Abstract
Objectives
To evaluate the risk of obstructive sleep apnea (OSA) in a primary care population of elderly Nigerians and to determine its correlates.
Methods
Clinical and demographic data of 414 elderly individuals in a primary care clinic were obtained. Their risk of OSA was estimated using Berlin questionnaire while Epworth sleepiness scale and the Center for Epidemiologic Studies Depression Scale (CESD-10) were also administered.
Results
Of the 414 subjects, 96 (23.2%) met the criteria for a high risk for OSA with a male to female ratio of 1:1. Subjects at high OSA risk (high OSA risk group) were younger than those at low OSA risk (low OSA risk group) (71.4±6.8 vs 73.6±7.7, p=0.011). Mean body mass index (BMI, kg/m2) (27.3±5.8 vs 24.7±5.1, p<0.001) and waist circumference (WC, cm) (90.7±13.1 vs 86.5±13.9, p=0.011) were higher in the high OSA risk group compared with the low OSA risk group. A total of 215 (51.9%) and 62 (15.0%) subjects had clinically significant depressive symptoms (CESD-10 score≥10) and excessive daytime sleepiness (EDS), respectively. On regression, the odds of EDS, depressive symptoms, increased BMI and younger age were significantly higher in the high OSA risk group compared with the low OSA risk group.
Conclusions
High risk for OSA and depressive symptoms are common in our sample of elderly Nigerians. Depressive symptoms, EDS, BMI and age independently predict high OSA risk in the elderly.
Keywords: Obstructive sleep apnea, Elderly, Nigeria, Prevalence, Primary care, Predictors
1. Introduction
Sleep-related disorders are common among the elderly. Studies in large populations of older adults have reported prevalence of sleep-related disturbances greater than 50% [1], [2]. Obstructive sleep apnea (OSA) is a breathing disorder of sleep that has gained significant recognition in recent years due to its associated morbidity and mortality worldwide. Individuals suffering from obstructive sleep apnea have a higher prevalence of obesity, diabetes, hypertension, dyslipidemia, metabolic syndrome and other related chronic medical conditions [3], [4], [5]. There had been a dearth of data from sub-Saharan Africa compared to the developed world in the OSA literature until recently [6]. There is now an increase in efforts in the direction of exploring factors that are related to the condition among various populations. The trend of recent research findings seems to suggest that OSA might be more frequent than had been previously reported in these populations [7], [8], [9], [10]. This justifies the need for more research that will further elucidate factors that are interrelated with the disorder more importantly among different populations in developing countries.
The prevalence of OSA has been studied across diverse populations worldwide. In a review of the epidemiology of OSA, an overall prevalence of 3–7% was reported across various countries, with a higher prevalence among men (3–7%) compared with women (2–5%) [7]. It was reported that these cut across populations in both the developing and the developed world. Among the factors that have been shown to be significantly associated with an increased risk of obstructive sleep disorders across diverse populations is advancing age. Several studies have replicated the finding that the disease prevalence steadily increases with age and plateaus at about 60 years [7]. This portends the need to critically explore the prevalence of OSA and its associated factors in the older adult population. Epidemiological data on OSA in the elderly in sub-Saharan African populations are scarce. This study aimed at quantifying the risk of OSA in a primary care population of elderly Nigerians and to determine its correlates.
2. Methods
This cross-sectional study was carried out at the outpatient clinic of State Hospital, Ilesa between June and October 2014. Ilesa, with a population of 212,225 as at 2006 [11], is one of the largest cities in the state of Osun located in South-western Nigeria. The outpatient clinic of the State Hospital, Ilesa, is one of the two largest primary care clinics in the city of Ilesa serving the entire population of Ilesa and some of the adjourning towns and villages.
2.1. Study population
The sample consisted of 414 consecutive community-living elderly men and women, aged 65–100 years. They were consecutively recruited from individuals who had been receiving monthly financial support from the Government of the state of Osun, South-Western Nigeria under a scheme which provided a monthly stipend to support their wellbeing.
2.2. Selection criteria
Ambulant, community-living black men and women of Nigerian extract, aged ≥65 years who gave their informed consent were consecutively enrolled. Non-ambulant, institutionalized and individuals of non-Nigerian origin as well as those who were not willing to participate in the study, were excluded. The eligibility of the subjects was first assessed by one of the investigators, a family physician, before they were recruited.
2.3. Subject assessment
Informed consent was obtained from all the subjects participating in the study. The study questionnaires were then administered to them by the investigators and trained research assistants.
2.3.1. Demographic and clinical data
Sociodemographic data including age, sex, highest formal educational level attained and ethnic group; while disease-related variables including history of chronic medical conditions such as hypertension, diabetes, dyslipidemia, coronary artery disease, intermittent claudication and chronic kidney disease were obtained. Other variables obtained include smoking and alcohol history and history of coffee and kolanut consumption. Sitting blood pressure and anthropometric measures including weight and height were measured. Body Mass Index (BMI), a measure of body adiposity, was calculated using the formula weight (kg) divided by the square of height (m2).
2.3.2. Evaluation of OSA risk
The risk of OSA was determined using the Berlin Questionnaire. The Berlin Questionnaire has three categories. Category 1 has five questions about snoring, Category 2 has three questions about daytime somnolence, and Category 3 has one question about the history of hypertension. In addition, the questionnaire also collects information about age, gender, height, and weight (to calculate the BMI). The overall score is based on the patient's response to each of the three categories. The patients are classified as high risk for OSA if two or more categories are positive and low risk if less than two categories are positive. This tool has reasonable sensitivity (68.9%), specificity (56.4%), and positive predictive value (77.9%) [12]. It has been used previously among populations in Nigeria [5], [13].
2.3.3. Assessment of excessive daytime sleepiness
The Epworth Sleepiness Scale (ESS) is an effective instrument used to measure average daytime sleepiness. The ESS differentiates between average sleepiness and excessive daytime sleepiness that requires intervention. The individual self-rates his/her likelihood of dozing in eight different situations. Scoring of the answers is 0–3, with 0 being “would never doze” and 3 being “high chance of dozing”. A sum of 11 or more from the eight individual scores reflects above normal daytime sleepiness and need for further evaluation [14]. The validity and reliability of ESS has been tested in different groups of individuals across the healthcare continuum [4]. It has also been used previously among populations in Nigeria [13], [15].
2.3.4. Assessment of depressive symptoms
The 10-item version of the Center for Epidemiologic Studies Depression Scale (CESD-10) was used to evaluate the participants for depression symptoms. The CESD-10 is a short-form scale with 10 items that is designed to identify depressive symptoms in the general population and has been used extensively in general patient and older adult populations [16], [17]. The CESD-10 has shown good predictive accuracy across different studies (kappa, 0.84–0.97) when compared with the full-length 20-item version of the CESD [17]. A 4-point Likert scale ranging from “rarely” (scored 0) to “all of the time” (scored 3), is used to score the responses giving a summed total of 0–30. Responses to at least 9 of the items were included in the analyses. Clinically relevant depressive symptoms were defined as CESD-10 score ≥10.
2.4. Statistical analysis
Data analyses was done using the Statistical Package for the Social Sciences (SPSS), version 16 (SPSS Inc., Chicago, IL, U.S.A.) and presented as means±SD and frequencies and percentages. For variables with normal distribution comparison between groups was performed using independent t-test. Relationship between categorical variables was assessed using chi-square test. A logistic regression model was constructed using OSA risk status as the dependent variable and variables that were significantly related to OSA risk on bivariate analysis as covariates. A 5% significance level (p<0.05) was considered significant.
2.5. Ethical clearance
Ethical clearance was obtained from the Ethics and Research Committee of the Hospitals Management Board of the State of Osun.
3. Results
3.1. Demographic and clinical profiles of the subjects
Out of the 414 subjects who participated in the study, 349 (84.3%) were women. The ages of the subjects ranged from 65 to 102 years with a mean of 73.1±7.5 years. Majority of the subjects (240, 59.4%) had no form of formal education. All were from the Yoruba tribe and resided in Ilesa town. Habitual use of kolanut, alcohol and tobacco was reported by 76 (18.8%), 62 (15.3%) and 28 (7.0%) of the subjects respectively. Coffee intake was reported by 20 (5.0%). A total of 134 (33.3%) subjects had a history of hypertension, while 29 (7.2%), 45 (11.6%) and 6 (1.5%) had a history of diabetes mellitus, intermittent claudication and stroke respectively. The study population had a mean systolic blood pressure (SBP), diastolic blood pressure (DBP) and BMI of 141.0±25.3 mmHg, 84.0±13.8 mmHg and 25.3±5.4 kg/m2 respectively (Table 1). While snoring was r1eported more frequently by the male subjects (29.2% vs 15.5%, p=0.004), witnessed apnea (23.9% vs 12.9%, p=0.055) and BMI >30 kg/m2 were more frequent among the female subjects (21.0% vs 6.3%, p=0.006). Overall, BMI was significantly higher among the female subjects (25.8±5.5 kg/m2) compared with the male subjects (22.8±3.8 kg/m2) (p<0.001). The male subjects were more likely to report a past diagnosis of hypertension (43.8% vs 31.3%, p=0.052).
Table 1.
Demographic and clinical characteristics of the study sample.
| Characteristics | Statistics |
|---|---|
| Age (years) (mean ± SD) | 73.1±7.5 |
| Age group (years) n (%) 65–74 75–84 ≥85 |
251 (60%) 135 (32.6) 28 (6.8) |
Sex n (%)
|
65 (15.7) 349 (84.3) |
Education n (%)
|
240 (59.4) 108 (26.7) 33 (8.2) 23 (5.7) |
| Smoking n (%) | 28 (7.0) |
| Alcohol n (%) | 62 (15.3) |
| Coffee (n) (%) | 20 (5.0) |
| Colanut (n) (%) | 76 (18.8) |
History of cardiovascular disease (n) (%)
|
134 (33.3) 29 (7.2) 6 (1.5) 45 (11.6) 0 (0) |
| Migraine (n) (%) | 4 (1.0) |
| Report of use of antidepressant | 0 (0) |
| BMI (kg/m2) (mean±SD) | 25.3±5.4 |
| WC (cm) (mean±SD) | 87.5±13.8 |
| SBP (mmHg) (mean±SD) | 141.0±25.3 |
| DBP (mmHg) (mean±SD) | 84.0±13.8 |
Other: coronary artery disease, myocardial infarction, dyslipidaemia and chronic Kidney Disease, WC waist circumference, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, CESD-10 10 item Center for Epidemiologic Studies Depression scale, ESS Epworth sleepiness scale.
3.2. Profiles of OSA risk, EDS and depression of the study population
Of the 414 subjects participating in this study, 96 (23.2%) met the Berlin questionnaire criteria indicating a high risk of (OSA) with a male to female ratio of 1:1. Excessive daytime sleepiness (ESS score ≥11) was indicated in 62 (15.0%) of the subjects with a mean ESS score of 5.4±4.9. A total of 215 (51.9%) of the subjects had clinically significant depressive symptoms (CESD-10 score ≥10). The mean score on the CESD-10 scale for clinically relevant depressive symptoms was 10.6±5.5.
3.3. Correlates of high risk for OSA
3.3.1. Demographic variables and the risk of OSA
There was no significant gender difference between subjects who had high OSA risk (high OSA risk group) and those with low risk (low OSA risk group) (p=0.643). However, the high OSA risk group was younger compared with the low OSA risk group (p=0.011). The proportion of subjects in the high OSA risk group decreased progressively as age increased, however, this was not statistically significant (p=0.069). Other socio-demographic variables that were assessed did not relate significantly with the risk of OSA (Table 2).
Table 2.
Demographic and clinical characteristics of the subjects by OSA risk status.
| Characteristics | OSA risk |
p value | |
|---|---|---|---|
| Low risk | High risk | ||
| Age (years) (mean ± SD) | 73.6±7.7 | 71.4±6.8 | 0.011 |
| Age group (years) n (%) | |||
|
184 (73.3) 109 (80.7) 25 (89.3) |
67 (26.7) 26 (19.3) 3 (10.7) |
0.069 |
| Gender n (%) | |||
| Male Female |
50 (83.9) 268 (76.8) |
15 (23.1) 81 (23.4) |
0.981 |
| Education (%) | |||
|
184 (76.7) 127 (77.4) |
56 (23.3) 37 (22.6) |
0.856 |
| Smoking n (%) | 21 (6.8) | 7 (7.6) | 0.800 |
| Alcohol n (%) | 50 (16.1) | 12 (12.9) | 0.178 |
| Coffee n (%) | 16 (5.1) | 4 (4.3) | 0.757 |
| Kolanut n (%) | 61 (19.6) | 15 (16.1) | 0.451 |
| History of cardiovascular disease | |||
|
99 (31.9) | 35 (37.6) | 0.306 |
|
21 (6.8) | 8 (8.6) | 0.647 |
|
4 (1.3) | 2 (2.2) | 0.625 |
|
30 (10.1) | 15 (16.3) | 0.104 |
|
0 (0) | 0 (0) | – |
| Migraine n (%) | 3 (1.0) | 1 (1.1) | 0.927 |
| WC cm (mean±SD) | 86.5±13.9 | 90.7±13.1 | 0.011 |
| BMI (kg/m2) (mean±SD) | 24.7±5.1 | 27.3±5.8 | <0.001 |
| BMI group n (%) | |||
|
27 (96.4) 140 (80.9) 91 (75.2) 46 (62.2) |
1 (3.6) 33 (19.1) 30 (24.8) 28 (37.8) |
0.001 |
| SBP(mmHg)(mean±SD) | 141.9±26.3 | 138.1±21.2 | 0.207 |
| DBP(mmHg)(mean±SD) | 84.3±14.2 | 83.1±11.9 | 0.460 |
| CESD-10 score (mean±SD) | 9.7±5.2 | 13.4±5.6 | <0.001 |
| ESS score (mean±SD) | 4.9±4.4 | 7.4±6.0 | 0.011 |
Other: coronary artery disease, myocardial infarction, dyslipidaemia and chronic Kidney Disease, WC waist circumference, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, CESD-10 10 item Center for Epidemiologic Studies Depression scale, ESS Epworth sleepiness scale.
3.3.2. Clinical variables and the risk of OSA
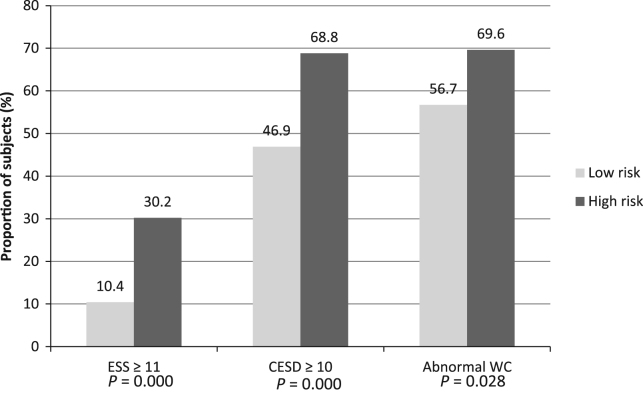
The high OSA risk group had higher mean body mass index (BMI) (p=0.011) and higher mean waist circumference (WC) (90.7±13.1 vs 86.5±13.9, p<0.001) compared to the low OSA risk group. When the subjects were re-stratified into two BMI groups (BMI ≥25 kg/m2 and <25 kg/m2), the high OSA group had a higher proportion of BMI ≥25 kg/m2 compared with the low OSA risk group (63.0% vs 45.1%, p < 0.001). Adjusting for gender, this difference remained significant in the female subjects (p<0.001) but disappeared in the male subjects (p=0.104). Similarly, when WC was categorized into normal and abnormal WC categories, the high OSA risk group had a significantly higher proportion of subjects in the abnormal WC category (69.6% vs 56.7%, p=0.029) (Fig. 1). Adjusting for gender, the difference was sustained in the female subjects (p=0.013) while it disappeared in the male subjects (p=0.887). None of the subjects reported use of an antidepressant.
Fig. 1.
Comparison of the prevalence of EDS and depression between subjects with high and low OSA risks.
3.3.3. Excessive daytime sleepiness, depressive symptoms and the risk of OSA
Subjects in the high OSA risk group were more likely to have excessive daytime sleepiness (ESS score ≥11) compared to the low risk group (30.2% vs 10.4%, p<0.001). Similarly, subjects in the high OSA risk group were more likely to be depressed (CESD score of ≥10) compared with those in the low risk group (68.8% vs 46.9%, p<0.001) (Fig. 1).
3.3.4. Logistic regression analysis
A logistic regression model was constructed using risk of OSA as the dependent variable and age, gender, WC, excessive daytime sleepiness (ESS score ≥11), depressive symptoms (CESD-10 score ≥10) as covariates. All but WC emerged as independent predictors of high OSA risk. When BMI was excluded from the model, all, except WC emerged as independent predictors of high OSA risk (Table 3).
Table 3.
Logistic regression of the predictors of high risk for OSA.
|
BMI included |
BMI excluded |
|||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p | OR | 95% CI | p |
| Age | 0.948 | 0.912–0.984 | 0.005 | 0.944 | 0.912–0.984 | 0.003 |
| Gender | 1.654 | 0.765–3.576 | 0.201 | 1.801 | 0.842–3.855 | 0.130 |
| ESS ≥11 | 3.930 | 1.881–6.941 | <0.001 | 3.565 | 1.910–6.655 | <0.001 |
| CESD ≥10 | 2.893 | 1.687–4.963 | <0.001 | 3.007 | 1.761–5.135 | <0.001 |
| WC | 1.037 | 0.499–2.155 | 0.922 | 1.680 | 0.945–2.988 | 0.077 |
| BMI >25 kg/m2 | 2.011 | 1.047–3.862 | 0.036 | |||
ESS Epworth sleepiness scale, CESD-10 10 item Center for Epidemiologic Studies Depression scale, WC waist circumference, BMI body mass index.
The odds of excessive daytime sleepiness (EDS) in subjects in the high OSA risk group were about 4 times compared with those in the low OSA risk group (p<0.001) Similarly, the odds of depression in participants in the OSA high risk group were about 3 times compared with those in the low risk low risk group (p<0.001). Older age was also independently related to lower odds OSA risk while BMI was associated with higher odds of OSA risk. The association between WC and OSA risk disappeared upon regression (Table 3). When BMI was removed from the model (in view of its inclusion in the estimation of OSA risk), the other variables, except WC, were not substantially modified (Table 3).
4. Discussions
4.1. Prevalence of high risk for OSA
In this primary care sample of elderly Nigerians, we found that about one out of every four (23.2%) of individuals were at a high risk for OSA. This is consistent with the findings of previous studies. In a large population-based study conducted in the United States [18], [19], 26% of adults were found to be at high risk of OSA. A similar study found a prevalence of high risk for OSA of 2 out of every 5 hospitalized adults aged >50 years and with a mean age of 65 years [20]. In a sample of community-dwelling elderly individuals studied by Hoch et al. [21], a prevalence of 26% was reported. Reports from the developing world also suggest a prevalence that falls within the range reported from the developed world [22]. In spite of the differences in methodology in exploring OSA risk and its related factors across various geographic regions and ethnic groups, it has been noted that comparisons of several epidemiologic studies have yielded similar prevalence rates [22].
4.2. Factors modifying the risk of OSA
4.2.1. Gender
In the present study, we found no significant gender difference in the risk of OSA while we found a male to female ratio of about 1:1. It is fairly well established that the risk, prevalence and severity of OSA have a male preponderance in the general population [23], however, these gender differences seem to disappear in the older age bracket [24]. This has been attributed, partly, to hormonal changes accompanying menopause which limited evidence suggests favors increased odds of OSA [24]. The male gender is under-represented in our study, constituting only 16.9% of the study population; this may have reduced likelihood of encountering individuals with high OSA risk.
4.2.2. Age
Age was significantly associated with OSA risk in this study such that the high OSA risk group was younger than the low OSA risk group. From evidence, it is generally held that the prevalence of snoring, high risk for OSA and, indeed, of OSA, increases with age, peaks in middle age and declines after age 65 years [18], [25]. However, the relationship between age and OSA risk in the elderly has not been commonly explored. Our finding is similar to that of a study conducted in the Southern stroke belt of the United States on a large sample with an age range of 49–93 years and a mean age of 67.5 years [26]. In the study, the subjects who were at a high risk for OSA were significantly younger than the low risk subjects. Some older studies have also demonstrated a decline in the prevalence of OSA after midlife [18].
4.2.3. Excessive daytime sleepiness
The prevalence of excessive daytime sleepiness (EDS) was high in our sample (15.0%) with a significantly higher prevalence in the high OSA risk group (30.2%) compared with the low OSA risk group (10.4%). When other factors were adjusted for, the odds of having EDS was four fold higher in the high OSA group compared with the low OSA group.
Excessive daytime sleepiness is common among elderly individuals [27], [28] with a prevalence of about 15%. Several recent reports have suggested that EDS could be a predictor of cognitive decline and possibly, a harbinger of dementia in the elderly [29], [30]. It has also been linked to increased risk of poor functional [31], psychosocial [32] and cardiovascular outcomes [33] and all-cause mortality [34]. Excessive daytime sleepiness is consistently reported as a strong association of OSA in the literature [35]. OSA is characterized by upper airway obstruction with consequent hypoxemia and sleep fragmentation; this leads to daytime somnolence and fatigue [36]. Excessive daytime sleepiness is such a cardinal feature of OSA that the American Academy of Sleep Medicine Task Force recommends an AHI ≥5 associated with the presence of symptoms such as excessive daytime sleepiness as a criterion for OSA diagnosis [37].
4.2.4. Depressive symptoms
In this study, the prevalence of depression was slightly above 1 out of every 2 individuals with none reporting being on an antidepressant agent. This is consistent with previous reports from Nigeria which suggest that depressive disorders are common, underdiagnosed and poorly treated with significantly high unmet needs for treatment in the older adult population in Nigeria [38], [39]. It is generally accepted that depression is not a normal part of aging, however, the various medical conditions associated with increasing age typically results in an increase in the risk of depression in older adults [40], [41].
Our analysis also showed that 2 out of every 3 subjects with a high OSA risk had clinically significant depressive symptoms. When other factors were controlled for, the odds of depression in the high OSA risk group was 3 that of the low risk group. The association between depressive symptoms and OSA risk was strong and independent. To the best of our knowledge, our study is the first study among populations of the aged in Nigeria to describe this relationship.
Our finding also mirrors reports from several previous studies that have consistently demonstrated a high prevalence (5–63%) of depression among individuals with OSA [42], [43]. While the exact pathophysiologic basis for the relationship between OSA and depression appears to be elusive, various explanations for their co-existence have been proposed. Firstly, the upper airway obstruction in OSA causes hypoxemia which results in sleep fragmentation which in turn causes excessive daytime sleepiness [36] with associated poor cognitive function and low mood [44]. Secondly and hypothetically, an abnormality in central and peripheral serotonin neurotransmission which has been implicated in depression also interferes with the hypoglossal nerve, impeding genioglossus muscle activity and compromising the airway [45]. Furthermore, a systematic review and meta-analysis of the results of randomized controlled trials established that the treatment of OSA with continuous positive airway pressure (CPAP) and mandibular advancement devices have significant and positive impact on depression [46], suggesting that an association may exist between OSA and depression. Nevertheless, the true nature of the interaction between OSA and depression remains, largely, unclear [42].
4.2.5. Adiposity and body fat distribution
We also found an association between BMI and WC and the risk of OSA on bivariate analysis. This association disappeared for WC on regression analysis but remained for BMI. As BMI increased, the proportion of subjects at a high risk for OSA increased from 3.6% in the underweight to 37.8% in the obese. This confirms previous studies which have repeatedly reported similar patterns [2]. Increased body adiposity is considered a strong risk factor for OSA. In fact, according to meta-analysis, up to 85% of OSA patients who underwent bariatric surgery had complete resolution of their sleep-disordered breathing after surgery [47]. When we removed BMI from the regression model, being one of the items in the Berlin questionnaire, WC remained non-significant in its contribution to the model but other variables remained independent in predicting OSA risk.
We found no association between hypertension and OSA risk in this study; this may be due to the effect of age. Reports from large population-based studies have indicated that a strong, graded association exists between hypertension and the risk of OSA with the prevalence of hypertension rising with increasing severity of OSA [48], [49]. However, this association is less prominent in the in the elderly compared with younger individuals [48], [49] and may be a consequence of a reduction in cardiovascular response to sleep arousal with increasing age [50].
4.3. Limitations
There are a few limitations which may reduce the generalization of our findings without negating the implication of our results. Our study was conducted only in one center, comprising mainly a tribe in Nigeria and excluding non-ambulant individuals. Using self-report might bring about recall bias among subjects. The subjects were however given adequate time to respond to the items in the questionnaires. This was also a cross-sectional study of the risk of OSA without objective sleep study. The Berlin questionnaire, in a recent study, demonstrated a low level of specificity (39%) in discriminating OSA in elderly individuals [51]. There is, therefore, a need for caution in ascribing causality to the associated factors. The evidence regarding the influence of gender on EDS and OSA risk in this study is further limited by the female preponderance in our sample. This reflects the gender distribution of the elderly population at the study site and not of the elderly population in Nigeria or the State of Osun [11]. Therefore, the findings from this study cannot be generalized to the elderly population in Nigeria.
5. Conclusions
The results of this study confirm that the risks of OSA and depression are high in the elderly. This study also provides evidence that OSA has significant negative impact on the mental health of the elderly as a high OSA risk independently increases the odds of depressive symptoms in them. As previously established, a direct and independent association was also found between excessive daytime sleepiness, increased body adiposity and increased risk of OSA. We also found that in people aged 65 years and above, the risk of OSA decreases with age as opposed to what has been documented in individuals under the age of 65 years. Larger community-based, prospective studies in sub-Saharan Africa are needed to confirm the findings of this study.
Acknowledgement
The authors wish to acknowledge the support of Dr Tola Oladimeji whose help with data processing was of immense value to the work.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
Contributor Information
Michael B. Fawale, Email: bimbofawale@yahoo.com.
Olanrewaju Ibigbami, Email: oibigbami@yahoo.com.
Ishaq Ismail, Email: Ishaqbola@yahoo.com.
Adekunle F. Mustapha, Email: mustakunle@yahoo.com.
Morenikeji A. Komolafe, Email: adeyoyin2001@yahoo.com.
Michael A. Olamoyegun, Email: mustakunle@yahoo.com.
Tewogbade A. Adedeji, Email: philipsade@yahoo.com.
References
- 1.Lindstrom V., Andersson K., Lintrup M., Holst G., Berglund J. Prevalence of sleep problems and pain among the elderly in Sweden. J Nutr Health Aging. 2012;16(2):180–183. doi: 10.1007/s12603-011-0356-2. [DOI] [PubMed] [Google Scholar]
- 2.Foley D.J., Monjan A.A., Brown S.L., Simonsick E.M., Wallace R.B., Blazer D.G. Sleep complaints among older persons: an epidemiological study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 3.Lam J.C., Sharma S.K., Lam B. Obstructive sleep apnoea: definitions, epidemiology and natural history. Indian J Med Res. 2010;131:165–170. [PubMed] [Google Scholar]
- 4.Drager L.F., Genta P.R., Pedrosa R.P., Nerbass F.B., Gonzaga C.C., Krieger E.M. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105(8):1135–1139. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Adewole O.O., Hakeem A., Fola A., Anteyi E., Ajuwon Z., Erhabor G. Obstructive sleep apnea among adults in Nigeria. J Natl Med Assoc. 2009;101(7):720–725. doi: 10.1016/s0027-9684(15)30983-4. [DOI] [PubMed] [Google Scholar]
- 6.Mbata G.C., Chukwuka J.C. Obstructive sleep apnea hypopnea syndrome. Ann Med Health Sci Res. 2012;2(1):74–77. doi: 10.4103/2141-9248.96943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Punjabi N.M. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinzer R., Vat S., Marques-Vidal P., Marti-Soler H., Andries D., Tobback N. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tufik S., Santos-Silva R., Taddei J.A., Bittencourt L.R. Obstructive sleep apnea syndrome in the Sao Paulo epidemiologic sleep study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commission NP . National Population Commission; Abuja, Nigeria: 2010. Federal Republic of Nigeria, 2006 population and housing census priority table volume III; population distribution by sex, state, LGA and senatorial district. [Google Scholar]
- 12.Chung F., Yegneswaran B., Liao P., Chung S.A., Vairavanathan S., Islam S. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822–830. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 13.Obaseki D., Kolawole B., Gomerep S., Obaseki J., Abidoye I., Ikem R. Prevalence and predictors of obstructive sleep apnea syndrome in a sample of patients with type 2 Diabetes Mellitus in Nigeria. Niger Med J. 2014;55(1):24–28. doi: 10.4103/0300-1652.128154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johns M. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Ozoh O., Okubadejo N., Akanbi M., Dania M. High-risk of obstructive sleep apnea and excessive daytime sleepiness among commercial intra-city drivers in Lagos metropolis. Niger Med J. 2013;54(4):224–229. doi: 10.4103/0300-1652.119607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andresen E.M., Malmgren J.A., Carter W.B. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 17.Boey K.W. Cross-validation of a short form of the CES-D in Chinese elderly. Int J Geriatr Psychiatry. 1999;14(8):608–617. doi: 10.1002/(sici)1099-1166(199908)14:8<608::aid-gps991>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Hiestand D.M., Britz P., Goldman M., Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130(3):780–786. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 19.Ancoli-Israel S., Kripke D.F., Klauber M.R., Mason W.J., Fell R., Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shear T.C., Balachandran J.S., Mokhlesi B., Spampinato L.M., Knutson K.L., Meltzer D.O. Risk of sleep apnea in hospitalized older patients. J Clin Sleep Med. 2014;10(10):1061–1066. doi: 10.5664/jcsm.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoch C.C., Reynolds C.F. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep. 1990;6:502–511. doi: 10.1093/sleep/13.6.502. [DOI] [PubMed] [Google Scholar]
- 22.Lee W., Nagubadi S., Kryger M.H., Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Punjabi N.M. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bixler E.O., Vgontzas A.N., Lin H.M., Ten Have T., Rein J., Vela-Bueno A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 25.Young T., Peppard P.E., Gottlieb D.J. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 26.Addison-Brown K.J., Letter A.J., Yaggi K., Mcclure L.A., Unverzagt F.W., Howard V.J. Age differences in the association of obstructive sleep apnea risk with cognition and quality of life. J Sleep Res. 2014;23(1):69–76. doi: 10.1111/jsr.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayley A.C., Williams L.J., Kennedy G.A., Berk M., Brennan S.L., Pasco J.A. Prevalence of excessive daytime sleepiness in a sample of the Australian adult population. Sleep Med. 2014;15(3):348–354. doi: 10.1016/j.sleep.2013.11.783. [DOI] [PubMed] [Google Scholar]
- 28.Vashum K.P., McEvoy M.A., Hancock S.J., Islam M.R., Peel R., Attia J.R. Prevalence of and associations with excessive daytime sleepiness in an Australian older population. Asia Pac J Public Health. 2015;27(2):2275–2284. doi: 10.1177/1010539513497783. [DOI] [PubMed] [Google Scholar]
- 29.Ohayon M.M., Vecchierini M.F. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162(2):201–208. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 30.Jaussent I., Bouyer J., Ancelin M.L., Berr C., Foubert-Samier A., Ritchie K. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35(9):1201–1207. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gooneratne N.S., Weaver T.E., Cater J.R., Pack F.M., Arner H.M., Greenberg A.S. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51(5):642–649. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsuno N., Jaussent I., Dauvilliers Y., Touchon J., Ritchie K., Besset A. Determinants of excessive daytime sleepiness in a French community-dwelling elderly population. J Sleep Res. 2007;16(4):364–371. doi: 10.1111/j.1365-2869.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- 33.Endeshaw Y., Rice T.B., Schwartz A.V., Stone K.L., Manini T.M., Satterfield S. Snoring, daytime sleepiness, and incident cardiovascular disease in the health, aging, and body composition study. Sleep. 2013;36(11):1737–1745. doi: 10.5665/sleep.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ensrud K.E., Blackwell T.L., Ancoli-Israel S., Redline S., Cawthon P.M., Paudel M.L. Sleep disturbances and risk of frailty and mortality in older men. Sleep Med. 2012;13(10):1217–1225. doi: 10.1016/j.sleep.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shear T.C., Balachandran J.S., Mokhlesi B., Spampinato L.M., Knutson K.L., Meltzer D.O. Risk of sleep apnea in hospitalized older patients. J Clin Sleep Med. 2014;10(10):1061–1066. doi: 10.5664/jcsm.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dempsey J.A., Veasey S.C., Morgan B.J., O’Donnell C.P. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Academy of Sleep Medicine . 3rd ed. American Academy of Sleep Medicine; Darien, IL: 2014. International classification of sleep disorders. [Google Scholar]
- 38.Baiyewu O., Smith-Gamble V., Lane K.A., Gureje O., Gao S., Ogunniyi A. Prevalence estimates of depression in elderly community-dwelling African Americans in Indianapolis and Yoruba in Ibadan, Nigeria. Int Psychogeriatr. 2007;19(4):679–689. doi: 10.1017/S1041610207005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gureje O., Kola L., Afolabi E. Epidemiology of major depressive disorder in elderly Nigerians in the Ibadan study of ageing: a community-based survey. Lancet. 2007;370(9591):957–964. doi: 10.1016/S0140-6736(07)61446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor W.D. Depression in the elderly. N Engl J Med. 2014;371(13):1228–1236. doi: 10.1056/NEJMcp1402180. [DOI] [PubMed] [Google Scholar]
- 41.Ismail Z., Fischer C., McCall W.V. What characterizes late-life depression? Psychiatr Clin North Am. 2013;36(4):483–496. doi: 10.1016/j.psc.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Ejaz S.M., Khawaja I.S., Bhatia S., Hurwitz T.D. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17–25. [PMC free article] [PubMed] [Google Scholar]
- 43.Gassino G., Cicolin A., Erovigni F., Carossa S., Preti G. Obstructive sleep apnea, depression, and oral status in elderly occupants of residential homes. Int J Prosthodont. 2005;18(4):316–322. [PubMed] [Google Scholar]
- 44.Bardwell W.A., Norman D., Ancoli-Israel S., Loredo J.S., Lowery A., Lim W. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med. 2007;5:21–38. doi: 10.1207/s15402010bsm0501_2. [DOI] [PubMed] [Google Scholar]
- 45.Jans L.A., Riedel W.J., Markus C.R., Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12(6):522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- 46.Povitz M., Bolo C.E., Heitman S.J., Tsai W.H., Wang J., James M.T. Effect of treatment of obstructive sleep apnea on depressive symptoms: systematic review and meta-analysis. PLoS Med. 2014;11(11):e1001762. doi: 10.1371/journal.pmed.1001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchwald H., Avidor Y., Braunwald E., Jensen M.D., Pories W., Fahrbach K. Bariatric surgery: a systematic review and metaanalysis. J Am Med Assoc. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 48.Bixler E.O., Vgontzas A.N., Lin H.-M., Ten Have T., Leiby B.E., Vela-Bueno A. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 49.Haas D.C., Foster G.L., Nieto F.J., Redline S., Resnick H.E., Robbins J.A. Age-dependent associations between sleep-disordered breathing and hypertension importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the sleep heart health study. Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 50.Goff E.A., O'Driscoll D.M., Simonds A.K., Trinder J., Morrell M.J. The cardiovascular response to arousal from sleep decreases with age in healthy adults. Sleep. 2008;31(7):1009–1017. [PMC free article] [PubMed] [Google Scholar]
- 51.Sforza E., Chouchou F., Pichot V., Herrmann F., Barthélémy J.C., Roche F. Is the berlin questionnaire a useful tool to diagnose obstructive sleep apnea in the elderly? Sleep Med. 2011;12:142–146. doi: 10.1016/j.sleep.2010.09.004. [DOI] [PubMed] [Google Scholar]