Abstract
The aim of this study was to evaluate the association between sleeping habits, Mediterranean diet pattern, and weight status in an adolescent population. The sample consisted of 1586 individuals aged 11–14 years attending 15 secondary schools of Sicily, Southern Italy. School were randomly selected and the data collected during two school years. Anthropometric data was collected and body composition was assessed by bioelectrical impedance analysis. Demographic information, sleep duration, pediatric daytime sleepiness questionnaire (PDSS), physical activity and dietary habits (including adherence to the Mediterranean Diet using the KIDMED score) were further collected. The mean age was 12±0.7 and about 24% were overweight and obese. An inverse correlation between total sleep time and body mass index (β=−0.829, P=0.021), fat mass (β=−0.526, P=0.025), and waist circumference (β=−0.426, P=0.045) was found. Similar results were found for weekdays sleep time, while an inverse relationship was found with PDSS score. Adherence to Mediterranean Diet was higher in under/normal weight adolescent with a significant linear association between the KIDMED score and the PDSS, weekdays sleep time and total sleep time. Sleep duration was also associated positively with fruits and vegetable intake and negatively with sweet and snack consumption and eating outside habits. Short sleep duration and poor sleep were associated with an increase in BMI and fat mass as well as to unhealthy eating behaviors. These findings suggest that sleep patterns could be a potential target for obesity prevention programs in young adolescence.
Keywords: Adolescents, Sleep, Obesity, KIDMED, PDSS, BMI
1. Introduction
Prevalence of obesity has reached epidemic proportions across all gender, age and ethnic groups. However, overweight and obesity in children is growing alarmingly. From 1990 to 2010 the global prevalence of childhood overweight and obesity increased from 4.2% to 6.7% and is expected to reach 9.1% in 2020 [1]. Overweight and obese children are more likely to be obese adults, with many repercussions on health including cardiovascular disease, metabolic syndrome and cancer. Genetic factors, dietary behaviors, and physical activity are the main factors influencing overweight and obesity in young adolescent. However, other factors related with modernization of life have been related to obesity and need to be better addressed [2].
Meta-analysis and systematic reviews have provided evidence that sleep patterns, sleep duration, and sleep quality are associated with overweight and obesity. Shorter sleepers had higher risk to be overweight/obese compared to longer ones, with a stronger association in boys than in girls [3], [4], [5]. Despite adolescents need about 9 to 10 h of sleep [6], the National Sleep Foundation and a recent meta-analysis, reported that teens tend to have irregular sleep patterns across the week and only the 20% had an optimal sleep duration [7], [8]. Short sleep duration may affect food intake, appetite, satiety and energy balance through the modification of hormonal responses [9], [10]. Moreover, alterations in sleep patterns and sleep efficiency is often associated with unhealthy habits and lifestyle modifications, such as lower physical activities, consumption of high calories foods and beverages [11], [12]. The aim of this study was to evaluate the association between sleeping habits, Mediterranean Diet pattern, and weight status using bioelectrical impedance analysis in an adolescent population.
2. Methods
2.1. Design, setting, participants
The sample was collected during two scholastic years (period October–May of 2012–13 and 2013–14) involving students 11–14 years old attending 15 secondary schools of Sicily, Southern Italy. Schools were randomly selected after stratification based on the socio-economic level of the ten districts of the municipality of Catania to obtain a diverse range of socio-economic status (SES) among the participants. Adolescents attending last year were invited to participate (n=1766) and 1643 (93%) provided informed consent from parents and oral consent themselves prior to filling out the questionnaire. A final number of 1586 was included in the analysis (57 subjects were excluded due to incomplete or inconsistent data from questionnaires). Participation was not compulsory and anonymity was preserved. The study was approved by the ethics committee of the University of Catania and the Department of School Policies of Catania.
2.2. Data collection
The questionnaire was administered during school hours, between 10 am and noon, in the classroom in presence of a teacher and researchers. There was no time restriction to complete the questionnaire. The clinical visit to registered the anthropometric measurements was performed after the questionnaire, in a separate room. Data collection was performed by three trained medical doctors and a member of the Department of the School Policies, following a specific protocol to ensure that the same conditions were met for all participants.
Demographic information, such as the adolescents’ age, their parent's education level and job were collected in the first part of the questionnaire. Educational level was categorized as follow: secondary or lower, high school, and university. Occupational level was categorized as follow: unemployed and unskilled professions (i.e., manual workers), partially skilled professions (i.e., professors, nurses, etc.), skilled professions and white collars (i.e., medical doctors, lawyers, managers, etc.). Physical activity status was evaluated by the Physical Activity Questionnaire for Adolescents (PAQ-A) [13]. The score ranged from one to five and higher scores indicate higher levels of physical activity.
Information related to daily sleep patterns were collected through six questions: What time do you usually go to bed?; what time do you usually wake up?; how many minutes did you sleep on any daytime naps?; both on weekdays and weekend day, as made in other studies [14], [15]. Sleep durations (hours) were calculated as the difference between self-reported bedtime and wake time, for weekdays, weekend, nap and the total week (the sum of the weekday sleep duration multiplied by five, and of the weekend sleep duration multiplied by two, was then divided by seven). Bedtime and wake time where then categorized as follows: before 21:00, between 21:00 and 22:00, between 22:01 and 23:00 and after 23:01 for bedtime; before 7:00, between 7:00 and 7:30 and after 7:30 for wake time. Moreover, adolescents were classified into one of four categories based on median splits (age and gender adjusted) of their bedtime and wake up time: EE: early bed–early rise (2210 h, 0700 h, sleep duration 8.5 h); EL: early bed–late rise (2215 h, 0730 h, sleep duration 9.15 h); LE: late bed–early rise (2245 h, 0710 h, sleep duration 8.3 h); LL: late bed–late rise (2259 h, 0755 h, sleep duration 8.5 h) [16].
To assess sleepiness, the Pediatric Daytime Sleepiness Scale (PDSS) [14], an 8-item instrument based on a Likert-scale ratings (never=0; seldom=1; sometimes=2; frequently=3; always=4) was used. Total scores could range from 0 to 32, with higher scores indicating higher levels of sleepiness.
Finally the last part of the questionnaire focused on the dietary assessment based on a revised version of other food frequency questionnaires (FFQs) developed for Italian adolescents [17], [18]. Data from that questionnaire were used to calculate the KIDMED score (Mediterranean Diet Quality Index for children and adolescent) [19] used to evaluate the different adherence to the Mediterranean diet by measuring the consumption of 16 components. Total KIDMED scores were classified as follows: 0–3 reflected a poor adherence to the Mediterranean diet; 4–7 described average adherence; and 8–12 described a good adherence.
2.3. Body composition assessment
Determination of body impendence was obtained using the foot to foot devices TANITA BC-420 MA (Tanita, Tokyo, Japan), with a 50 kHz frequency; According to manufacturer's instructions, skin-to skin contact was avoided in all measurements. To compute Total Body Water (TBW), the equation proposed by Bray et al. [20] was used instead the manufacturers’ one. Then, a hydration fraction of 0.732 ml/g was used to compute TBW in Free Fat Mass (FFM).
Body weight (BW, Kg), body height (HT, cm) and waist circumference (WC, cm) were measured to the nearest 100 g and 0.5 cm respectively. BMI was computed as weight in kilograms divided by the square of height in meters, and international age- and gender-specific cut-off points for children according to the International Obesity Task Force were used to define their weight status in terms of underweight, overweight and obesity [21].
2.4. Statistical analysis
Continuous variables are presented as means and standard deviations (SDs), categorical variables are presented as absolute frequencies and percentages. Normality of variables' distribution was tested by Kolmogorov-Smirnov test. Differences between categorical variables were tested by Chi-square test. Respectively, Kruskall-Wallis test and one-way ANOVA (with Bonferroni correction) were used for multiple comparisons. The associations among the dependent variables BMI, fat mass (FM), free FM (FFM), waist circumference (WC) and selected weight-related food groups and the independent variable sleep-wake behavior and PDSS were examined using multivariate linear regression models. SPSS version 20.0 (IMB SPSS Inc) was used for all statistical evaluations.
3. Results
The mean age of the adolescents was 12.0±0.7. Nearly 27% of participants were overweight and 24.5% were obese. Boys had a large proportion of overweight and obesity compared with girls (p<0.001). No other gender difference was found for the variables of interest. Moreover, adolescent who have parents with a higher education or a skilled profession were more likely to be under/normal weight. Physical activity was found to be lower in overweight and obese adolescent, while almost a half of the under/normal weight subject had a medium physical activities level. Only 84 adolescents (6%) had a good adherence to the Mediterranean Diet, with under/normal weight subject showing, on average, a greater adherence. The demographic characteristics of the 1586 adolescents (870 boys and 716 girls) distributed by BMI classification are showed in Table 1.
Table 1.
Demographic characteristics by BMI classification (N=1586).
| Under/Normal weight N=755 | Overweight N=442 | Obese N=389 | P | |
|---|---|---|---|---|
| Age (years) | 12.1±0.7 | 12.0±0.7 | 12.1±0.6 | 0.655 |
| Gender | <0.001 | |||
| Male | 343 (45.5) | 268 (60.6) | 259 (66.6) | |
| Female | 412 (54.6) | 174 (39.4) | 130 (33.4) | |
| BMI | 18.5±1.9 | 23.2±1.4 | 28.1±2.8 | <0.001 |
| Parents’ education | 0.024 | |||
| Secondary or lower | 305 (40.4) | 255 (57.7) | 220 (56.6) | |
| High school | 284 (37.6) | 115 (26.0) | 108 (27.8) | |
| University | 166 (22.0) | 72 (16.3) | 61 (15.7) | |
| Parents’ occupation | 0.026 | |||
| Unskilled professions | 370 (40.9) | 291 (65.8) | 254 (65.3) | |
| Partially skilled professions | 291 (38.5) | 119 (26.9) | 112 (28.8) | |
| Skilled professions | 94 (12.5) | 32 (7.2) | 23 (5.9) | |
| Physical activity | <0.001 | |||
| Low | 221 (29.3) | 202 (45.7) | 197 (50.6) | |
| Medium | 340 (45.0) | 176 (39.8) | 156 (40.1) | |
| High | 194 (24.7) | 64 (14.5) | 36 (9.3) | |
| KIDMED score | 0.003 | |||
| Poor | 299 (39.6) | 212 (48.0) | 200 (51.4) | |
| Average | 371 (49.1) | 201 (45.5) | 174 (44.7) | |
| Good | 85 (11.3) | 29 (6.6) | 15 (3.9) |
Data are presented as number (%) or mean±SD; ANOVA was used to compare age and BMI; χ2 for all others variables.
Table 2 presents the sleep habits of the sample according to BMI category. The three groups shared similar bed times and wake times, with no significant differences. Under/normal weight adolescent had longer sleep time duration, both as total and weekdays sleep time, compared to the overweight and obese groups. Sleep duration was prolonged during weekends indifferently in all the three groups. When weekdays sleep time and weekend sleep time was compared within the three BMI category, a significant difference was found in all the groups, indicating a possible sleep deprivation pattern in all sample (p<0.001, data not shown). No difference in nap habits or length was found. The PDSS score increased among the three groups with a mean of 13.3±6.3 for under/normal weight, 9.8±5.9 for overweight and 14.7±6.7 for obese adolescent. Anthropometric characteristics and weight-related KIDMED score items by sleep timing behavior category are shown in Table 3. BMI, FM% and WC resulted significantly lower in EL category, while no association was found with KIDMED items or physical activities.
Table 2.
Sleep time habits according to BMI category (N=1586).
| Under/Normal weight N=755 | Overweight N=442 | Obese N=389 | P | |
|---|---|---|---|---|
| Sleep quantity, hh | ||||
| Total sleep time | 9.0±1.3 | 8.5±1.2a | 8.5±1.6a | <0.001 |
| Weekdays time | 8.3±1.1 | 7.9±1.0a | 7.8±1.1a | <0.001 |
| Weekend sleep time | 10.0±1.5 | 9.8±1.8 | 9.9±1.9 | 0.778 |
| Nap time on weekdays | 1.5±0.8 | 1.4±0.7 | 1.4±0.7 | 0.645 |
| Nap time on weekend | 1.6±0.9 | 1.5±0.8 | 1.6±0.7 | 0.792 |
| Sleep quality | ||||
| PDSS score | 13.3±6.3 | 14.5±5.9a | 14.7±6.7a | 0.001 |
| Bedtimes weekdays | 0.256 | |||
| <21:00 | 29 (3.8) | 18 (4.1) | 13 (3.3) | |
| 21:00–22:00 | 131 (17.4) | 69 (15.6) | 38 (9.8) | |
| 22:01–23:00 | 483 (64.0) | 260 (58.8) | 263 (67.6) | |
| >23:00 | 112 (14.8) | 95 (21.5) | 75 (19.3) | |
| Wake times weekdays | 0.544 | |||
| <7:00 | 208 (27.5) | 145 (32.8) | 121 (31.1) | |
| 7:00–7:30 | 371 (49.1) | 209 (47.3) | 188 (48.3) | |
| >7:30 | 176 (23.3) | 88 (19.9) | 80 (20.6) | |
| Bedtimes weekend | 0.195 | |||
| <22:00 | 120 (15.9) | 64 (14.5) | 56 (14.4) | |
| 22:00–23:00 | 264 (35.0) | 145 (32.8) | 131 (33.7) | |
| 23:01–24:00 | 255 (33.8) | 149 (33.7) | 136 (35.0) | |
| >24:00 | 116 (15.4) | 84 (19.0) | 66 (17.0) | |
| Wake times weekend | 0.432 | |||
| <9:00 | 109 (14.4) | 84 (19.0) | 99 (25.4) | |
| 9:00–10:00 | 274 (36.3) | 132 (29.9) | 95 (24.4) | |
| >10:00 | 372 (49.3) | 226 (51.1) | 195 (50.1) | |
| Nap on weekdays, yes | 198 (26.2) | 121 (27.3) | 101 (25.9) | 0.678 |
| Nap on weekend, yes | 207 (27.4) | 122 (27.7) | 103 (26.5) | 0.746 |
Data are presented as number (%) or mean±SD; ANOVA was used to compare sleep quantity and quality; χ2 for all others variables;
Significantly different from Under/ Normal weight (P<0.05); Hh, hours; PDSS, Pediatric Daytime Sleepiness Scale.
Table 3.
Anthropometric characteristics and weight-related KIDMED items by sleep timing behavior category (N=1586).
| EEN=459 | EL N=286 | LE N=349 | LL N=492 | P | |
|---|---|---|---|---|---|
| BMI | 22.2±2.1 | 22.0±2.3 | 22.4±2.0a | 22.2±2.4 | 0.041 |
| FM% | 23.7±9.3 | 23.1±9.1 | 23.9±9.6a | 23.5±8.7 | 0.047 |
| FFM% | 40.2±7.5 | 40.7±7.7 | 39.8±8.0 | 40.1±8.3 | 0.357 |
| WC | 73.5±11.2 | 73.1±13.4 | 73.6±12.7a | 73.4±10.9 | 0.049 |
| KIDMED score | 4.3±2.3 | 4.4±2.4 | 4.2±2.5 | 4.3±2.7 | 0.246 |
| Fruit (serving/day) | 1.6±1.8 | 1.7±2.0 | 1.6±1.9 | 1.5±1.9 | 0.145 |
| Vegetable(serving/day) | 1.4±2.0 | 1.5±1.9 | 1.3±2.1 | 1.4±1.8 | 0.153 |
| Breakfast (times/w) | 5.5±1.8 | 5.4±1.5 | 5.2±1.7 | 5.4±1.8 | 0.514 |
| Sweet and snack (serving/day) | 3.2±2.2 | 3.1±2.3 | 3.1±2.2 | 3.2±2.4 | 0.333 |
| Eating outside (times/w) | 1.6±0.9 | 1.5±1.1 | 1.7±1.0 | 1.5±1.2 | 0.256 |
| Physical activity | 3.1±0.8 | 3.2±0.7 | 2.9±0.9 | 3.0±0.8 | 0.565 |
Data are presented as mean ± SD; ANOVA test was used for all the comparison.
Significantly different from EL (P<0.05); EE, early bed–early rise; EL, early bed–late rise; FFM, free fat mass; FM, free fat mass; LE, late bed–early rise; LL, late bed–late rise; WC, waist circumference.
The linear regression showed an inverse correlation between the total sleep time and BMI (β=−0.829, P=0.021), FM% (β=−0.526, P=0.025) and WC (β=−0.426, P=0.045). Similar results were found also for weekdays sleep time (Table 4). Instead a direct association was found between the PDSS score and BMI (β=0.529, P=0.041) and FM% (β=0.435, P=0.039), but not with WC and FFM. Regarding selected weight-related food groups and behaviors, fruit and vegetable intake was positively associated with total and weekdays sleep time, but not with weekend sleep time and PDSS. Consumption of sweets, snacks, and the eating outside habit were more frequent in shorter sleep adolescent and directly related with PDSS. On the contrary, breakfast habit and physical activity were not related to sleep duration or PDSS.
Table 4.
Multivariate linear regression between sleep patterns, anthropometric characteristics and weight-related KIDMED items.
| Sleep quantity |
Sleep quality | |||||||
|---|---|---|---|---|---|---|---|---|
| Total sleep time |
Weekdays time |
Weekend sleep time |
PDSS score |
|||||
| Beta | P | Beta | P | Beta | P | Beta | P | |
| BMI | −0.829 | 0.021 | −0.926 | 0.011 | −0.007 | 0.421 | 0.529 | 0.041 |
| FM% | −0.526 | 0.025 | −0.601 | 0.016 | −0.004 | 0.325 | 0.435 | 0.039 |
| FFM% | 0.361 | 0.345 | 0.255 | 0.436 | 0.332 | 0.526 | 0.565 | 0.432 |
| WC | −0.426 | 0.045 | −0.569 | 0.024 | −0.021 | 0.623 | 0.351 | 0.061 |
| KIDMED items | ||||||||
| Fruit (serving/day) | 0.021 | 0.035 | 0.063 | 0.041 | 0.032 | 0.063 | 0.184 | 0.564 |
| Vegetable(serving/day) | 0.033 | 0.029 | 0.092 | 0.036 | 0.011 | 0.059 | −0.265 | 0.359 |
| Breakfast (times/w) | 0.095 | 0.231 | 0.063 | 0.337 | 0.049 | 0.433 | 0.199 | 0.065 |
| Sweet and snack (serving/day) | −0.037 | 0.044 | −0.028 | 0.039 | −0.015 | 0.112 | 0.015 | 0.045 |
| Eating outside (times/w) | −0.044 | 0.025 | −0.012 | 0.027 | −0.002 | 0.041 | 0.197 | 0.035 |
| Physical activity | 0.101 | 0.146 | 0.203 | 0.442 | 0.320 | 0.233 | 0.264 | 0.169 |
Adjusted for: Age, gender, physical activities and parents’ education and occupation; FFM, free fat mass; FM, free fat mass; WC, waist circumference;
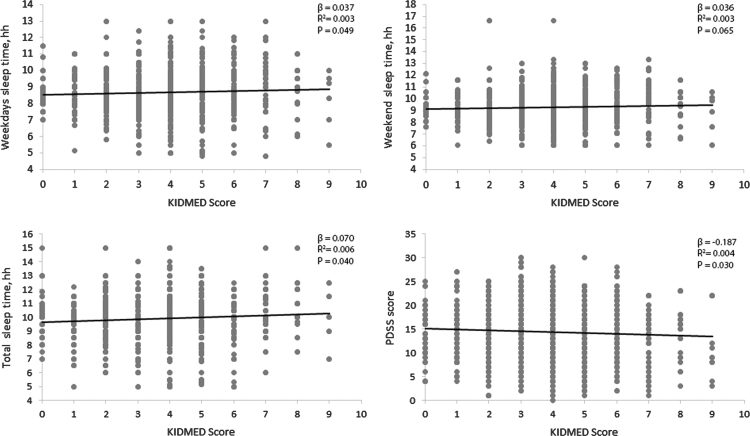
The assumption of linearity for the continuous variables was confirmed by plotting the residuals versus the predicted values (data not shown). A significant linear direct relation between the KIDMED score and PDSS, weekdays sleep time and total sleep time was found (Fig. 1).
Fig. 1.
Linear regression analyzes of association between sleep patterns and KIDMED score.
4. Discussion
This study investigated the relationship between sleep patterns, body composition, and dietary habits in a sample of healthy adolescent aged 11–14 years. To our knowledge, this study is the first study investigating the association between sleep patterns, weight status, and Mediterranean Diet in adolescent using bioelectrical impedance analysis.
The result showed that male adolescents were significantly more likely to develop overweight and obesity compared to females. This data are consistent with recent investigations assessing a high prevalence of overweight and obesity in Southern Italy and Italian islands, especially among boys [22]. This might be due to a higher attention to physical appearance, weight and body shape among teenage girls, but also hormonal differences and maturational status could have an important effect in both sleep and metabolic control [23]. Adolescents reporting higher level of parents’ education and occupation were significantly less likely to be overweight/obese. Lifestyle habits of young adolescents dependent on parental control with a progressive decrease with age [24]. Parents with a higher level of education and SES seems to provide their children food of a higher nutritional quality, including more fruits and vegetables [25]. However, it has been estimated than more than a half of parents underestimate their children's weight, encouraging their sons to eat more [26].
Weekdays, total sleep duration and EL sleeping category were inversely associated with BMI, FM% and WC. Although the association between short sleep duration and adolescent obesity has been reported by several studies, the potential mechanism remains unclear. We investigated not only BMI levels but also effective FM because it has been shown to be better correlated with hormonal and metabolic alteration in human body [27], [28]. In fact, imbalance of two opposite hormones, leptin and ghrelin involved in food intake and energy balance, has been reported in many studies investigating sleep duration and weight gain [29], [30], [31], [32]. Leptin is secreted from white adipocytes, and acts on the central nervous system, in the arcuate nucleus of the hypothalamus, through the inhibition of hunger and by the stimulation of energy expenditure. Moreover, leptin is involved in other pathophysiological processes including atherosclerosis, tolerance and insulin sensitivity and also is liked to cardiovascular diseases and the metabolic syndrome [33], [34]. On the contrary, ghrelin is mainly secreted from the stomach and by acting on the hypothalamus, stimulates hunger and fat production. Short sleep has been associated with decreased circulating leptin level and increased ghrelin, a hormonal pattern leading to an increased appetite and fat production with a decreased energy expenditure [29]. Although this pathophysiological mechanism seems to be the one with the greater consensus, other studies showed conflicting results [35], [36]. Moreover, not only sleep duration may induce hormonal changes, but also sleep quality seems to lead to a pro-fat hormonal pattern. Sleep disturbance increase morning cortisol levels, reduce insulin sensitivity and growth hormone secretion [37], [38], supporting the associations between poor sleep quality and obesity, as found in other studies [39], [40].
Bedtime and wake time were not related to overweight or obesity in our study, however an EL sleep timing behavior, corresponding to a longer duration of sleep, resulted in a better weight control compared to the others sleep timing behaviors. In a recent Australian cross-sectional study, adolescent with a late bed–late rise pattern were 1.47 times more likely to be overweight or obese than those with early bed–early rise one [41]. Moreover, adolescents with late bedtime had a higher intake of extra foods (energy-dense, nutrient-poor foods) while adolescent with an early bedtime consume more fruit and vegetables [41]. Prospective studies showed how a chronic pattern of late sleep timing is linked with an increase of BMI [42] and metabolic disorders [43].
Regarding energy expenditure, we did not found significant differences between physical activities and BMI groups as strong as sleep characteristics. In contrast, sleep quality and duration was inversely associated with the total score and some weight-related items of the KIDMED score. There is evidence of a progressive abandonment of traditional eating pattern in the Mediterranean region [44], [45], [46], [47]. In this context, adolescent who habitually sleep less are more likely to consume less vegetable and fruits and eating out more frequently. These findings are consistent with those of other studies suggesting an association between sleep restriction and the consumption of high-calorie foods, sugared beverages and snacks [48], [49]. In this study we investigated the adherence to a Mediterranean dietary pattern, which has been associated with a number of healthy outcomes, including metabolic disorders, decreased risk of cardiovascular disease (CVD) and certain cancers [50], [51], [52]. There is also evidence of benefits of some peculiar characteristics of the Italian Mediterranean diet, such as the high intake of omega-3 rich fish, which seem to exert anti-obesogenic action [53], [54]. Despite its association with BMI in adolescents is still unclear [55], a high adherence to a healthy dietary pattern, such as the Mediterranean diet, may be associated with a whole healthier lifestyle, which include also better sleep quality. As final results, this condition may decrease the overall risk of non-communicable disease.
Findings of this study should be considered in light of some limitations. Due to the cross-sectional design, it is not possible to define the causality of the associations. Second, both sleep and dietary patterns are affected by parental control, parental dietary habits and parental weight status, which were not investigated in this study. Third, sleep duration calculated as the difference between bed time and wake up time, could overestimate sleep duration due to sleep latency and wake after sleep onset time. Finally, the results may be affected by recall bias and social desirability bias.
In conclusion, less sleep and poor sleep were associated with a lower adherence to Mediterranean Diet, an increase in unhealthy eating behaviors and overweigh/obese status. These findings highlight that the prevention of adolescent obesity needs a multidisciplinary approach considering not only physical activities and diet, but also other lifestyle interventions such as improvements in sleeping habits. Further studies are needed to clarify the multiple mechanisms involved between diet, lifestyle behaviors and sleep patterns in adolescents.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.de Onis M., Blossner M., Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 2.Di Renzo L., Tyndall E., Gualtieri P., Carboni C., Valente R. Association of body composition and eating behavior in the normal weight obese syndrome. Eat Weight Disord. 2016;21:99–106. doi: 10.1007/s40519-015-0215-y. [DOI] [PubMed] [Google Scholar]
- 3.Chen X., Beydoun M.A., Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 4.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006;91:881–884. doi: 10.1136/adc.2005.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel S.R., Hu F.B. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iglowstein I., Jenni O.G., Molinari L., Largo R.H. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 7.National Sleep Foundation. Teens and Sleep: 2006; Available from: 〈www.sleepfoundation.org〉.
- 8.Gradisar M., Gardner G., Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–118. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Vgontzas A.N., Bixler E.O., Chrousos G.P. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 10.St-Onge M.P., Shechter A. Sleep restriction in adolescents: forging the path towards obesity and diabetes? Sleep. 2013;36:813–814. doi: 10.5665/sleep.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Hazzaa H.M., Musaiger A.O., Abahussain N.A., Al-Sobayel H.I., Qahwaji D.M. Lifestyle correlates of self-reported sleep duration among Saudi adolescents: a multicentre school-based cross-sectional study. Child Care Health Dev. 2014;40:533–542. doi: 10.1111/cch.12051. [DOI] [PubMed] [Google Scholar]
- 12.Westerlund L., Ray C., Roos E. Associations between sleeping habits and food consumption patterns among 10–11-year-old children in Finland. Br J Nutr. 2009;102:1531–1537. doi: 10.1017/S0007114509990730. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski K.C., Crocker R.E., Donen R.M. Universtiy of Saskatchewan; Saskatoon, Canada: 2004. The physical activity questionnaire for older children (PAC-C) and adolescents (PAQ-A) manual. [Google Scholar]
- 14.Drake C., Nickel C., Burduvali E., Roth T., Jefferson C. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26:455–458. [PubMed] [Google Scholar]
- 15.Zhang B., Hao Y., Zhou J., Jia F., Li X. The association between sleep patterns and overweight/obesity in Chinese children: a cross-sectional study. Neuropsychiatr Dis Treat. 2015;11:2209–2216. doi: 10.2147/NDT.S90838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touchette E., Mongrain V., Petit D., Tremblay R.E., Montplaisir J.Y. Development of sleep-wake schedules during childhood and relationship with sleep duration. Arch Pediatr Adolesc Med. 2008;162:343–349. doi: 10.1001/archpedi.162.4.343. [DOI] [PubMed] [Google Scholar]
- 17.Grosso G., Mistretta A., Turconi G., Cena H., Roggi C. Nutrition knowledge and other determinants of food intake and lifestyle habits in children and young adolescents living in a rural area of Sicily, South Italy. Public Health Nutr. 2013;16:1827–1836. doi: 10.1017/S1368980012003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turconi G., Bazzano R., Roggi C., Cena H. Reliability and relative validity of a quantitative food-frequency questionnaire for use among adults in Italian population. Int J Food Sci Nutr. 2010;61:846–862. doi: 10.3109/09637486.2010.495329. [DOI] [PubMed] [Google Scholar]
- 19.Serra-Majem L., Ribas L., Ngo J., Ortega R.M., Garcia A. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, mediterranean diet quality index in children and adolescents. Public Health Nutr. 2004;7:931–935. doi: 10.1079/phn2004556. [DOI] [PubMed] [Google Scholar]
- 20.Bray G.A., DeLany J.P., Harsha D.W., Volaufova J., Champagne C.C. Evaluation of body fat in fatter and leaner 10-y-old African American and white children: the Baton rouge children’s study. Am J Clin Nutr. 2001;73:687–702. doi: 10.1093/ajcn/73.4.687. [DOI] [PubMed] [Google Scholar]
- 21.Cole T.J., Bellizzi M.C., Flegal K.M., Dietz W.H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bibiloni Mdel M., Pons A., Tur J.A. Prevalence of overweight and obesity in adolescents: a systematic review. ISRN Obes. 2013;2013:392747. doi: 10.1155/2013/392747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowley S.J., Acebo C., Carskadon M.A. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Grosso G., Marventano S., Nolfo F., Rametta S., Bandini L. Personal eating, lifestyle, and family-related behaviors correlate with fruit and vegetable consumption in adolescents living in sicily, Southern Italy. Int J Vitam Nutr Res. 2013;83:355–366. doi: 10.1024/0300-9831/a000177. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Alvira J.M., Mouratidou T., Bammann K., Hebestreit A., Barba G. Parental education and frequency of food consumption in European children: the IDEFICS study. Public Health Nutr. 2013;16:487–498. doi: 10.1017/S136898001200290X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundahl A., Kidwell K.M., Nelson T.D. Parental underestimates of child weight: a meta-analysis. Pediatrics. 2014;133:e689–e703. doi: 10.1542/peds.2013-2690. [DOI] [PubMed] [Google Scholar]
- 27.De Lorenzo A., Bianchi A., Maroni P., Iannarelli A., Di Daniele N. Adiposity rather than BMI determines metabolic risk. Int J Cardiol. 2013;166:111–117. doi: 10.1016/j.ijcard.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 28.De Lorenzo A., Nardi A., Iacopino L., Domino E., Murdolo G. A new predictive equation for evaluating women body fat percentage and obesity-related cardiovascular disease risk. J Endocrinol Invest. 2014;37:511–524. doi: 10.1007/s40618-013-0048-3. [DOI] [PubMed] [Google Scholar]
- 29.Taheri S., Lin L., Austin D., Young T., Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. Plos Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bornhorst C., Hense S., Ahrens W., Hebestreit A., Reisch L. From sleep duration to childhood obesity – what are the pathways? Eur J Pediatr. 2012;171:1029–1038. doi: 10.1007/s00431-011-1670-8. [DOI] [PubMed] [Google Scholar]
- 31.Miller A.L., Lumeng J.C., LeBourgeois M.K. Sleep patterns and obesity in childhood. Curr Opin Endocrinol Diabetes Obes. 2015;22:41–47. doi: 10.1097/MED.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Disi D., Al-Daghri N., Khanam L., Al-Othman A., Al-Saif M. Subjective sleep duration and quality influence diet composition and circulating adipocytokines and ghrelin levels in teen-age girls. Endocr J. 2010;57:915–923. doi: 10.1507/endocrj.k10e-145. [DOI] [PubMed] [Google Scholar]
- 33.Correia M.L., Rahmouni K. Role of leptin in the cardiovascular and endocrine complications of metabolic syndrome. Diabetes Obes Metab. 2006;8:603–610. doi: 10.1111/j.1463-1326.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 34.Ceddia R.B. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int J Obes (Lond) 2005;29:1175–1183. doi: 10.1038/sj.ijo.0803025. [DOI] [PubMed] [Google Scholar]
- 35.St-Onge M.P., O’Keeffe M., Roberts A.L., RoyChoudhury A., Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–1510. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson N.S., Banks S., Dinges D.F. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;12:47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamatakis K.A., Punjabi N.M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michels N., Verbeiren A., Ahrens W., De Henauw S., Sioen I. Children’s sleep quality: relation with sleep duration and adiposity. Public Health. 2014;128:488–490. doi: 10.1016/j.puhe.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Bawazeer N.M., Al-Daghri N.M., Valsamakis G., Al-Rubeaan K.A., Sabico S.L. Sleep duration and quality associated with obesity among Arab children. Obesity (Silver Spring) 2009;17:2251–2253. doi: 10.1038/oby.2009.169. [DOI] [PubMed] [Google Scholar]
- 40.Liu X., Forbes E.E., Ryan N.D., Rofey D., Hannon T.S. Rapid eye movement sleep in relation to overweight in children and adolescents. Arch Gen Psychiatry. 2008;65:924–932. doi: 10.1001/archpsyc.65.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golley R.K., Maher C.A., Matricciani L., Olds T.S. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond) 2013;37:546–551. doi: 10.1038/ijo.2012.212. [DOI] [PubMed] [Google Scholar]
- 42.Asarnow L.D., McGlinchey E., Harvey A.G. Evidence for a possible link between bedtime and change in body mass index. Sleep. 2015;38:1523–1527. doi: 10.5665/sleep.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonnissen H.K., Rutters F., Mazuy C., Martens E.A., Adam T.C. Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr. 2012;96:689–697. doi: 10.3945/ajcn.112.037192. [DOI] [PubMed] [Google Scholar]
- 44.Grosso G., Marventano S., Giorgianni G., Raciti T., Galvano F. Mediterranean diet adherence rates in Sicily, Southern Italy. Public Health Nutr. 2014;17:2001–2009. doi: 10.1017/S1368980013002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarou C., Panagiotakos D.B., Matalas A.L. Level of adherence to the Mediterranean diet among children from Cyprus: the CYKIDS study. Public Health Nutr. 2009;12:991–1000. doi: 10.1017/S1368980008003431. [DOI] [PubMed] [Google Scholar]
- 46.Ozen A.E., Bibiloni Mdel M., Murcia M.A., Pons A., Tur J.A. Adherence to the Mediterranean diet and consumption of functional foods among the Balearic Islands’ adolescent population. Public Health Nutr. 2015;18:659–668. doi: 10.1017/S1368980014000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grosso G G.F. Mediterranean diet adherence in children and adolescents in Southern European countries. NFS J. 2016;3:13–19. [Google Scholar]
- 48.McDonald L., Wardle J., Llewellyn C.H., Fisher A. Nighttime sleep duration and hedonic eating in childhood. Int J Obes (Lond) 2015;39:1463–1466. doi: 10.1038/ijo.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss A., Xu F., Storfer-Isser A., Thomas A., Ievers-Landis C.E. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010;33:1201–1209. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grosso G., Buscemi S., Galvano F., Mistretta A., Marventano S. Mediterranean diet and cancer: epidemiological evidence and mechanism of selected aspects. BMC Surg. 2013;13(Suppl 2):S14. doi: 10.1186/1471-2482-13-S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grosso G., Marventano S., Yang J., Micek A., Pajak A. A comprehensive meta-analysis on evidence of mediterranean diet and cardiovascular disease: are individual components equal? Crit Rev Food Sci Nutr. 2015:0. doi: 10.1080/10408398.2015.1107021. [DOI] [PubMed] [Google Scholar]
- 52.Grosso G., Mistretta A., Marventano S., Purrello A., Vitaglione P. Beneficial effects of the mediterranean diet on metabolic syndrome. Curr Pharm Des. 2014;20:5039–5044. doi: 10.2174/1381612819666131206112144. [DOI] [PubMed] [Google Scholar]
- 53.De Lorenzo A., Noce A., Bigioni M., Calabrese V., Della Rocca D.G. The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr Pharm Des. 2010;16:814–824. doi: 10.2174/138161210790883561. [DOI] [PubMed] [Google Scholar]
- 54.Di Daniele N., Petramala L., Di Renzo L., Sarlo F., Della Rocca D.G. Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean Diet in obese patients with metabolic syndrome. Acta Diabetol. 2013;50:409–416. doi: 10.1007/s00592-012-0445-7. [DOI] [PubMed] [Google Scholar]
- 55.Grosso G., Marventano S., Buscemi S., Scuderi A., Matalone M. Factors associated with adherence to the Mediterranean diet among adolescents living in Sicily, Southern Italy. Nutrients. 2013;5:4908–4923. doi: 10.3390/nu5124908. [DOI] [PMC free article] [PubMed] [Google Scholar]