Abstract
Currently no effective vaccine is available for human visceral leishmaniasis(VL) caused by Leishmania donovani. Previously, we showed that centrin1 and p27gene deleted live attenuated Leishmania parasites (LdCen1−/− and Ldp27−/−) are safe, immunogenic and protective in animal models. Here, to assess the correlates of protection, we evaluated immune responses induced by LdCen1−/− and Ldp27−/− in human blood samples obtained from healthy, healed VL (HVL), post kala-azar dermal leishmaniasis(PKDL) and VL subjects. Both parasites infected human macrophages, as effectively as the wild type parasites. Further, LdCen1−/− and Ldp27−/− strongly stimulated production of pro-inflammatory cytokines including, IL-12, IFN-γ, TNF-α, IL-2, IL-6 and IL-17 in the PBMCs obtained from individuals with a prior exposure to Leishmania (HVL and PKDL). There was no significant stimulation of anti-inflammatory cytokines (IL-4 and IL-10). Induction of Th1 biased immune responses was supported by a remarkable increase in IFN-γ secreting CD4+ and CD8+ T cells and IL-17 secreting CD4+ cells in PBMCs from HVL cases with no increase in IL-10 secreting T cells. Hence, LdCen1−/− and Ldp27−/− are promising as live vaccine candidates against VL since they elicit strong protective immune response in human PBMCs from HVL, similar to the wild type parasite infection, mimicking a naturally acquired protection following cure.
Human visceral leishmaniasis (VL) or kala-azar is a potentially fatal disease with an estimated incidence of 0.2 to 0.4 million cases worldwide, causing 20,000–40,000 deaths every year1,2. Following therapeutic cure of VL, approximately 5–15% cases in India and 50–60% of cases in Sudan develop post kala-azar dermal leishmaniasis (PKDL), which manifests as a dermatitis3. PKDL patients are considered to be a major parasite reservoir particularly in India where transmission of VL occurs through anthroponotic route4. At present, there is no effective vaccine available to treat or prevent human VL5,6. The epidemiological observations that individuals recovered from a Leishmania infection develop lifelong immunity against reinfection, suggests the possibility of developing a prophylactic vaccine.
Till now in humans the best protection against leishmaniasis has been achieved by leishmanization, which represents inoculation of a low dose of live Leishmania promastigotes at the chosen site, usually the arm7,8,9,10, however; due to issues related to safety, this practice has been abandoned11. Studies have also shown that the limited persistence of parasite is an important factor to develop long lasting immunity12,13,14 and durable protective immunity can be induced by live attenuated parasites15,16. The major advantage is that these parasites are taken by the host cells similar to virulent parasites, deliver several antigens and do not require adjuvant as compared to the subunit or recombinant vaccine. Live attenuated parasites developed by genetically defined irreversible mutations would be safer compared to the parasite lines that have been developed through other means including long term culture, irradiation or chemical treatment. Limited persistence of the parasites and the immunomodulatory functions exerted on the antigen presenting cells by the attenuated parasites17 might help the host to develop a strong memory response.
We have developed two genetically defined live attenuated Leishmania donovani parasites, one lacking centrin1, a growth regulating gene (Ldcen1−/−)18 and another lacking p27 gene (Ldp27−/−)19, an essential component of cytochrome c oxidase complex, involved in oxidative phosphorylation. Attenuation of growth and virulence in both LdCen1−/− and Ldp27−/− occur specifically at the intracellular amastigote stage, hence the major advantage is that the parasites can be easily propagated as promastigotes and upon infection of host cells would undergo limited replication as amastigotes without causing pathology. As vaccine candidates, both LdCen1−/− and Ldp27−/− have been found to be safe, immunogenic and protective in various animal models15,16,19,20,21. Furthermore, both LdCen1−/− and Ldp27−/− induced production of pro-inflammatory cytokines in murine macrophages by classical activation and also skewed antigen presentation abilities of the macrophages more towards a Th1 response that favors development of protective immunity17. Lack of knowledge regarding clear biomarkers associated with immunological protection in humans remains a barrier for development of an effective vaccine. Previous studies have shown that Leishmania antigens that elicit higher IFN-γ and TNF-α response in healed VL (HVL) as compared to active VL cases have better potential as vaccine candidates22 and the vaccine candidates that provide strong protective efficacy in experimental models, generally induce Th1 recall response in PBMCs isolated from HVLcases23.
As the animal models do not fully recapitulate the full spectrum of human-parasite interactions, translation of results obtained from studies in the animal models remains a major challenge24,25. We have undertaken studies using human blood samples obtained from different clinical groups to identify the correlates of protection using two live attenuated parasites (LdCen1−/− and Ldp27−/−). Our results demonstrated a strong Th1 response to infection with the live attenuated parasites in the individuals pre-exposed to Leishmania parasite. Moreover, the infectivity to macrophages and the immune responses elicited in human PBMCs by the live attenuated parasites was similar to that by the wild type parasite.
Results
LdCen1−/− and Ldp27−/− parasites infect human macrophages similar to wild type
Previously we have shown that both LdCen1−/− and Ldp27−/− infect mouse macrophages effectively15,17,19. In the present study, we have determined infectivity of the parasites and compared to the wild type infection, in macrophages obtained by differentiation of human PBMCs. Macrophages were infected with early stationary phase promastigotes at 1:10 macrophage to parasite ratio. Following this protocol, >70% of the population was infected after 6 hours of infection with either LdCen1−/− or Ldp27−/− with an average of 5 to 6 parasites phagocytosed per cell. Further, the percentage of macrophages infected with the live attenuated parasites was similar to that of the wild type (Fig. 1).
Figure 1. In vitro infectivity of live attenuated Leishmania parasites.
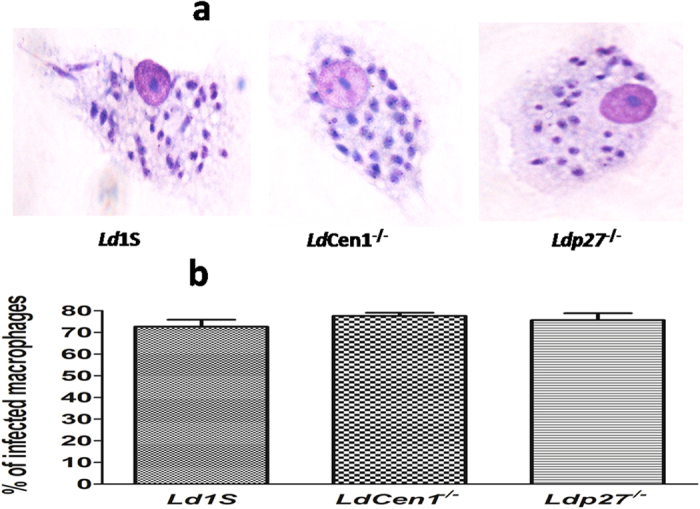
Human PBMCs differentiated macrophages were infected with wild type (Ld1S), LdCen1−/− or Ldp27−/− parasites for 6 hours in a ratio of 10:1 (parasites:macrophage). (a) Macrophages infected with Ld1S, LdCen1−/− and Ldp27−/− respectively after staining with Diff-Quik, (b) Percentages of infected macrophages determined by counting a minimum of 300 macrophages per sample under microscope (100X). Results are shown as mean ± SEM for three cover slips for each treatment and are pooled from three different experiments.
LdCen1−/− and Ldp27−/− strongly induce pro-inflammatory cytokines in PBMCs from healed VL and PKDL subjects
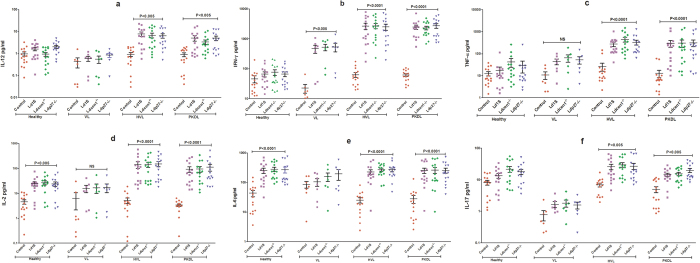
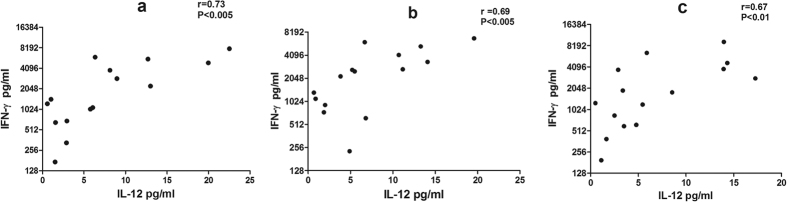
Cytokine response to LdCen1−/− and Ldp27−/− was evaluated in the culture supernatant of blood PBMCs of 15 Naïve healthy, 15 HVL, 15 PKDL and 7 active VL patients. TH1 response was evaluated in culture supernatants by measuring level of cytokines IL-12, IFN-γ, TNF-α and IL-2, (Fig. 2). We observed that both LdCen1−/− and Ldp27−/− induced significantly higher levels of IL-12 in the PBMCs from the HVL and PKDL subjects when compared to the control uninfected cells (P < 0.005) (Fig. 2a). No significant stimulation of IL-12 was observed in the VL group or healthy naive subjects (Fig. 2a). Further, both LdCen1−/− and Ldp27−/− stimulated very high levels of IFN-γ in HVL and PKDL as compared to the control uninfected cells (P < 0.0001) (Fig. 2b). In our study we found significantly higher stimulation of IFN-γ in response to LdCen1−/− and Ldp27−/− in the VL group also (P < 0.005), although level of stimulation was lower in comparison to HVL and PKDL (Fig. 2b). Significantly higher stimulation of TNF-α was found only in HVL and PKDL (P < 0.0001) group in comparison to the control uninfected cells (Fig. 2c). Additionally, significantly higher stimulation of IL-2 in response to parasite exposure was observed in HVL, PKDL and healthy subjects; however, level of significance was higher in HVL and PKDL (P < 0.0001) as compared to the healthy subjects (P < 0.005) (Fig. 2d). IL-6 showed significantly higher level of stimulation (P < 0.001) in HVL, PKDL and healthy groups with similar level of stimulation in all the three groups (Fig. 2e). Significantly higher level of stimulation of IL-17 was seen only in HVL and PKDL (P < 0.005) while no stimulation was seen in healthy and VL groups (Fig. 2f). Moreover, stimulation of all cytokines measured in the study showed by both LdCen1−/− and Ldp27−/− parasites was similar to that by the wild type parasites with no significant difference among the attenuated parasite and virulent infections (Fig. 2). As expected, PHA used as a positive control showed very high stimulation as compared to the control unstimulated cells for IFN-γ, TNF-α, IL-6 and IL-17 in PBMCs of all groups (data not shown). Furthermore, the data was compared between different clinical groups and we observed that stimulation in response to LdCen1−/− and Ldp27−/− and wild type parasites in HVL and PKDL groups was significantly higher for IL-12, IL-17, IFN-γ and TNF-α as compared to the healthy group; however, no difference was found in the level of significance for IL-2 and IL-6 (Supplementary Fig. 1). As compared to the VL group stimulation of all cytokines was significantly higher for HVL and PKDL groups. No significant difference in stimulation of cytokines was found between healthy and VL group except for IFN-γ (Supplementary Fig. 1). Of note, we also determined the correlation between different cytokines and found that PBMCs of HVL group showed positive pair wise correlation between the two critical TH1 cytokines i.e. IL-12 and IFN-γ after exposure to Ld1S (r = 0.73, P < 0.005), LdCen1−/− (r = 0.69, P < 0.005) and Ldp27−/− (r = 0.67, P < 0.005) (Fig. 3). However, no significant correlation was found among other cytokines (Data not shown).
Figure 2. Level of pro-inflammatory cytokines stimulated by wild type (Ld1S), LdCen1−/− and Ldp27−/− parasites in culture supernatant of PBMCs obtained from Healthy, HVL, VL and PKDL patients.
The results are expressed as scattering of individual values and data are given as Mean ± SEM (pg/ml) of (a) IL-12, (b) IFN-γ, (c) TNF-α, (d) IL-2, (e) IL-6 and (f) IL-17. Significance was determined by Mann-Whitney U test. P < 0.05 is considered statistically significant.
Figure 3.
Pairwise correlation of IL-12 and IFN-γ cytokines stimulated in response to (a) wild type (Ld1S), (b) LdCen1−/− and (c) Ldp27−/− parasites in culture supernatant of PBMCs obtained from HVL. Significance was determined by Spearman’s rank correlation test. P < 0.05 is considered statistically significant.
LdCen1−/− and Ldp27−/− parasites do not induce disease promoting anti-inflammatory cytokines in PBMCs of any group
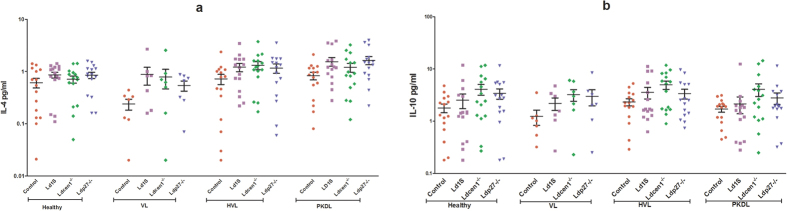
Anti-inflammatory response was evaluated by analyzing stimulation of IL-4 and IL-10, both being immunosuppressive cytokines that promote VL pathogenesis. There was no induction in levels of IL-4 or IL-10 in response to LdCen1−/− or Ldp27−/− in any of the groups examined in comparison with the control uninfected cells (P > 0.05), similar to the response to wild type parasite (Fig. 4). Positive control with PHA showed significantly higher stimulation only for IL-10 and this stimulation was found in all the groups including healthy and VL (data not shown). Further, no difference was found in stimulation of IL4 and IL-10 among healthy, VL, HVL and PKDL groups (Supplementary Fig. 2).
Figure 4. Level of anti-inflammatory cytokines stimulated by wild type (Ld1S), LdCen1−/− and Ldp27−/− parasites in culture supernatant of PBMCs obtained from Healthy, HVL, VL and PKDL patients.
The results are expressed as scattering of individual values and data are given in Mean ± SEM (pg/ml) of (a) IL-4 and (b) IL-10. Significance was determined by Mann-Whitney U test. P < 0.05 is considered statistically significant.
CD4+ and CD8+ T cells produce IFN-γ and IL-17 in response to live attenuated parasite in HVL group
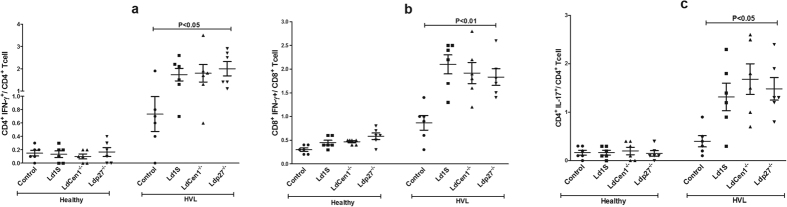
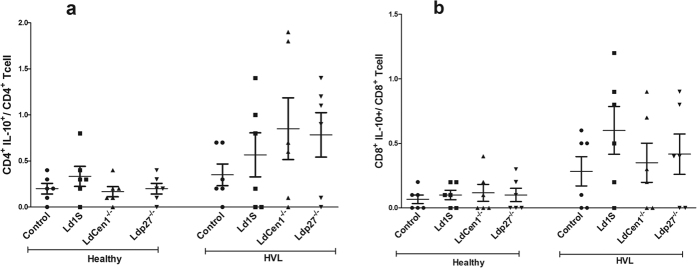
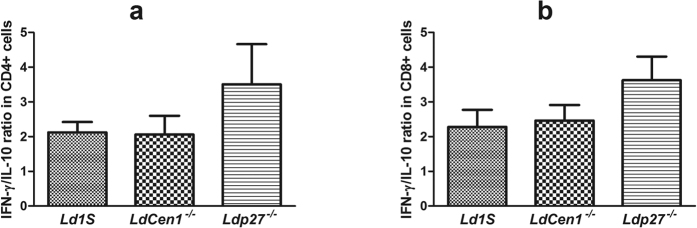
We analyzed the phenotype of cytokine producing cells in response to parasite exposure in healthy and HVL PBMCs. In HVL group, we observed that percentage of IFN-γ producing CD4+ and CD8+ T cells was significantly increased (P < 0.05) after stimulation with the live attenuated parasites as compared to the control unstimulated cells and the increase in CD8+ T cells was more significant (P < 0.01) as compared to the CD4+ T cells (P < 0.05). No significant increase was found in the percentage of IFN-γ producing CD4+ or CD8+ T cells in the healthy group (Fig. 5a,b). We also found significant increase in the percentage of IL-17 producing CD4+ T cells after live parasite stimulation in cultures from HVL individuals, in comparison to non-stimulated culture (P < 0.05) (Fig. 5c). Further, we evaluated the effect of parasite exposure on the percentage of IL-10 producing CD4+ and CD8+ T cells, and found no significant increase in the percentage of IL-10 producing CD4+ or CD8+ T cells in either healthy or HVL group (P > 0.05) (Fig. 6a,b). An increased ratio of IFN-γ/IL-10 has been correlated with parasite clearance in VL15. Parasite exposed PBMCs of HVL group showed an elevated ratio of IFN-γ/IL-10 producing CD4+ and CD8+ T-cells (Fig. 7a,b), suggesting that exposure of PBMCs to LdCen1−/− and Ldp27−/− live attenuated parasites induced a host-protective cell-mediated pro-inflammatory cytokines producing CD4+ and CD8+ T cells.
Figure 5. Percentage of IFN-γ and IL-17 -producing cells after stimulation of blood PBMCs obtained from healthy and HVL with wild type (Ld1S), LdCen1−/− and Ldp27−/− parasites.
The results are expressed as scattering of individual values and data are given in Mean ± SEM (pg/ml) of (a) % CD4+IFN-γ+/CD4+ cells, (b) % CD8+IFN-γ+/CD8+ cells and (c) % CD4+IL-17+/CD4+ cells.
Figure 6. Percentage of IL-10 producing cells after stimulation of blood PBMCs obtained from healthy and HVL with wild type (Ld1S), LdCen1−/− and Ldp27−/− parasites.
The results are expressed as scattering of individual values and data are given in Mean ± SEM (pg/ml) of (a) % CD4+IL-10+/CD4+ cells and (b) % CD8+IL-10+/CD8+ cell.
Figure 7. Ratio of IFN-γ/IL-10 producing cells after stimulation of blood PBMCs obtained from HVL with wild type (Ld1S), LdCen1−/− and Ldp27−/− parasites.
The results are expressed as bar graph and data are given in Mean ± SEM (pg/ml) of (a) Ratio IFN-γ/IL-10 producing CD4+ cells and (b) Ratio IFN-γ/IL-10 producing CD8+ cells.
Discussion
VL is one of the world’s most neglected parasitic diseases second only to malaria26. In this study, we report the immune response generated by two distinct live attenuated vaccine candidates (Ldcen1−/− and Ldp27−/−) in human PBMCs obtained from different clinical groups in comparison with the immune response generated by ex vivo infection with the wild type parasite.
We found that macrophage infectivity of both LdCen1−/− and Ldp27−/− was comparable to that of the wild type parasite, indicating that the attenuation did not limit the ability to infect human macrophages, consistent with the findings in animal models15,17,19. Previously, we have shown that infection by LdCen1−/− and Ldp27−/− parasites resulted in classical activation of murine macrophages (M1 phenotype) as reflected by increased production of pro-inflammatory cytokines, chemokines, reactive oxygen species, nitric oxide and reduced production of anti-inflammatory cytokines and arginaseactivity17. In our study the observed increase in pro-inflammatory cytokines production by Ldcen1−/− and Ldp27−/− in PBMCs culture from pre-exposed group is consistent with that observed in classically activated murine macrophages.
Studies have shown that the leishmaniasis outcome is mainly determined by Th1/Th2 balance and Th1 response is known to provide protection by an induction of pro-inflammatory response leading to macrophage activation and elimination of intracellular parasite, whereas a Th2 response increases susceptibility to the disease23,27,28. Live attenuated parasites that can predominantly elicit a Th1 response in individuals previously exposed to Leishmania infection would be good candidates for prophylactic and/or therapeutic vaccines, as was shown in similar studies with other vaccine formulations including recombinant antigen vaccines23. We evaluated pro-inflammatory response by measuring IL-12, IL-2, TNF-α, IFN-γ, IL-6 and IL-17 cytokines and found that all were significantly elevated in HVL and PKDL groups, suggesting that the live attenuated parasite strains induced a Th1 biased immunity.
IL-12 is a critical cytokine that helps in differentiation of naive CD4+ T cells into Th1 cells and plays a major immune-regulatory role in the development of cell-mediated immunity (CMI) during intracellular bacterial or parasitic infections by activating macrophages to produce IFN- γ and TNF-α29,30. Previously, we had shown that the PBMCs of LdCen1−/− immunized dogs20 and Ldp27−/− immunized mice16 produced elevated level of IL-12 in response to Leishmania antigen. Here we found that both LdCen1−/− and Ldp27−/− stimulated significantly higher level of IL-12 in PBMCs from HVL and PKDL cases that are already exposed to Leishmania parasite and mimic a naturally protected individual. IL-12 is secreted by antigen presenting cells (APCs) upon activation and dendritic cells serve as primary APCs in the initial phase of Leishmania infection31. The observed increase in production of IL-12 after infection with LdCen1−/− and Ldp27−/− indicates that the de novo antigen presenting function of APC may be fully functional in HVL and PKDL and that unlike virulent parasites, LdCen1−/− and Ldp27−/− parasites do not induce immunesuppressive activities. In contrast, IL-12 production in active VL cases was low suggesting a poor function of dendritic cells in that group. Our studies in mice have shown that the infection of macrophages with LdCen1−/− or Ldp27−/− does not alter the membrane architecture, hence does not affect the antigen presentation ability17. However, wild type Leishmania parasite depletes membrane cholesterol of macrophages, resulting in defective antigen presentation to T cells32. The robust production of IL-12 in our ex vivo studies with human PBMCs indicates that the attenuated parasites showed similar macrophage/dendritic cells activation as observed in murine studies. In our previous studies we have shown that both LdCen1−/− and Ldp27−/− can induce IFN-γ production in animal models15,16,17. In the present study, we observed significantly higher stimulation of IFN-γ in HVL and PKDL group as compared to the control uninfected cells and healthy group. Leishmanial antigens have previously been shown to induce stimulation of IFN-γ in PKDL and healed cases of VL, asymptomatic VL, cutaneous and mucocutaneous leishmaniasis33,34,35,36,37. In the present study significantly higher stimulation of IFN-γ was also found in the VL group as compared to the healthy group. PBMCs from VL cases do not generally produce IFN-γ in response to Leishmania antigen33,38,39, however, some studies have shown that human whole blood/PBMCs can indeed produce IFN-γ in active VL40,41,42,43. It is important to note that the level of stimulation of IFN-γ observed in the active VL samples were much lower than in HVL and PKDL samples. It is also pertinent to highlight that unlike most previous studies where antigenic re-stimulation was done using soluble Leishmania antigen, our study used live parasites (virulent and attenuated) for this purpose suggesting that immunomodulatory activities of the live parasites could be responsible for the observed levels of IFN-γ.
Evaluation of 6 defined Leishmania antigen vaccine candidates revealed that only 2 antigens, which were the most immunogenic and protective in murine model, induced IFN-γ production in HVL cases, indicating that Leishmania antigens that are protective in experimental models, do not necessarily induce immune response in HVL23. Recognition of a single antigen by T cells from individuals with different immunologic and genetic background cannot be always expected, suggesting that the appropriately modified whole parasites would make a better vaccine23. IL-12 is a potent inducer of IFN-γ44 and our findings also point towards a positive pair wise correlation between IL-12 and IFN-γ. Therefore, it is likely that the increased production of IL-12 observed here also induced production of IFN-γ, which further would activate macrophages for generation of ROS and NO for subsequent killing of intracellular Leishmania parasite after a virulent infection17. We analyzed the capacity of LdCen1−/− and Ldp27−/− to stimulate TNF-α and found that similar to IL-12, significantly higher stimulation of TNF-α was seen only in the HVL and PKDL groups. The observed increase in production of Th1 cytokines (IFN-γ, TNF-α and IL-12) in Leishmania exposed group (HVL or PKDL) compared to naive or active VL individual in response to live attenuated or wild type Leishmania parasites, is in accordance with earlier studies carried out with Leishmania antigen in HVL and PKDL groups33,34,40,44. Further, this increase in Th1 cytokine production in pre-exposed group suggested that the live attenuated parasites can induce protective effector response from memory response generated during resolution of infection in HVL individuals.
Th-17 cells play complementary role along with Th1 to provide protection against VL by producing IL-17 and IL-22 cytokines45. IL-17 is a pro-inflammatory cytokine, although little is known about its role during VL infection. Studies have shown that it provides protection against VL45 by activating innate immunity and inducing expression of innate inflammatory mediators, including IL-6, GM-CSF, IL-1, IL-8, TNF-α and inducible nitric oxide synthase (iNOS)46. Further, it acts synergistically with IFN-γ to strengthen Th1 response and also prevents Treg and IL-10+ cell expansion, which helps in controlling parasite replication47. In our study, both live attenuated parasites significantly stimulated IL-17 from blood PBMCs of HVL and PKDL groups, which will further promote the leishmanicidal activity of macrophages and improve TH1 response. Anti-inflammatory response induced by LdCen1−/− and Ldp27−/− in PBMCs was evaluated by measuring IL-4 and IL-10. Both IL-4 and IL-10 increase susceptibility for Leishmania infection by inducing a Th2 response and are important for the prediction of vaccine success22. We observed no significant difference in stimulation of IL-4 and IL-10 between parasite exposed and control-uninfected cells in any study group. Our group has previously shown that, production of IL-4 and IL-10 was not induced in animal models vaccinated with these live attenuated parasites after virulent challenge15,16,21. Previous studies have also shown that PBMCs from cured VL and naïve individual failed to stimulate IL-10 in response to Leishmania antigen vaccine candidates22,33. Importantly, lack of significant stimulation of IL-4 and IL-10 upon infection with LdCen1−/−, Ldp27−/− and wild type infection suggests that the two attenuated parasites do not induce a disease promoting immune response significantly different than that of the wild type infection indicating the safety of these attenuated parasites.
In order to assess the intracellular cytokines producing cells after exposure to the parasites, we analyzed the production of IFN-γ, IL-17 and IL-10 by CD4+ and CD8+ T cells. CD4+ cells with the Th1 type cytokine profile such as IFN-γ provide protection against leishmaniasis and it is well established that CD8+ T cells play a potential role in the cure of leishmaniasis, particularly VL by exerting its cytotoxic effect33,42. We found that PBMCs infected with live attenuated and wild type parasite displayed higher frequency of CD4+ T cells expressing IL-17 and both CD4+ as well as CD8+ T cells expressing IFN-γ in HVL, while no significant increase was seen in IL-10 secreting cells. This suggests that similar to a naturally occurring exposure to virulent parasites of the HVL individuals following cure in Leishmania endemic areas, ex vivo infection with LdCen1−/− and Ldp27−/− also produced an immune response consistent with a protection outcome. Similar results were observed in mice and dogs immunized with these live attenuated parasites where stimulation was found in Leishmania antigen experienced IFN-γ secreting effector CD4+ and CD8+ T cells but not in IL-10 secreting cells15,16,20. Previous studies with human subjects have also shown that the increase in IFN-γ producing CD4+ and CD8+ T cells in response to Leishmania antigen was stimulated mainly in PBMCs of individuals cured of VL42,48 and CL49 infection. CD8+ T-cells were shown to be exhausted in VL cases hence failed to produce IFN-γ in response to Leishmania antigen in whole blood cultures; however, the ability of CD8+ T-cells to produce antigen specific IFN-γ was restored following clinical cure (HVL)42. Another study with whole Leishmania parasite (live/dead) showed that proliferation of CD4+ and CD8+ T cells was significantly higher in cured CL individuals as compared to the healthy individuals. Further, the stimulation of IFN-γ secreting CD4+ and CD8+ T cells was higher with live attenuated parasites as compared to the killed parasites, providing another evidence that live attenuated Leishmania parasites are better in inducing protective immune response as compared to the killed parasites50. This increase in percentage of pro-inflammatory cytokine producing CD4+ and CD8+ T cells upon stimulation with live attenuated parasites in HVL group corroborates with our cytokine profile data observed in PBMCs culture supernatants. As IFN-γ and IL-10 are the two main regulatory cytokines governing the fate of the infection in VL, their ratio has been correlated to parasites elimination and indicator of vaccine success51. The increased ratio of IFN-γ: IL-10 producing CD4+ and CD8+ T cells in HVL after infection with LdCen1−/− and Ldp27−/− provide another correlate of protection. Previously, we have shown similar polarization to increased ratio of IFN-γ: IL-10 producing CD4+ and CD8+ T cells after parasite challenge in mice immunized with LdCen1−/− and Ldp27−/− 15,16.
One of the major challenges for elimination of VL is the presence of asymptomatic carriers that are difficult to identify and might serve as a parasite reservoir52. Asymptomatic individuals reach a state of acquired protection against leishmaniasis due to low level parasite infection as suggested by earlier studies53. The nature and durability of this protection is indeterminate, however, it is reasonable to argue that vaccination of asymptomatic carriers with live attenuated parasites would be beneficial in maintaining or perhaps adding to the protective immunity due to a favorable immune environment induced by the previous exposure to low level of virulent parasites. Previous analyses have indicated that in the absence of drug treatment, vaccination of asymptomatic carriers presents the best approach to prevent and eliminate VL54,55.
In summary, this study evaluated the capacity of LdCen1−/− and Ldp27−/− live attenuated vaccine candidates to elicit immune responses in PMBCs obtained from individuals with distinct clinical status, including naïve healthy, active VL, HVL and PKDL groups. Previously, we have established that LdCen1−/− and Ldp27−/− are immunogenic, protective and safe in animal models. The present work is a step forward, demonstrating that LdCen1−/− and Ldp27−/− are safe, immunogenic and induce protective immune response in human PBMCs comparable to the wild type parasite, therefore both have great potential as live attenuated vaccine against VL.
Material and Methods
Study subjects and ethical consideration
Study was carried out in 4 groups; Naïve healthy (n = 15), Healed VL (n = 15), PKDL (n = 15) and active VL (n = 7) patients. Individuals unexposed to Leishmania parasite i.e. living in VL non-endemic areas and negative for rk39 strip test were included in the naïve healthy group while those having a previous history of VL that was cured at least 1 year before the recruitment in the study were included in the HVL group. Patients reported at the Safdarjung hospital, New Delhi, showing clinical symptom of the disease and positive for PCR/ LD bodies or both in tissue biopsy/BMA were included in PKDL and VL group. Naïve healthy individuals were included because they are never exposed to Leishmania donovani and serve as negative controls while HVL and PKDL cases are already exposed and are presumably immune to recurrence of VL. The study was approved by and carried out under the guidelines of the Ethical Committee of the Safdarjung Hospital, India. Written informed consent for participation was obtained from all participants including the healthy subjects.
Parasite culture
Leishmania donovani promastigotes were cultured according to the procedure previously described56,57. In brief, wild type Ld1S was cultured in M199 medium containing 10% heat inactivated fetal bovine serum while LdCen1−/− and Ldp27−/− were grown in the same medium with added antibiotics; hygromycin (40 μg/ml) and G418 (40 μg/ml) for LdCen1−/− and G418 (40 μg/ml) for Ldp27−/−. All parasite cultures were incubated at 26 °C in a BOD incubator and early stationary phase promastigotes were used in the study.
PBMCs isolation and stimulation with parasites
Heparinized blood was collected from all the study subjects and PBMCs were isolated from blood by density gradient centrifugation with Ficoll-Hypaque (Sigma-Aldrich) method. The cells were cultured in RPMI 1640 supplemented with 10% FCS, glutamine, HEPES and penicillin (100 U/ml), and streptomycin (100 μg/ml). 2 × 105 PBMCs were plated in each well of 96 well flat bottom tissue culture plates and incubated in triplicate with (i) only media as unstimulated control, (ii) PHA (1 μg ⁄mL) as a positive control, (iii) 1 × 104 live wild type L. donovani (Ld1S), (iv) 1 × 104 live LdCen1−/− or (v) 1 × 104 live Ldp27−/− parasites. All parasite cultures were washed three times with PBS before incubation with PBMCs. After 120 hours of incubation in a 5% CO2 humidified atmosphere at 37 °C, supernatants were collected and stored at −70 °C until further analysis.
Multiplex ELISA for cytokine estimation
Cytokine levels in PBMC culture supernatants were determined using Human Cytokines, Bio-PlexProTM (Bio-Rad) kit according to the manufacturer’s protocol. Briefly, a 50 μl cell supernatant sample was incubated with antibody coupled beads. Immune complexes were washed, incubated with biotinylated detection antibody and finally, with streptavidin-phycoerythrin prior to assessing cytokine concentration titers. Manufacturer provided standards were used to prepare the standard curve for each cytokine. A total of 8 human cytokines representing either a pro-inflammatory (IL12, IL2, TNF-α, IFN-γ, IL6 and IL17) or an anti-inflammatory (IL4 and IL10) immune response were analyzed. Cytokine levels were determined using a multiplex array reader from Luminex™ Instrumentation System (Bio-Plex Workstation from Bio-Rad Laboratories). The cytokines concentration was calculated using software provided by the manufacturer (Bio-Plex Manager Software).
Preparation of human peripheral blood macrophages and in vitro macrophage infections
Infectivity of WT, LdCen1−/− and Ldp27−/− parasites was investigated using human PBMCs derived macrophages. PBMCs were isolated from the healthy blood sample as described above and plated in 24 well (1.5 × 106/well) plate containing lysine coated cover slips and incubated for adherence at 37 °C with 5% CO2 for 4 hours in RPMI 1640 with 10% FCS. After removing the non-adherent cells by washing with PBS, the plate was further incubated for 8 days in complete RPMI medium supplemented with human MCSF (50 ng/ml) for differentiation of macrophages. After every 48 hours’ cells were washed and fresh medium was added. On day-8 macrophages were infected with early stationary phase parasites at 1:10 macrophage to parasite ratio. After 6 hours of infection, cells were washed to remove non-internalized parasites. Cells adhered to cover slips were fixed in 100% methanol and stained with Diff Quik staining. Percentages of infected macrophages were determined by counting a minimum of 300 macrophages per sample under microscope (100X). Macrophage infectivity assay was performed in three technical and biological replicates for each parasite.
Intracellular staining and flow cytometry
To determine the cellular source/s and levels of cytokines, flow cytometry was performed with the blood PBMCs obtained from naïve (n = 6) and HVL (n = 6) individuals. PBMCs were stimulated with parasites as described above. Phorbolmyristate acetate (PMA) (50 ng/ml; Sigma-Aldrich) and ionomycin (500 ng/ml; Sigma-Aldrich) were added for 6 hours in PBMCs culture and used as positive controls. To block cytokine secretion, cultures were treated with GolgiStop (1 ug/ml, BD Biosciences) and further incubated for an additional 4 hours. After incubation, cells were washed and surface stained with the following antibodies: CD3-PerCPcy5.5, CD4-PE-CF594 and CD8-APC-H7 for 20 minutes at 4 °C. Surface stained cells were fixed and permeabilized using BD Cytoperm/cytofix kit (BD Biosciences) according to manufacturer’s instructions, washed in permeabilization buffer (BD) and stained for the presence of intracellular IFN-γ, IL-17 and IL-10 using PE-Cy7, Alexa Fluor® 647 and PE conjugated antibodies (BD) respectively. Isotype matched control antibodies were used to detect non-specific binding to the cells. Following intra cellular staining, samples were acquired on FACS Aria and analyzed using FACS Diva software(BD Biosciences). 7AAD staining (BD Biosciences) of a limited number of samples confirmed that the gated lymphocytes were >95% viable for both healthy and HVL group stimulated with parasite. Lymphocytes were gated on the basis of forward and side scatter. From these lymphocyte population CD3+ T-cells were gated to determine frequencies of CD4+ and CD8+ T-cells and for further analysis of IFN-γ, IL-10 and IL-17 expression.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism 5 software (San Diego, USA). Statistical significance was determined by Mann-Whitney U test. Correlation was evaluated using Spearman’s rank correlation test. P values of less than 0.05 were considered significant.
Additional Information
How to cite this article: Avishek, K. et al. Gene deleted live attenuated Leishmania vaccine candidates against visceral leishmaniasis elicit pro-inflammatory cytokines response in human PBMCs. Sci. Rep. 6, 33059; doi: 10.1038/srep33059 (2016).
Supplementary Material
Acknowledgments
This work was funded by grants from Department of Biotechnology (DBT), Government of India. K.A. and H.K. are grateful to the Council for Scientific and Industrial Research (CSIR) and the Indian Council of Medical Research (ICMR), India, for fellowships. We are thankful to T.A. Nagarajuna and Dr. Vinay Gupta of BD Academy, Jamia Hamdard, India for helpful discussions.
Footnotes
Author Contributions K.A., S.G., R.D., A.S., H.L.N. and P.S. conceived and designed the experiments. K.A. and H.K. performed the experiments. V.R., N.S.N., S.G., R.D., A.S., H.L.N. and P.S. contributed reagents/materials/analysis tools. K.A. and P.S. wrote the manuscript in consultation with H.L.N., S.G., R.D., A.S. and U.S.D. All authors read and approved the final manuscript.
References
- Nylén S. & Eidsmo L. Tissue damage and immunity in cutaneous leishmaniasis. Parasite Immunol. 34, 551–561 (2012). [DOI] [PubMed] [Google Scholar]
- WHO Fact sheet No 375 (2016).
- World Health Organization (WHO). Post kala-azar dermal leishmaniasis (PKDL): A manual for case management and control. Report of a WHO consultative meeting. Kolkata, India, 6–13 (2012).
- Singh R. et al. Visceral leishmaniasis, or kala-azar (KA): high incidence of refractoriness to antimony is contributed by anthroponotic transmission via post-KA dermal leishmaniasis. J. Infect. Dis. 194, 302–6 (2006). [DOI] [PubMed] [Google Scholar]
- Kedzierski L., Zhu Y. & Handman E. Leishmania vaccines: progress and problems. Parasitology 133, S87–112 (2006). [DOI] [PubMed] [Google Scholar]
- Gannavaram S. et al. Biomarkers of safety and immune protection for genetically modified live attenuated Leishmania vaccines against visceral leishmaniasis - discovery and implications. Front. Immunol. 5, 241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall L.-I., Zhang W.-W., Ranasinghe S. & Matlashewski G. Leishmanization revisited: Immunization with a naturally attenuated cutaneous Leishmania donovani isolate from Sri Lanka protects against visceral leishmaniasis. Vaccine 31, 1420–25 (2013). [DOI] [PubMed] [Google Scholar]
- Greenblatt C. L. The present and future of vaccination for cutaneous leishmaniasis. Prog. Clin. Biol. Res. 47, 259–85 (1980). [PubMed] [Google Scholar]
- Handman E. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14, 229–43 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellina O. I. Problem and current lines in investigations on the epidemiology of leishmaniasis and its control in the USSR. Bull. Soc. Pathol. Exot. Fil. 74, 306–18 (1981). [PubMed] [Google Scholar]
- Okwor I. & Uzonna J. Vaccines and vaccination strategies against human cutaneous leishmaniasis. Human Vaccines 5, 291–301 (2009). [DOI] [PubMed] [Google Scholar]
- The working group on research priorities for development of leishmaniasis vaccines et al. Vaccines for the leishmaniases: proposals for a research agenda. PLoS Negl. Trop. Dis. 5, e943 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwor I. & Uzonna J. Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies. Immunol. Res. 41, 123–36 (2008). [DOI] [PubMed] [Google Scholar]
- Bogdan C. Mechanisms and consequences of persistence of intra-cellular pathogens: leishmaniasis as an example. Cell. Microbiol. 10, 1221–34 (2008). [DOI] [PubMed] [Google Scholar]
- Selvapandiyan A. et al. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J. Immunol. 183, 1813–20 (2009). [DOI] [PubMed] [Google Scholar]
- Dey R. et al. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 190, 2138–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P. et al. Genetically modified live attenuated Leishmania donovani parasites induce innate immunity through classical activation of macrophages that direct the Th1 response in mice. Infect. Immun. 83, 3800–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvapandiyan A. et al. Centrin gene disruption impairs stage-specific basal body duplication and cell cycle progression in Leishmania. J. Biol. Chem. 279, 25703–10 (2004). [DOI] [PubMed] [Google Scholar]
- Dey R. et al. Characterization of a Leishmania stage-specific mitochondrial membrane protein that enhances the activity of cytochrome c oxidase and its role in virulence. Mol. Microbiol. 77, 399–14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza J. A. et al. Induction of immunogenicity by live attenuated Leishmania donovani centrin deleted parasites in dogs. Vaccine 31, 1785–92 (2013). [DOI] [PubMed] [Google Scholar]
- Fiuza J. A. et al. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine 33, 280–88 (2015). [DOI] [PubMed] [Google Scholar]
- Singh O. P., Stober C. B., Singh A. K., Blackwell J. M. & Sundar S. Cytokine responses to novel antigens in an Indian population living in an area endemic for visceral leishmaniasis. Plos Negl. Trop. Dis. 6, e1874 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R. et al. Evaluation of ex vivo human immune response against candidate antigens for a visceral leishmaniasis vaccine. Am. J. Trop. Med. Hyg. 82, 808–13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski L., Zhu Y. & Handman E. Leishmania vaccines: progress and problems. Parasitology 133, S87–112 (2006). [DOI] [PubMed] [Google Scholar]
- Garg R. & Dube A. Animal models for vaccine studies for visceral leishmaniasis. Indian J. Med. Res. 123, 439–54 (2006). [PubMed] [Google Scholar]
- Van Griensven J. & Diro E. Visceral leishmaniasis. Infect. Dis. Clin. North. Am. 26, 309–22 (2012). [DOI] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E. & Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168, 1675–84 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Heinzel F. P., Sadick M. D., Holaday B. J. & Gardner K. D. Jr. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T cell subsets. Ann. Inst. Paster. Immunol. 138, 744–9 (1987). [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84, 4008–27 (1994). [PubMed] [Google Scholar]
- Watford W. T., Moriguchi M., Morinobu A. & O’Shea J. J. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 14, 361–68 (2003). [DOI] [PubMed] [Google Scholar]
- Gorak P. M., Engwerda C. R. & Kaye P. M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28, 687–95 (1998). [DOI] [PubMed] [Google Scholar]
- Banerjee S. et al. Designing therapies against experimental visceral leishmaniasis by modulating the membrane fluidity of antigen-presenting cells. Infect. Immun. 77, 2330–42 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal H. et al. Role of CD8 (+) T cells in protection against Leishmania donovani infection in healed visceral leishmaniasis individuals. BMC Infect. Dis. 14, 653 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal H. et al. Evaluation of cellular immunological responses in mono- and polymorphic clinical forms of post kala-azar dermal leishmaniasis in India. Clin. Exp. Immunol. 185, 50–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. M. Jr. et al. Characterization of a membrane antigen of Leishmania amazonensis that stimulates human immune responses. J. Immunol. 146, 742–48 (1991). [PubMed] [Google Scholar]
- Russo D. M. et al. Human T cell responses to gp63, a surface antigen of Leishmania. J. Immunol. 147, 3575–80 (1991). [PubMed] [Google Scholar]
- Dos Santos P. L. et al. The severity of visceral leishmaniasis correlates with elevated levels of serum IL-6, IL-27 and sCD14. Plos Negl. Trop. Dis. 10, e0004375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S. et al. IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: the significance of amphotericin B in the control of Leishmania donovani infection in India. J. Immunol. 179, 5592–603 (2007). [DOI] [PubMed] [Google Scholar]
- Nylén S. & Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 28, 378–84 (2007). [DOI] [PubMed] [Google Scholar]
- Singh O. P. et al. Reassessment of immune correlates in human visceral leishmaniasis as defined by cytokine release in whole blood. Clin. Vaccine Immunol. 19, 961–66 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari N. et al. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J. Immunol. 186, 3977–85 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam S. et al. CD8 T cell exhaustion in human visceral leishmaniasis. J. Infect. Dis. 209, 290–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidwani K., Jones S., Kumar R., Boelaert M. & Sundar S. Interferon-gamma release assay (modified QuantiFERON) as a potential marker of infection for Leishmania donovani, a proof of concept study. Plos Negl. Trop. Dis. 5, e1042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P., Ray S., Sunder S., Dube A. & Naik S. Identification of Leishmania donovani antigens stimulating cellular immune responses in exposed immune individuals. Clin. Exp. Immunol. 143, 380–8 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitta M. G. et al. IL-17 and IL-22 are associated with protection against human kala-azar caused by Leishmania donovani. J. Clin. Invest. 19, 2379–87 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls J. K. & Linden A. Interleukin-17 family members and inflammation. Immunity 21, 467–76 (2004). [DOI] [PubMed] [Google Scholar]
- Nascimento M. S. et al. Interleukin 17A acts synergistically with interferon γ to promote protection against Leishmania infantum infection. J. Infect. Dis. 211, 1015–26 (2015). [DOI] [PubMed] [Google Scholar]
- Chamakh-Ayari R. et al. In Vitro evaluation of a soluble Leishmania promastigote surface antigen as a potential vaccine candidate against human leishmaniasis. Plos One 9, e92708 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naouar I. et al. Involvement of different CD4 (+) T cell subsets producing granzyme B in the immune response to Leishmania major antigens. Mediators Inflamm. 2014, 636039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nategi R. M. et al. Differential in vitro CD4+/CD8+ T-cell response to live vs. killed Leishmania major. Parasite immunol. 32, 101–10 (2010). [DOI] [PubMed] [Google Scholar]
- Silvestre R. et al. SIR2-deficient Leishmania infantum induces a defined IFN-γ/IL-10 pattern that correlates with protection. J. Immunol. 179, 3161–70 (2007). [DOI] [PubMed] [Google Scholar]
- Singh O. P., Hasker E., Sacks D., Boelaert M. & Sundar S. Asymptomatic Leishmania infection: a new challenge for Leishmania control. Clin. Infect. Dis. 58, 1424–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall L. I., Zhang W. W., Ranasinghe S. & Matlashewski G. Leishmanization revisited: immunization with a naturally attenuated cutaneous Leishmania donovani isolate from Sri Lanka protects against visceral leishmaniasis. Vaccine 31, 1420–5 (2013). [DOI] [PubMed] [Google Scholar]
- Das S., Matlashewski G., Bhunia G. S., Kesari S. & Das P. Asymptomatic Leishmania infections in northern India: a threat for the elimination programme? Trans. R. Soc. Trop. Med. Hyg. 108, 679–84 (2014). [DOI] [PubMed] [Google Scholar]
- Lee B. Y. et al. The economic value of a visceral leishmaniasis vaccine in Bihar State, India. Am. J. Trop. Med. Hyg. 86, 417–25 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvapandiyan A. et al. Expression of a mutant form of Leishmania donovani centrin reduces the growth of the parasite. J. Biol. Chem. 276, 43253–61 (2001). [DOI] [PubMed] [Google Scholar]
- Bhandari V. et al. Increased parasite surface antigen-2 expression in clinical isolates of Leishmania donovani augments antimony resistance. Biochem. Biophys. Res. Commun. 440, 646–51 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.