleep onset [32], [33]. Specifically, SWA in non-rapid eye movement (NREM) sleep increases significantly during the initial sleep cycles of recovery sleep. Conversely, an evening nap reduces power density in NREM sleep in the delta and theta bands in EEG [34]. It was found that the duration of sleep changes proportional to the duration of wakefulness and that periods of silence of the cortical neurons are long and frequent during NREM sleep following sustained wakefulness [34], [35]. This will reduce the instances of lateral activation of IPLs activating the inter-LINKed postsynaptic terminals at several upstream neural pathways.
Since a large proportion of the lateral activation of the inter-LINKed postsynaptic terminals takes place by continuous arrival of stimuli from the environment, removing the animals from their natural environment is likely to reduce the duration of sleep. In one space mission study, it was found by both subjective and objective methods that the duration of sleep during space stay is approximately 6.5 h per day, which is less than expected [36]. Several previous space mission studies have also shown a consistent reduction in the duration of sleep between 6 and 6.5 h while living in space for nearly two weeks [37], [38], [39], [40], [41], [42]. Along with the reduction in the number of environmental stimuli, a large number of sensory inputs from within the body will also be reduced due to the absence of gravity. For example, the tendon reflex, which is a stretch reflex, is reduced after remaining in space [43]. The duration of lateral activation of inter-LINKed postsynaptic terminals is expected to be reduced in space due to deficient sensory stimuli in comparison to that available on earth. This may explain why the duration of sleep is reduced during space stay and supports the present theoretical observations.
4. Conclusion
The present work has provided a theoretical reasoning for the systems requirement for sleep. The work also highlights the absence of any natural molecules on Earth that can completely block the miniature postsynaptic potentials, making it a favorable environment where any nervous systems operate. Since maintaining the duration of sleep is essential for the very property of inducing semblances responsible for the internal sensation of various higher brain functions, sleep cannot be substituted with anything else, including stimulants such as caffeine. Since the requirement for sleep is expected to be reduced during space flight, using medications to increase the duration of sleep is not necessary. However, maintaining sleep hygiene by reducing sensory stimuli during sleep will be essential. Attempting to associate new sensory stimuli and challenging to retrieve memories of them is expected to increase pressure on the system to undergo adequate sleep. The present work invites the question whether an engineered system built to simulate the nervous system will require sleep for its functions. Since sleep is an indispensable requirement for systems operations, that even evolution could not eliminate, cut short or bypass, artificial systems are expected to require a state of sleep. Experiments can be undertaken to examine whether increasing the frequency and number of quantal releases per unit area can reduce the duration of the required sleep. The present explanation evolved from examining the nervous system functions from a first-person frame of reference using the semblance hypothesis and can be subjected to further verifications.
Competing interests
Author has applied for U.S. patent (number 14/068,835) for an electronic circuit model of the inter-postsynaptic functional LINK.
Acknowledgements
Author acknowledges the support from Neurosearch Center (Grant number: 3:24/2014).
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Siegel J.M. Clues to the functions of mammalian sleep. Nature. 2005;437(7063):1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris J.J., Jolivet R., Attwell D. Synaptic energy use and supply. Neuron. 2012;75(5):762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Netchiporouk L., Shram N., Salvert D., Cespuglio R. Brain extracellular glucose assessed by voltammetry throughout the rat sleep-wake cycle. Eur J Neurosci. 2001;13(7):1429–1434. doi: 10.1046/j.0953-816x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 4.Wisor J.P. A metabolic-transcriptional network links sleep and cellular energetics in the brain. Pflug Arch. 2012;463(1):15–22. doi: 10.1007/s00424-011-1030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seibt J., Dumoulin M.C., Aton S.J., Coleman T., Watson A., Naidoo N., Frank M.G. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol. 2012;22(8):676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D.J., Nicholson C., Iliff J.J., Takano T., Deane R., Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maquet P. The role of sleep in learning and memory. Science. 2001;294(5544):1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 8.Diekelmann S., Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 9.Aton S.J., Seibt J., Dumoulin M., Jha S.K., Steinmetz N., Coleman T., Naidoo N., Frank M.G. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61(3):454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aton S.J., Suresh A., Broussard C., Frank M.G. Sleep promotes cortical response potentiation following visual experience. Sleep. 2014;37(7):1163–1170. doi: 10.5665/sleep.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger J.M., Frank M.G., Wisor J.P., Roy S. Sleep function: toward elucidating an enigma. Sleep Med Rev. 2015;28:42–50. doi: 10.1016/j.smrv.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Y., Jones C.R., Fujiki N., Xu Y., Guo B., Holder J.L., Jr, Rossner M.J., Nishino S., Fu Y.H. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325(5942):866–870. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rial R.V., Nicolau M.C., Gamundí A., Akaârir M., Aparicio S., Garau C., Tejada S., Roca C., Gené L., Moranta D., Esteban S. The trivial function of sleep. Sleep Med Rev. 2007;11(4):311–325. doi: 10.1016/j.smrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Siegel J.M. Sleep viewed as a state of adaptive inactivity. Nat Rev Neurosci. 2009;10(10):747–753. doi: 10.1038/nrn2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendricks J.C., Finn S.M., Panckeri K.A., Chavkin J., Williams J.A., Sehgal A., Pack A.I. Rest in Drosophila is a sleep-like state. Neuron. 2000;25(1):129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 16.Shaw P.J. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287(5459):1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 17.Trojanowski N.F., Raizen D.M. Call it worm sleep. Trends Neurosci. 2016;39(2):54–62. doi: 10.1016/j.tins.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolau M.C., Akaârir M., Gamundí A., González J., Rial R.V. Why we sleep: the evolutionary pathway to the mammalian sleep. Prog Neurobiol. 2000;62(4):379–406. doi: 10.1016/s0301-0082(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 19.Vadakkan KI. 2007. Semblance of Activity at the shared Postsynaptic Terminals and Extracellular Matrices – A (structure) (function hypothesis of memory. iUniverse publishers). ISBN: 978-0-595-47002-0
- 20.Vadakkan K.I. A supplementary circuit rule-set for the neuronal wiring. Front Hum Neurosci. 2013;7:170. doi: 10.3389/fnhum.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercer A. Electrically coupled excitatory neurons in cortical regions. Brain Res. 2012;1487:192–197. doi: 10.1016/j.brainres.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 22.Minsky M. K-lines: a theory of memory. Cognit Sci. 1980;4:117–133. [Google Scholar]
- 23.Vadakkan K.I. The functional role of all postsynaptic potentials examined from a first-person frame of reference. Rev Neurosci. 2015;27(2):159–184. doi: 10.1515/revneuro-2015-0036. [DOI] [PubMed] [Google Scholar]
- 24.De Gennaro L., Ferrara M., Bertini M. The boundary between wakefulness and sleep: quantitative electroencephalographic changes during the sleep onset period. Neuroscience. 2001;107(1):1–11. doi: 10.1016/s0306-4522(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 25.Durmer J.S., Dinges D.F. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 26.Alhola P., Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3(5):553–567. [PMC free article] [PubMed] [Google Scholar]
- 27.Turner T.H., Drummond S.P., Salamat J.S., Brown G.G. Effects of 42 h of total sleep deprivation on component processes of verbal working memory. Neuropsychology. 2007;21(6):787–795. doi: 10.1037/0894-4105.21.6.787. [DOI] [PubMed] [Google Scholar]
- 28.Vadakkan K.I. Framework of consciousness from semblance of activity at functionally LINKed postsynaptic membranes. Front Psychol. 2010;1:168. doi: 10.3389/fpsyg.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rechtschaffen A., Gilliland M.A., Bergmann B.M., Winter J.B. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221(4606):182–184. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 30.Mahowald M.W., Schenck C.H. Insights from studying human sleep disorders. Nature. 2005;437(7063):1279–1285. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- 31.Austin G.M., Grant F.C. Physiologic observations following total hemispherectomy in man. Surgery. 1958;38:239–258. [PubMed] [Google Scholar]
- 32.Borbély A.A., Baumann F., Brandeis D., Strauch I., Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51(5):483–495. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 33.Dijk D.J., Brunner D.P., Borbély A.A. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258(3 Pt 2):R650–R661. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 34.Werth E., Dijk D.J., Achermann P., Borbély A.A. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271(3 Pt 2):R501–R510. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 35.Vyazovskiy V.V., Olcese U., Lazimy Y.M., Faraguna U., Esser S.K., Williams J.C., Cirelli C., Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63(6):865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dijk D.J., Neri D.F., Wyatt J.K., Ronda J.M., Riel E., Ritz-De Cecco A., Hughes R.J., Elliott A.R., Prisk G.K., West J.B., Czeisler C.A. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1647–R1664. doi: 10.1152/ajpregu.2001.281.5.R1647. [DOI] [PubMed] [Google Scholar]
- 37.Frost J.D., Jr, Shumate W.H., Salamy J.G., Booher C.R. Sleep monitoring: the second manned Skylab mission. Aviat Space Environ Med. 1976;47(4):372–382. [PubMed] [Google Scholar]
- 38.Gundel A., Nalishiti V., Reucher E., Vejvoda M., Zulley J. Sleep and circadian rhythm during a short space mission. Clin Investig. 1993;71(9):718–724. doi: 10.1007/BF00209726. [DOI] [PubMed] [Google Scholar]
- 39.Gundel A., Polyakov V.V., Zulley J. The alteration of human sleep and circadian rhythms during spaceflight. J Sleep Res. 1997;6(1):1–8. doi: 10.1046/j.1365-2869.1997.00028.x. [DOI] [PubMed] [Google Scholar]
- 40.Monk T.H., Buysse D.J., Billy B.D., Kennedy K.S., Willrich L.M. Sleep and circadian rhythms in four orbiting astronauts. J Biol Rhythm. 1998;13:188–201. doi: 10.1177/074873098129000039. [DOI] [PubMed] [Google Scholar]
- 41.Santy P.A., Kapanka H., Davis J.R., Stewart D.F. Analysis of sleep on Shuttle missions. Aviat Space Environ Med. 1998;59(11 Pt 1):1094–1097. [PubMed] [Google Scholar]
- 42.Gundel A., Drescher J., Polyaki Quantity and quality of sleep during the record manned space flight of 438 days. Hum Factors Aerosp Saf. 2001;1:87–98. [Google Scholar]
- 43.Baker J.T., Nicogossian A.E., Hoffler G.W., Johnson R.L. Measurement of a single tendon reflex in conjunction with a myogram: the second manned Skylab mission. Aviat Space Environ Med. 1976;47(4):400–402. [PubMed] [Google Scholar]